Abstract
Reversible protein phosphorylation has critical functions in the eukaryotic circadian negative feedback loops. In Neurospora, the FREQUENCY protein closes the circadian negative feedback loop by promoting the phosphorylation of its transcription activator, the WHITE COLLAR complex (WCC) and consequently inhibiting WCC activity. Here we show that protein phosphatase 4 is a novel component of the Neurospora clock by regulating both processes of the circadian negative feedback loop. The disruption of pp4 results in short period rhythms with low amplitude. In addition to its role in regulating FRQ phosphorylation and stability, PP4 also dephosphorylates and activates WCC. In contrast to PP2A, another phosphatase that activates WCC, PP4 has a major function in promoting nuclear entry of WCC. PKA, a WC kinase, inhibits WC nuclear localization. Furthermore, the FRQ-dependent WC phosphorylation promotes WCC cytosolic localization. Together, these results revealed WCC nucleocytoplasmic shuttling as an important step in the circadian negative feedback process and delineated the FRQ-dependent WCC inhibition as a two-step process: the inhibition of WCC DNA-binding activity followed by sequestration of WCC into the cytoplasm.
Keywords: circadian clock, frequency, Neurospora, protein phosphatase, WHITE COLLAR
Introduction
Eukaryotic circadian oscillators consist of autoregulatory negative feedback loops in which there are positive and negative elements (Dunlap, 1999; Allada et al, 2001; Young and Kay, 2001; Sehgal, 2004). In these negative feedback loops, the positive elements activate the transcription of the negative elements, whereas the protein products of the negative elements inhibit the activity of the positive elements. In all circadian model systems examined, posttranslational modification of clock proteins by phosphorylation has essential functions in clock functions (Price et al, 1998; Lowrey et al, 2000; Lin et al, 2002; Sathyanarayanan et al, 2004; Liu, 2005; Nakajima et al, 2005; Mori et al, 2007). Despite the evolutionary distances among eukaryotic organisms, there are remarkable similarities of the circadian clock mechanisms from fungi to animals (Liu and Bell-Pedersen, 2006; Heintzen and Liu, 2007).
In the filamentous fungus Neurospora crassa, two PAS-domain containing transcription factors, WHITE COLLAR-1 (WC-1) and WC-2, form a heteromeric WC complex (WCC) that activates the transcription of the frequency (frq) gene by binding to the Clock box (C-box) of its promoter (Crosthwaite et al, 1997; Loros and Dunlap, 2001; Cheng et al, 2001b, 2002; Froehlich et al, 2003; He and Liu, 2005; He et al, 2006; Belden et al, 2007). On the other hand, FRQ and FRH (a FRQ-interacting RNA helicase) form another complex (FFC) that represses frq transcription by inhibiting WCC activity through their physical interaction (Aronson et al, 1994a; Merrow et al, 1997; Denault et al, 2001; Cheng et al, 2001a, 2005; Froehlich et al, 2003; Schafmeier et al, 2005; He et al, 2006).
Reversible protein phosphorylation has critical functions in both processes of the Neurospora circadian negative feedback loop. Phosphorylation of WCC inhibits its activity and FRQ promotes the WCC phosphorylation by recruiting CK-1a (the homologue of human CKI delta/epsilon) and CKII to the WCC (He and Liu, 2005; He et al, 2005b, 2006; Schafmeier et al, 2005, 2006). The phosphorylation of WC-1 occurs sequentially: first by Protein Kinase A and then by the FRQ-recruited casein kinases (Huang et al, 2007). To reactivate WCC, protein phosphatase 2A (PP2A) counters the function of kinases and activates WCC by dephosphorylation (Yang et al, 2004; Schafmeier et al, 2005). The direct inhibition of the WCC biochemical activity by phosphorylation was thought to be the main cause that represses its function in vivo as dephosphorylation of WCC significantly enhanced its DNA-binding activity (He and Liu, 2005).
The same kinases that phosphorylate WCC also phosphorylate FRQ progressively (Garceau et al, 1997; Gorl et al, 2001; Yang et al, 2001, 2002, 2003; He et al, 2006). Phosphorylation of FRQ by CK-1a and CKII promotes its degradation through the ubiquitin-proteasome pathway mediated by ubiquitin E3 ligase SCFFWD-1 (He et al, 2003, 2005a), whereas PKA counters the role of the casein kinases by stabilizing FRQ (Huang et al, 2007). We have previously shown that two protein phosphatases, PP1 and PP2A, dephosphorylate FRQ and PP1 opposes the role of the casein kinases by stabilizing FRQ (Yang et al, 2004). The FRQ phosphorylation-degradation pathway has a major function in defining the circadian period length (Liu et al, 2000; Yang et al, 2003).
In this study, we identified protein phosphatase 4 (PP4) as a critical component of the Neurospora circadian clock by regulating both arms of the circadian negative feedback loop. PP4 dephosphorylates and stabilizes FRQ. More importantly, PP4 regulates WCC phosphorylation and strongly promotes its nuclear entry. Thus, our results uncover an important step in the negative feedback process and suggest that FRQ-dependent WCC inhibition is a two step process: first by removal of WCC from the DNA and then by sequestration of WCC in the cytoplasm.
