Abstract
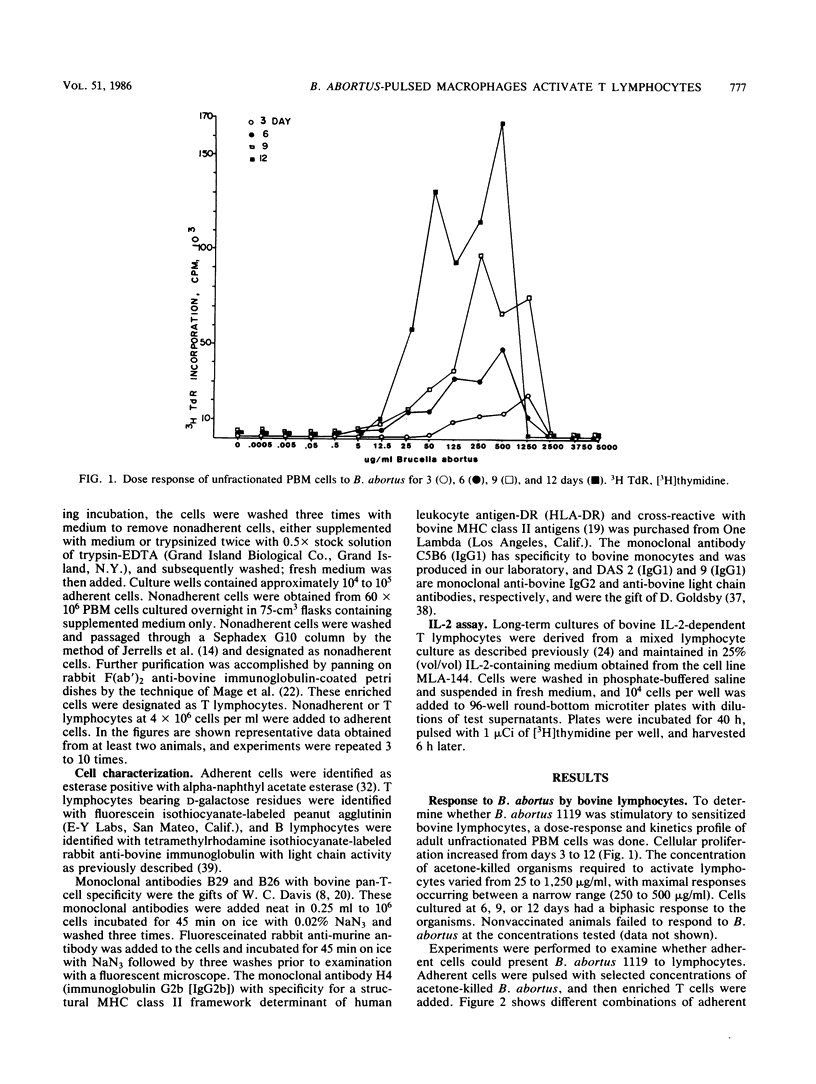
Brucella abortus-induced bovine macrophage-T-lymphocyte collaboration was studied as a prerequisite for the eventual clearance of this infectious organism. Esterase-positive, peripheral blood monocytes functioned as an adherent antigen-presenting cell population. A dual requirement for expression of bacterial antigens in combination with self major histocompatibility complex class II products was required by adherent cells for the activation of T lymphocytes. Comparison of antigen-presenting cell populations that were either trypsinized or nontrypsinized following B. abortus ingestion substantiated the need for phagocytosis and antigen processing. A monoclonal antibody (H4) directed against major histocompatibility complex class II determinants was able to block or, with complement, to abrogate T-lymphocyte responses. Measurement of both proliferation and interleukin 2 production via [3H]thymidine incorporation confirmed specific activation of an enriched T-lymphocyte population. These results indicate that in vivo-primed T lymphocytes of peripheral blood origin recognize phagocytized bacterial components of the facultative intracellular bacterium B. abortus and may contribute to the removal of the bacteria. Furthermore, bovine peripheral blood-adherent cells function as classic antigen-presenting cells, which suggests that macrophages are capable of processing this bacteria. Therefore, any lymphocyte-mediated dysfunction attributable to B. abortus most likely occurs at some point in the cascade of immune events following initial macrophage-T-lymphocyte collaboration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen P. M., Beller D. I., Braun J., Unanue E. R. The handling of Listeria monocytogenes by macrophages: the search for an immunogenic molecule in antigen presentation. J Immunol. 1984 Jan;132(1):323–331. [PubMed] [Google Scholar]
- Baldwin C. L., Winter A. J. Blastogenic response of bovine lymphocytes to Brucella abortus lipopolysaccharide. Infect Immun. 1985 Feb;47(2):570–572. doi: 10.1128/iai.47.2.570-572.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham J. R., Jeska E. L. Characterization of macrophage functions in mice infected with Brucella abortus. Infect Immun. 1981 Jun;32(3):1079–1083. doi: 10.1128/iai.32.3.1079-1083.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- Cheers C., Pagram F. Macrophage activation during experimental murine brucellosis: a basis for chronic infection. Infect Immun. 1979 Feb;23(2):197–205. doi: 10.1128/iai.23.2.197-205.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Campbell S. G. Immunity to intracellular bacteria. Vet Immunol Immunopathol. 1982 Jan;3(1-2):5–66. doi: 10.1016/0165-2427(82)90031-9. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Cellular antimicrobial immunity. CRC Crit Rev Microbiol. 1978;7(1):27–91. doi: 10.3109/10408417909101177. [DOI] [PubMed] [Google Scholar]
- Eibl M., Mannhalter J. W., Ahmad R. Macrophage-lymphocyte interaction in response to a bacterial antigen (E. coli). Clin Exp Immunol. 1982 Feb;47(2):260–268. [PMC free article] [PubMed] [Google Scholar]
- Farr A. G., Kiely J. M., Unanue E. R. Macrophage-T cell interactions involving Listeria monocytogenes--role of the H-2 gene complex. J Immunol. 1979 Jun;122(6):2395–2404. [PubMed] [Google Scholar]
- Fitzgeorge R. B., Solotorovsky M., Smith H. The behaviour of Brucella abortus within macrophages separated from the blood of normal and immune cattle by adherence to glass. Br J Exp Pathol. 1967 Oct;48(5):522–528. [PMC free article] [PubMed] [Google Scholar]