Results
Disruption of pp4 results in a short period and low amplitude rhythm
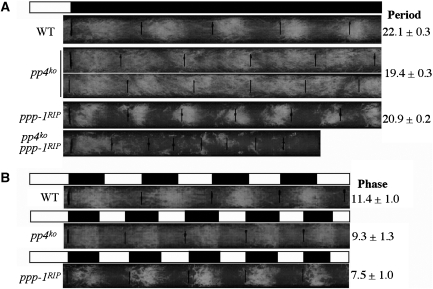
To further understand the role of protein phosphorylation in the Neurospora circadian clock, we carried out systematic deletion analyses of all potential serine/threonine protein phosphatases. One of these potential phosphatases (NCU08301.1) was named PP4 because it is homologous to the catalytic subunit of the human protein phosphatase 4 (PP4; also known as PPX or PPP4) with 60% amino acid identity and 72% similarity. PP4 is a highly conserved PP2A-related eukaryotic protein phosphatase and is known to have many PP2A-independent cellular roles (Cohen et al, 2005). The disruption of the Neurospora pp4 by homologous recombination revealed that it is not required for essential cell functions as the homokaryotic pp4ko strains have only mild growth defects (Figure 1A). Race tube assay is the most commonly used method to monitor the Neurospora circadian rhythm of conidiation and allows for the determination of period, phase and amplitude of the clock (Loros and Dunlap, 2001). Examination by race tube assay of the circadian conidiation rhythm of the pp4ko strains showed that they exhibited in constant darkness (DD) a rhythm that was ∼3 h shorter than that of the wild-type strain (Figure 1A). In contrast to the high amplitude robust rhythm in the wild-type strain, the amplitude of the conidiation rhythm of pp4ko strains was also very low, indicating that PP4 regulates both the period and amplitude of the rhythm. Similar to our previous result (Yang et al, 2004), the ppp-1RIP strain, which harbours hypomorphic mutations in the PP1 catalytic subunit gene, has a short period but exhibits robust circadian conidiation rhythm. Furthermore, the pp4ko ppp-1RIP double mutants showed an arrhythmic phenotype with significant growth defects. Under 12/12 h light/dark (LD) cycles, the phase of the conidiation rhythm of the pp4ko strain was ∼2 h earlier than the wild-type strain but was ∼2 h later than the ppp-1RIP strain (Figure 1B). This result contrasts with the rhythm exhibited in DD where the period of the pp4ko strain was shorter than the ppp-1RIP strain. Together, these race tube results suggest that PP4 is an important circadian clock component in Neurospora.
Figure 1.
Disruption of pp4 results in a short period and low amplitude circadian conidiation rhythm. Race tube assays showing the conidiation rhythms of the indicated strains in DD (A) and LD 12/12 (B) conditions. The period length or phase of the strains are indicated on the right.
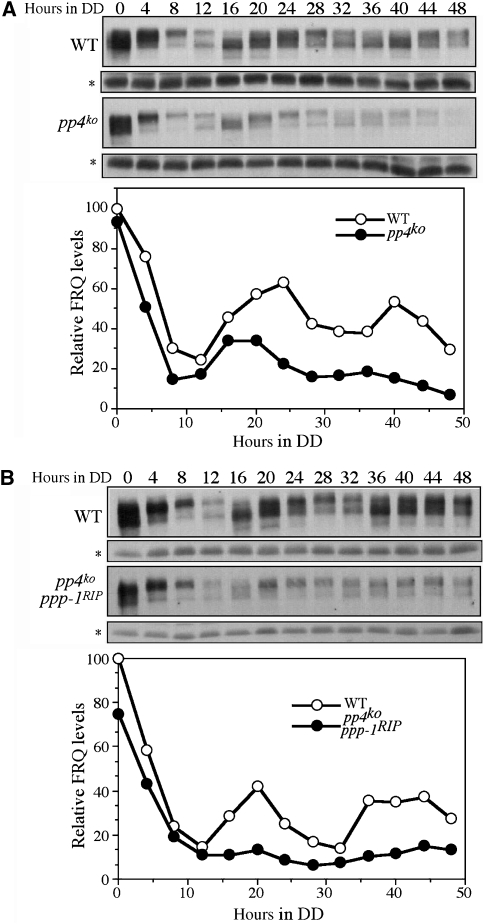
We then compared the circadian rhythms of FRQ expression in DD in wild-type and pp4ko strains. The levels of FRQ along with phosphorylation status in the wild-type strain oscillate in constant darkness (DD) (Figure 2A). In the pp4ko mutant, the overall FRQ levels were significantly lower than the wild-type strain and FRQ oscillates with low amplitude. In addition, although the phases of the FRQ oscillation were similar in both strains after the initial LD transition, FRQ became rapidly phosphorylated in the pp4ko mutant by DD16 and the newly synthesized hypophosphorylated FRQ started to appear at DD28, consistent with its shorter period phenotype on race tubes. Constant low levels of FRQ were also seen in the pp4ko ppp-1RIP double mutant after the LD transition and FRQ protein stayed hyperphosphorylated and arrhythmic in DD (Figure 2B). These results further support the importance of PP4 in the Neurospora circadian clock.
Figure 2.
Low amplitude or arrhythmic FRQ expression in the pp4KO strains. (A) Western blot analysis showing that FRQ oscillates with a low amplitude and short period in the pp4KO strain. (B) Western blot analysis showing the loss of FRQ oscillation in the pp4KO ppp-1RIP strain. The densitometric analyses of these experiments are shown below. A constantly expressed protein was indicated by the asterisks.
FRQ is hyperphosphorylated and unstable in the pp4ko strains and FRQ associates with PP4
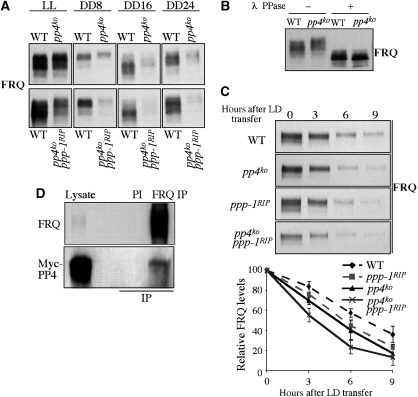
The short period rhythm of the pp4ko strain suggests that PP4 may regulate FRQ phosphorylation and FRQ stability. Indeed, hyperphosphorylation of FRQ was observed in the pp4ko single and pp4ko ppp-1RIP double mutants for protein samples harvested in LL and DD by side-by-side comparison (Figure 3A and B). Furthermore, the levels of FRQ in DD were also significantly lower in these mutants comparing to the wild-type strain. As we previously showed that FRQ levels in the ppp-1RIP mutant was comparable to the wild-type strain (Yang et al, 2004), this indicates that PP4 has a more important function in maintaining the FRQ levels. To determine whether the short period rhythm of the pp4ko mutant is due to increased FRQ degradation rate, we measured FRQ degradation rate after LD transfer, a process that triggers FRQ degradation (Liu et al, 2000; He et al, 2003). As shown in Figure 3C, the FRQ degradation rate was faster in the pp4ko mutant than the wild-type strain and was further increased in the pp4ko ppp-1RIP double mutant. These results indicate that PP4, as PP1, inhibits FRQ phosphorylation and stabilizes FRQ protein.
Figure 3.
FRQ is hyperphosphorylated and rapidly degraded in the pp4KO strains. (A) Western blot analysis showing that FRQ levels are low and hyperphosphorylated in the pp4KO and pp4KO ppp-1RIP strains. (B) Mobility shifts of FRQ protein in SDS–PAGE could be reversed by λ phosphatase treatments. (C) Western blot analysis showing the degradation of FRQ after a LD transition in the indicated strains. The densitometric analyses from three independent experiments are shown below. (D) Immunoprecipitation assay showing that FRQ associates with PP4 in vivo. IP using preimmune (PI) serum was used as the negative control.
The hyperphosphorylation of FRQ in the pp4ko mutant suggests that FRQ is a PP4 substrate. To examine this, we performed immunoprecipitation assay using a strain that expresses Myc-tagged PP4. As shown in Figure 3D, immunoprecipiation by FRQ antibody but not FRQ preimmune serum specifically pulled down Myc-PP4, indicating that FRQ associates with PP4. These results suggest that FRQ is a PP4 substrate in vivo.
WC hyperphosphorylation and reduced WCC activity in the pp4ko strain
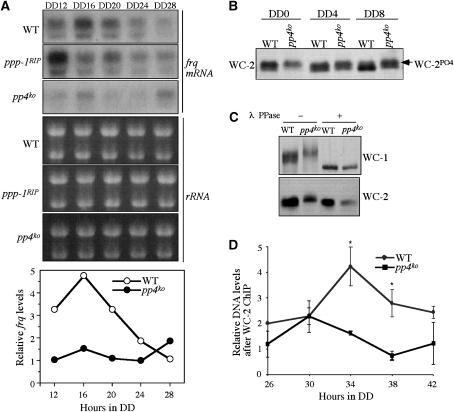
Although PP4 and PP1 are both involved in regulating FRQ phosphorylation and stability, the low amplitude rhythms in the pp4ko mutant suggest that PP4 has an additional role in the circadian clock. To determine such a role, we compared the levels of frq mRNA in the wild-type, pp4ko and ppp-1RIP strains in DD over a circadian cycle (Figure 4A). We found that despite the earlier phase, the levels of frq mRNA in the ppp-1RIP strain were comparable to the wild-type strain. In contrast, frq mRNA in pp4ko strain was maintained low levels at these time points, suggesting that frq transcription is regulated by PP4.
Figure 4.
Hyperphosphorylation of WC-2 and reduced WCC activity in the pp4KO strain. (A) Northern blot analyses showing the frq mRNA expression levels in DD in the indicated strains. (B) Western blot analysis showing that WC-2 is hyperphosphorylated in the pp4KO strain. To analyse the phosphorylation profile of WC-2, 10% SDS–PAGE gels containing a ratio of 139:1 acrylamide/bisacrylamide was used. (C) Western blot analysis showing that WC-1 and WC-2 are hyperphosphorylated in the pp4KO strain. Strains were cultured in LL. λ phosphatase treatment showed that the mobility shifts were due to phosphorylations. (D) The results of ChIP assays showing that WC-2 binds to the frq C-box at low levels in the pp4KO strain. Three independent experiments were performed and the error bars indicate the standard deviations (*P<0.05).