- Gullberg M., Ivars F., Coutinho A., Larsson E. L. Regulation of T cell growth factor production: arrest of TCGF production after 18 hours in normal lectin-stimulated mouse spleen cell cultures. J Immunol. 1981 Aug;127(2):407–411. [PubMed] [Google Scholar]
- Halliburton B. L., Hinsdill R. D. Recall of acquired cellular resistance in mice by antigens from killed Brucella. Infect Immun. 1972 Jan;5(1):42–47. doi: 10.1128/iai.5.1.42-47.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells T. R., Dean J. H., Richardson G. L., Herberman R. B. Depletion of monocytes from human peripheral blood mononuclear leukocytes: comparison of the sephadex G-10 column method with other commonly used techniques. J Immunol Methods. 1980;32(1):11–29. doi: 10.1016/0022-1759(80)90113-1. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Unanue E. R. Critical role of determinant presentation in the induction of specific responses in immunocompetent lymphocytes. J Exp Med. 1973 Apr 1;137(4):967–990. doi: 10.1084/jem.137.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H. Biological functions of t cell lines with specificity for the intracellular bacterium Listeria monocytogenes in vitro and in vivo. J Exp Med. 1982 Jun 1;155(6):1754–1765. doi: 10.1084/jem.155.6.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H., Simon M. M., Röllinghoff M., Wagner H. Interleukin 2 induction in Lyt 1+ 23- T cells from Listeria monocytogenes-immune mice. Infect Immun. 1982 Sep;37(3):1292–1294. doi: 10.1128/iai.37.3.1292-1294.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Simon M. M., Hahn H. Regulatory interactions between macrophages and T-cell subsets in Listeria monocytogenes-specific T-cell activation. Infect Immun. 1982 Dec;38(3):907–913. doi: 10.1128/iai.38.3.907-913.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin H. A., Calvert C. C., Bernoco D. Cross-reactivity of a monoclonal antibody with bovine, equine, ovine, and porcine peripheral blood B lymphocytes. Am J Vet Res. 1985 Apr;46(4):785–788. [PubMed] [Google Scholar]
- Lewin H. A., Davis W. C., Bernoco D. Monoclonal antibodies that distinguish bovine T and B lymphocytes. Vet Immunol Immunopathol. 1985 May;9(1):87–102. doi: 10.1016/0165-2427(85)90132-1. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mage M. G., McHugh L. L., Rothstein T. L. Mouse lymphocytes with and without surface immunoglobulin: preparative scale separation in polystyrene tissue culture dishes coated with specifically purified anti-immunoglobulin. J Immunol Methods. 1977;15(1):47–56. doi: 10.1016/0022-1759(77)90016-3. [DOI] [PubMed] [Google Scholar]
- Mannhalter J. W., Zlabinger G. J., Ahmad R., Eibl M. M. Human T cell proliferation in response to E. coli presented by autologous macrophages is antigen specific. Clin Exp Immunol. 1983 Oct;54(1):95–102. [PMC free article] [PubMed] [Google Scholar]
- Miller-Edge M., Splitter G. A. Bovine interleukin 2 (IL-2) production and activity on bovine and murine cell lines. Vet Immunol Immunopathol. 1984 Sep;7(2):119–130. doi: 10.1016/0165-2427(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Moreno E., Kurtz R. S., Berman D. T. Induction of immune and adjuvant immunoglobulin G responses in mice by Brucella lipopolysaccharide. Infect Immun. 1984 Oct;46(1):74–80. doi: 10.1128/iai.46.1.74-80.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Effects of nonionic, ionic, and dipolar ionic detergents and EDTA on the Brucella cell envelope. J Bacteriol. 1982 Nov;152(2):822–828. doi: 10.1128/jb.152.2.822-828.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscoplat C. C., Klausner D. J., Brunner C. J., Sloane E. D., Johnson D. W. Regulation of mitogen- and antigen-stimulated lymphocyte blastogenesis by prostaglandins. Infect Immun. 1979 Oct;26(1):311–315. doi: 10.1128/iai.26.1.311-315.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninnemann J. L., Stockland A. E., Condie J. T. Induction of prostaglandin synthesis-dependent suppressor cells with endotoxin: occurrence in patients with thermal injuries. J Clin Immunol. 1983 Apr;3(2):142–150. doi: 10.1007/BF00915485. [DOI] [PubMed] [Google Scholar]