The low levels of frq mRNA and FRQ protein suggest WCC activity is impaired in the pp4 mutant. As phosphorylation of WCC inhibits its function in activating frq transcription, we examined whether WC phosphorylation is affected in the pp4ko mutant. Under constant light condition (LL), WCs are hyperphosphorylated and become dephosphorylated after the LD transition (He and Liu, 2005). Thus, we compared the WC-2 phosphorylation profiles in the wildtype and pp4ko strains during the dephosphorylation process. As shown in Figure 4B, WC-2 became dephosphorylated after 8 h in DD in the wild-type strain. In contrast, significant amount of hyperphosphorylated WC-2 could still be seen at DD8 in the pp4 mutant. To compare WC-1 phosphorylated profiles, longer eletrophoresis was used. Similar to WC-2, WC-1 was also found to be hyperphosphorylated in the pp4ko strain (Figure 4C). The dephosphroylation of Wcs by lamda phosphatase showed that the mobility shifts of WCs are indeed due to phosphorylation. This result indicates that PP4 counteracts WC phosphorylation and the WCs may be PP4 substrates. On the other hand, WC phosphorylation profiles were not significantly affected in the ppp-1RIP strain (data not shown), suggesting that PP1 is not a major WC phosphatase.
To demonstrate whether WCC's ability to bind DNA is inhibited in the pp4ko strain, we performed chromatin immunoprecipitation (ChIP) assay to examine the WCC binding to the C-box of frq promoter in vivo. Consistent with previous results (He et al, 2006; Belden et al, 2007), WCC binding to the C-box was rhythmic in the wild-type strain, peaking around DD34 (Figure 4D). For the pp4ko strain, the levels of WCC binding to the C-box of frq promoter were maintained at low levels at these time points. This indicates that despite comparable WC levels in the pp4ko strain to wild-type, WCC cannot bind to the frq promoter efficiently, providing a molecular explanation for the low amplitude rhythms in the pp4ko strain.
PP4 is required for nuclear enrichment of WCC
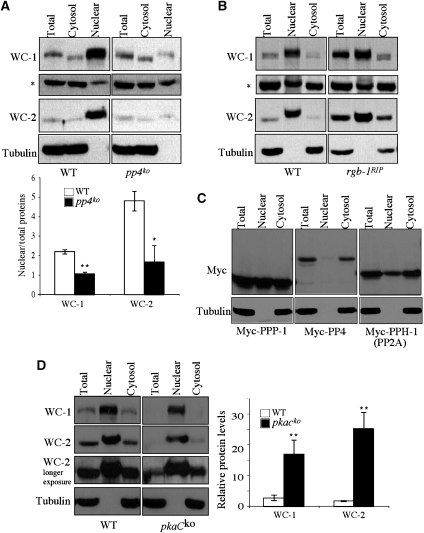
We and others have previously shown that the phosphorylation of WCC inhibits its DNA binding ability in vivo and the desphosphorylation of WCC significantly enhances its DNA-binding activity in vitro (He and Liu, 2005; He et al, 2005b, 2006; Schafmeier et al, 2005; Huang et al, 2007). Although the phosphorylation-promoted removal of the WCC from the promoter DNA should have an important function in the inhibition of WCC activity, we wondered whether an additional mechanism also contributes to the inhibition of WCC. One possible mechanism is to regulate the cellular localization of WCC by preventing its entry into the nucleus. To test this possibility, we compared the WC localization between the wild-type and pp4ko strains by fractionation. The absence of the cytoplasmic marker tubulin in the nuclear fractions indicated that our nuclear fractions were free of cytoplasmic contamination (Figure 5). Consistent with previous results (Talora et al, 1999; Schwerdtfeger and Linden, 2000; Cheng et al, 2003), we found that in the wild-type strain, both WCs are highly enriched in the nucleus, indicating that they are mostly nuclear proteins (Figure 5A). However, low levels of WCs could be seen in the cytoplasmic fraction, suggesting that the WCs may shuttle between nucleus and cytoplasm. In contrast to the wild-type strain, the nuclear enrichment of the WCs was abolished in the pp4ko strain and the nuclear levels of both WCs were comparable to those in the cytosolic fractions, despite similar WC levels in total extracts in these two strains. This result indicates that PP4 is required for the nuclear enrichment of WCC and the weak WCC binding to the frq promoter in the pp4ko strain is mostly due to its low nuclear WC levels. On the other hand, FRQ localization was not affected in the pp4ko strain (data not shown), suggesting that PP4 specifically regulates WC localization.
Figure 5.
The loss of nuclear enrichment of WCC in the pp4KO strain. Western blot analyses showing the levels of WC-1 and WC-2 in the total extracts, nuclear or cytosolic fractions in the pp4KO (A) and rgb-1RIP (B) strains. The tubulin was used to show that the nuclear fractions were free of cytosolic proteins. The asterisk indicates a protein band presented in both nuclear and cytosolic fractions non-specifically detected by our WC-1 antibody. Densitometric analyses from three independent experiments were shown for pp4KO strain (*P<0.05, **P<0.01). The error bars indicate the standard deviations. (C) Western blot analyses showing the levels of Myc-tagged PPP-1, PP4 and PP2A catalytic subunits in different cellular fractions. Cultures were grown in LL. (D) Western blot analysis comparing the levels of WC-1 and WC-2 in different cellular fractions between the wild-type and pkaCKO strains in LL. Note that pkaCKO has much lower levels of WC proteins in the total extract than the wild-type, but they had comparable WC amounts in the nuclear fractions. The densitometric analyses from three independent experiments were shown (**P<0.01).
Similar to PP4, PP2A was also shown to dephosphorylate the WCs and activate its activity (Yang et al, 2004; Schafmeier et al, 2005). However, the WC localization in the rgb-1RIP strain, which lacks one of the PP2A regulatory subunit, was similar to that of the wild-type strain with high levels of WCC accumulated in the nucleus (Figure 5B). Thus, PP2A does not have a major function in regulating WCC localization. These results suggest that despite the common role of PP2A and PP4 in activating WCC activity by dephosphorylation in vivo, they mainly function at two distinct steps: PP2A activates the DNA-binding activity of WCC, whereas PP4 mainly promotes the nuclear entry of WCC.
We then examined the cellular localization of different phosphatases by expressing c-Myc epitope tagged full-length proteins in a wild-type strain. As shown in Figure 5C, PPP-1 and PPH-1 (the catalytic subunit of PP2A) were found present in both nuclear and cytosolic fractions. However, most of the PP4 existed in the cytosolic fraction. This result suggests that PP4 carries out most of its functions in the cytoplasm, consistent with its role in promoting WCC nuclear entry by dephosphorylating WCs in the cytosplasm. On the other hand, PP2A may dephosphorylate WCC in both nucleus and cytoplasm.
PKA promotes cytoplasmic location of WCC
To further examine the role of WCC phosphorylation in regulating its localization, we reasoned that a mutant with impaired WC phosphorylation should show result that is opposite of what we found in the pp4 mutant. Thus, we examined the WCC localization in a PKA catalytic subunit (pkac) mutant. We have previously shown that PKA functions as a priming kinase for WC phosphorylation and WCs are hypophosphorylated in a pkacko mutant (Huang et al, 2007). In the pkacko strain, WC levels are very low but highly active in frq activation. As shown in Figure 5D, despite of its extremely low WC levels, the nuclear WC levels in the pkacko mutant are comparable to those of the wild-type strain, indicating that WCC is significantly more enriched in the nucleus in the pkacko mutant than in the wild-type strain. This result provides a molecular explanation for the high WCC activity in the pkacko mutant and further supports the conclusion that WCC phosphorylation promotes its cytoplasmic localization.
FRQ-dependent WCC phosphorylation inhibits its nuclear enrichment
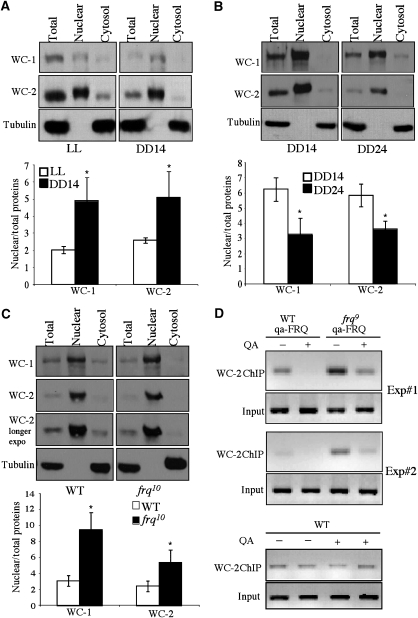
To further demonstrate the role of WC phosphorylation in inhibiting WC nuclear localization, we also compared the WCC localization between cultures grown in LL and DD14. In LL, WCs are hyperphosphorylated, whereas WCs are hypophosphorylated at DD14 (He and Liu, 2005; Schafmeier et al, 2005; He et al, 2006). As shown in Figure 6A, the nuclear enrichment of both WC-1 and WC-2 were consistently higher at DD14 than in LL. This result is in agreement with our previous ChIP results showing that the levels of WCC binding to the frq C-box at DD14 is higher than those in LL (He et al, 2006; Huang et al, 2007) and further suggests that light-induced WC phosphorylation is part of the photoadaptation mechanism.
Figure 6.
Phosphorylation and temporal-regulated WCC cellular localization. (A, B) Western blot analyses showing the levels of WC-1 and WC-2 in different cellular fractions. In these gels, the amounts of the nuclear extracts loaded were 1/3 of those in total and cytosolic fractions. Wild-type cultures grown in LL and DD14 were used in (A). In (B), wild-type cultures grown in DD14 and DD24 were compared. The densitometric analyses from three independent experiments were shown below (*P<0.05). The error bars indicate the standard deviations. (C) Western blot analyses comparing the levels of WC-1 and WC-2 in different cellular fractions between the wild-type and frq10 strains. The densitometric analyses from three independent experiments were shown below (*P<0.05). (D) ChIP assay using WC-2 antibody comparing the levels of WCC binding to the frq C box in the wild-type, WT,qa-FRQ and frq9,qa-FRQ strains. For experiments in (C) & (D), cultures were harvested at DD24.