- POMALES-LEBRON A., STINEBRING W. R. Intracellular multiplication of Brucella abortus in normal and immune mononuclear phagocytes. Proc Soc Exp Biol Med. 1957 Jan;94(1):78–83. doi: 10.3181/00379727-94-22860. [DOI] [PubMed] [Google Scholar]
- Raff H. V., Cochrum K. C., Stobo J. D. Macrophage-T cell interactions in the Con A induction of human suppressive T cells. J Immunol. 1978 Dec;121(6):2311–2315. [PubMed] [Google Scholar]
- Rosenstreich D. L., Mizel S. B. The participation of macrophages and macrophage cell lines in the activation of T lymphocytes by mitogens. Immunol Rev. 1978;40:102–135. doi: 10.1111/j.1600-065x.1978.tb00403.x. [DOI] [PubMed] [Google Scholar]
- Schlake W., Meyer E. M., Grundmann E. Histochemical identification of T and B areas in paraffin-embedded lymphoid tissues by demonstration of alpha-naphthyl acetate esterase (ANAE) activity. Pathol Res Pract. 1978 Oct;163(2):173–182. doi: 10.1016/s0344-0338(78)80087-9. [DOI] [PubMed] [Google Scholar]
- Smith H. Microbial interference with host defence mechanisms. Monogr Allergy. 1975;9:13–38. [PubMed] [Google Scholar]
- Smith W. G., Usinger W. R., Splitter G. A. Bovine con A-induced suppressor cells: generation, macrophage requirements and possible mechanisms of regulatory action. Immunology. 1981 May;43(1):91–100. [PMC free article] [PubMed] [Google Scholar]
- Splitter G. A., Everlith K. M. Suppression of bovine T- and B-lymphocyte responses by fetuin, a bovine glycoprotein. Cell Immunol. 1982 Jul 1;70(2):205–218. doi: 10.1016/0008-8749(82)90322-7. [DOI] [PubMed] [Google Scholar]
- Srikumaran S., Guidry A. J., Goldsby R. A. Production and characterization of monoclonal antibodies to bovine immunoglobulin G2. Am J Vet Res. 1982 Jan;43(1):21–25. [PubMed] [Google Scholar]
- Srikumaran S., Guidry A. J., Goldsby R. A. Production and characterization of monoclonal bovine immunoglobulins G1, G2 and M from bovine x murine hybridomas. Vet Immunol Immunopathol. 1984 Mar;5(4):323–342. doi: 10.1016/0165-2427(84)90002-3. [DOI] [PubMed] [Google Scholar]
- Usinger W. R., Splitter G. A. Two molecularly independent surface receptors identify bovine T lymphocytes. J Immunol Methods. 1981;45(3):209–219. doi: 10.1016/0022-1759(81)90299-4. [DOI] [PubMed] [Google Scholar]
- Vuento R., Eskola J., Leino R., Viander M., Toivanen A. Role of Ia-positive cells in the lymphocyte responses to Yersinia. Scand J Immunol. 1984 Aug;20(2):141–147. doi: 10.1111/j.1365-3083.1984.tb00987.x. [DOI] [PubMed] [Google Scholar]
- Weinberg D. S., Unanue E. R. Antigen-presenting function of alveolar macrophages: uptake and presentation of Listeria monocytogenes. J Immunol. 1981 Feb;126(2):794–799. [PubMed] [Google Scholar]
- Winter A. J., Verstreate D. R., Hall C. E., Jacobson R. H., Castleman W. L., Meredith M. P., McLaughlin C. A. Immune response to porin in cattle immunized with whole cell, outer membrane, and outer membrane protein antigens of Brucella abortus combined with trehalose dimycolate and muramyl dipeptide adjuvants. Infect Immun. 1983 Dec;42(3):1159–1167. doi: 10.1128/iai.42.3.1159-1167.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. The specific binding of Listeria monocytogenes-immune T lymphocytes to macrophages. I. Quantitation and role of H-2 gene products. J Exp Med. 1979 Nov 1;150(5):1143–1160. doi: 10.1084/jem.150.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]