The FRQ-dependent-WC phosphorylation states exhibit circadian rhythms: WCs are hypophosphorylated at ∼DD14 (the peak of its DNA-binding activity) and become extensively phosphorylated at ∼DD24 (the trough of its DNA-binding activity) (Schafmeier et al, 2005; He et al, 2006; Huang et al, 2007). Because phosphorylation of WCC regulates its localization, we expected that the nuclear enrichment of WCC should also exhibit a circadian rhythm. As expected, the ratios of the nuclear/total WC proteins were higher at DD14 than at DD24 (Figure 6B), indicating that there were more nuclear WCC when it is active and less nuclear WCC when it is inhibited. Consistent with this result, more cytosolic WC-1 was seen at DD24, a time point when WCs are extensively phosphorylated.
To further demonstrate the role of FRQ in regulating WCC nuclear localization, we compared WC localization between the wild-type and frq-null (frq10) strains. In the frq10 strain, WCs are constantly hypophosphorylated (Schafmeier et al, 2005). As shown in Figure 6C, despite the lower WC levels in the frq10 strain in the total extract, the nuclear levels of WCs were comparable to those of the wild-type strain. In addition, the WC levels in the cytosolic fractions in the frq10 strain were significantly lower than those in the wild-type strain. The differences of WC phosphorylation profile between these two strains were not obvious in these experiments due to the condition used for electrophoresis (He et al, 2006). Consistent with this result, FRQ was shown to promote the nuclear clearance of WCC in another independent study (Hong et al). Taken together, these results suggest that the FRQ-dependent WC phosphorylation promotes the cytosolic localization of WCC.
To demonstrate that the loss of FRQ in frq-null strain results in high WCC activity in vivo, we performed ChIP assay using WC-2 antibody to compare the levels of WCC binding to the frq C box between the wild-type and frq9 strain. frq9 strain behaves similar to a frq-null strain due to a frame-shift mutation, which results in truncated and non-functional FRQ protein (Aronson et al, 1994b). In both strains, a construct that can inducibly express FRQ (qa-FRQ) was introduced at the his-3 locus (Aronson et al, 1994a). The induction of FRQ results in extensive WC phosphorylation (Schafmeier et al, 2006). As shown in Figure 6D, in the absence of the QA (quinic acid) inducer, the levels of WCC binding to the frq C box were higher in the frq9 strain than those in the wild-type strain, indicating high WCC activity in the frq9 strain. When QA was included in the medium, the levels of WCC DNA binding decreased in both strains, indicating that the induction of FRQ expression resulted in the inhibition of WCC activity. In contrast, QA had no effects on WCC binding in the wild-type strain without the qa-FRQ construct. These results demonstrate that FRQ inhibits WCC binding to the C-box in vivo.
Discussion
FRQ-dependent WCC phosphorylation results in the closing of the circadian negative feedback loop. In this study, we demonstrated that the Neurospora PP4 is a critical component of the clock by regulating two essential processes in the core circadian negative feedback loop. Our results suggest that PP4, similar to PP1, dephosphorylates FRQ, resulting in the stabilization of FRQ. In addition, PP4 also regulates WC phosphorylation and activates WCC activity in vivo. But unlike PP2A, which mostly activates WCC DNA-binding activity, PP4 executes its role by promoting the nuclear localization of WCC. In addition, we found that PKA counters the role of PP4 by promoting the WCC cytoplasmic localization. These studies established that WCC phosphorylation promotes its cytosolic localization, a key step in the circadian negative feedback process.
Mechanisms that close the circadian negative feedback loop
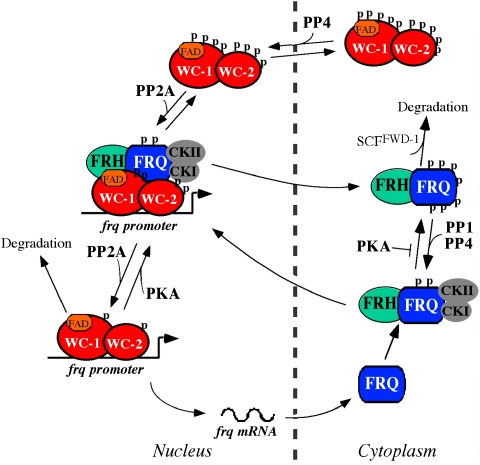
The results presented here provide important insights into the mechanism of the circadian negative feedback process. Based on these results and previous studies, we conclude that the inactivation of WCC activity by FRQ, the key process in the circadian negative feedback loop, is a two-step process (Figure 7). First, FRQ-dependent phosphorylation of WCC by the casein kinases directly inhibits its DNA-binding activity so that WCC can be removed from the DNA (He and Liu, 2005; Schafmeier et al, 2005; He et al, 2006; Huang et al, 2007). Second, as we demonstrated in this study, we established that FRQ suppresses the WCC activity by promoting WCC phosphorylation and its sequestration in the cytoplasm. We showed that extensive phosphorylation of WCs results in reduced nuclear enrichment of WCC. In the pp4 mutant, the nuclear enrichment of WCC is completely abolished, resulting in low WCC activity and low amplitude rhythms. In contrast, WCC is highly enriched in the nucleus in the pkac mutant despite its low WC levels. In addition, WCC nuclear enrichment is rhythmically regulated in DD: WCC is highly enriched in the nucleus when it is active and hypophosphorylated. Furthermore, we showed that FRQ inhibits the WCC nuclear localization. Together, these results suggest that the FRQ-dependent WC phosphorylation first removes WCC from the DNA due to its reduced DNA-binding activity, afterwards WCC is then shuttled into cytoplasm to further inhibit its function (Figure 7).
Figure 7.
A current model of the Neurospora circadian negative feedback loop. WCC functions as the positive element of the loop, whereas FRQ and FRH forms the FFC complex to function as the negative element. FFC inhibits WCC activity by recruiting casein kinases to phosphorylate WCC, resulting the removal of WCC from the DNA and its sequestration in the cytoplasm. To restart a circadian cycle, PP4 promotes the nuclear entry of WCC, whereas PP2A activates WCC DNA-binding activity. The casein kinases also phosphorylate FRQ, which promotes FRQ degradation through the ubiquitin-proteasome pathway. PP1 and PP4 counter the role of the casein kinases and stabilize FRQ. The stability of FRQ determines the period length of the clock. A full-colour version of this figure is available at The EMBO Journal Online.
In addition to these two mechanisms, high WCC activity also results in low WC levels probably due to increased WC degradation rate, which can partially suppress WCC activity. This conclusion is supported by the presence of low levels of WCs observed in the frq-null strain and in strains where WCC is highly active (Lee et al, 2000; Cheng et al, 2001b; He et al, 2005b, 2006; Schafmeier et al, 2006; Huang et al, 2007). This process may be also mediated by WC phosphorylation as light-induced phosphorylation of WCs is associated with increased WC degradation rate (Talora et al, 1999; Lee et al, 2000; He and Liu, 2005). In this view, the positive effects of FRQ on WC levels may be in part due to its inhibition of WCC activity.
Reactivation of WCC by PP2A and PP4 after its inhibition
The restart of another circadian cycle requires the reactivation of WCC and dephosphorylation of WCC by PP2A and PP4 has a major function in this process. In the PP2A mutant, WCC activity is low and WCs exhibit increased phosphorylation (Yang et al, 2004; Schafmeier et al, 2005). Similarly, the hyperphosphorylation of WCC in the pp4 mutant also results in low frq mRNA transcription and low WCC activity (Figure 4). Thus, both PP2A and PP4 are involved in the dephosphorylation and activation of WCC. However, our results presented above suggest that despite the common function of PP2A and PP4, they function on two different steps in this process. In the pp4 mutant, the nuclear enrichment of WCC is abolished, whereas in the rgb-1 mutant, the level of WCC in the nucleus was similar to a wild-type strain (Figure 5). Together with previous studies, these results suggest that PP4 is the major protein phosphatase that promotes the nuclear re-entry of WCC and PP2A mainly activates the WCC-binding activity. The different functions of these two phosphatases may be due to dephosphorylation of different WC phosphorylation sites or their different cellular localization. PP4 is a ubiquitous protein phosphatase in eukaryotic organisms. Although PP4 is related to PP2A, it is known to have many PP2A-independent functions in cellular processes, including signalling and organelle assembly (Cohen et al, 2005). The conservation of the posttranslational regulators in the circadian systems from Neurospora to animals suggests that PP4 may be another common clock component in eukaryotic circadian clocks.
FRQ stability, a major period length determinant, is regulated by both kinases and phosphatases
We have previously shown that progressive phosphorylation of FRQ triggers its degradation through the ubiquitin-proteasome pathway and FRQ stability is a major determinant in circadian period length (Liu et al, 2000; Gorl et al, 2001; Yang et al, 2002, 2003; He et al, 2003, 2005a). FRQ phosphorylation by CK-1a and CKII promotes its degradation, whereas PKA stabilizes FRQ protein (Yang et al, 2002; He et al, 2006; Huang et al, 2007). In this study, we showed that PP4, in addition to its role regulating WCC localization, also has an important function in determining FRQ stability. PP4 works together with PP1 to counter the effect of the casein kinases by dephosphorylating and stabilizing FRQ (Yang et al, 2004). The regulation of FRQ phosphorylation by multiple kinases and phosphatases with opposing roles allows the fine-tuning of the FRQ stability, resulting in a precise oscillation with circadian period length.
There are remarkable similarities between the circadian oscillators of Neurospora and those of Drosophila and mammals and the similarities are especially apparent in the posttranslational regulatory mechanisms (Liu and Bell-Pedersen, 2006; Heintzen and Liu, 2007). As with FRQ, PERIOD (PER) proteins in animals are progressively phosphorylated leading to their ubiquitination and proteasome-mediated degradation. This process is similarly regulated by CKI and PP1 and PP2A (Young and Kay, 2001; Grima et al, 2002; Ko et al, 2002; He et al, 2003; Sathyanarayanan et al, 2004; Eide et al, 2005; Fang et al, 2007). As for the circadian negative feedback process, the phosphorylation state of Drosophila CLOCK (CLK), such as those of WCs, exhibit a circadian rhythm and PER recruits DBT (a CK-1a homologue) to promote CLK phosphorylation and inhibition (Kim and Edery, 2006; Yu et al, 2006; Kim et al, 2007). Similar to that in Neurospora, the Drosophila PP2A counters the function of DBT in CLK phosphorylation. In addition, PER also promotes the cytoplasmic localization of CLK in cultured insect S2 cells (Kim and Edery, 2006). Similarly, transient transfection experiments in mammalian NIH 3T3 cells also suggest that the nucleocytoplasmic shuttling of BMAL1 is critical for its function (Kwon et al, 2006). Although the physiological relevance of these latter results are still not clear, our results presented here along with the conservation of the circadian systems suggest a similar mechanism for the closing of the circadian negative feedback loop in higher eukaryotic organisms.
Materials and methods
Strains and culture conditions
87-3 (bd a) was used as a wild type strain in this study. The bd pp4ko strain was created by deleting the entire pp4 (NCU08301.1) ORF by homologous recombination as described previously (Ninomiya et al, 2004; He et al, 2006). A positive transformant was crossed with a wild-type strain to generate the homokaryotic bd pp4ko strain. 322-3 (bd ppp-1RIP a), a ppp-1 mutant, was previously generated (Yang et al, 2004) and was crossed with a bd pp4ko strain to generate ppp-1RIP pp4ko double mutants. All strains were confirmed by Southern hybridization and PCR.
Liquid cultures were grown in minimal media (1 × Vogel's, 2% glucose). When QA was used, liquid cultures were grown in 0.01 M QA (pH 5.8), 1 × Vogel's, 0.5% glucose, and 0.17% arginine. Race tube media contained 1 × Vogel's, 0.1% glucose, 0.17% arginine, 50 ng/ml biotin, and 1.5% agar. Calculations of period length and phase were performed as previously described (Roenneberg and Taylor, 2000).
Protein and RNA analyses
Protein extraction, western blot analysis, and immunoprecipitation assays were performed as described previously (Garceau et al, 1997; Cheng et al, 2001a). Equal amounts of total protein (50 μg) were loaded in each lane of SDS–PAGE and western blot analyses were performed. The phosphorylation profiles of WC-2 were examined as previously described using 10% SDS–PAGE gels containing a ratio of 139:1 acrylamide/bisacrylamide (He et al, 2006). For other western blot analyses, 7.5% SDS–PAGE gels containing a ratio of 37.5:1 acrylamide/bisacrylamide were used. Phosphatase inhibitors (2 mM Na3VO4, 25 mM NaF, and 10 mM Na4P2O7) were included in protein extraction buffer where indicated.
Nuclear and cytoplasmic protein extracts were prepared as previously described (Luo et al, 1998) and each fraction (10 μg) was loaded onto SDS–PAGE for western blot analysis.
RNA extraction and Northern blot analyses were performed as previously described (Aronson et al, 1994a). Equal amounts of total RNA (20 μg) were loaded onto agarose gels for electrophoresis, and the gels were blotted and probed with an RNA probes specific for frq.
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed as previously described (He and Liu, 2005). PCR products were resolved by electrophoresis on 2% agarose gels. Densitometry of gels were performed using NIH image. Each experiment was independently performed three times, and immunoprecipitation without WC-2 antibody was used as the negative controls.
Expression of Myc-tagged protein phosphatases in Neurospora
A PCR fragment containing the entire open reading frame and 3′ UTR of pp4 gene was cloned into pqa.5Myc.6His vector (Cheng et al, 2001b; He et al, 2005a). The following primers were used: 5′-ATTGGCGCGCCTCTGACCTCTTTGGTGGATAGG-3′ and 5′-ATTGGCGCGCCTGAAGAGCCCACCTTCCATGAC-3′. The ppp-1 and pph-1 expression vectors were previously described (Yang et al, 2004). The resulting plasmids were transformed into a wild-type strain (301-6) at his-3 locus. The expression of Myc-tagged proteins in these transformants was confirmed by western blot analysis using a monoclonal c-Myc antibody (9E10, Roche).
Acknowledgments
We thank Haiyan Yuan for excellent technical assistance and Chi-Tai Tang for critical comments to the manuscript. This research was supported by grants from National Institutes of Health and Welch Foundation to Yi Liu. Y Liu is the Louise W Kahn Endowed Scholar in Biomedical Research at University of Texas Southwestern Medical Center.
References
- Allada R, Emery P, Takahashi JS, Rosbash M (2001) Stopping time: the genetics of fly and mouse circadian clocks. Annu Rev Neurosci 24: 1091–1119 [DOI] [PubMed] [Google Scholar]
- Aronson B, Johnson K, Loros JJ, Dunlap JC (1994a) Negative feedback defining a circadian clock: autoregulation in the clock gene frequency. Science 263: 1578–1584 [DOI] [PubMed] [Google Scholar]
- Aronson BD, Johnson KA, Dunlap JC (1994b) The circadian clock locus frequency: a single ORF defines period length and temperature compensation. Proc Natl Acad Sci USA 91: 7683–7687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belden WJ, Loros JJ, Dunlap JC (2007) Execution of the circadian negative feedback loop in Neurospora requires the ATP-dependent chromatin-remodeling enzyme CLOCKSWITCH. Mol Cell 25: 587–600 [DOI] [PubMed] [Google Scholar]
- Cheng P, He Q, He Q, Wang L, Liu Y (2005) Regulation of the Neurospora circadian clock by an RNA helicase. Genes Dev 19: 234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Yang Y, Gardner KH, Liu Y (2002) PAS domain-mediated WC-1/WC-2 interaction is essential for maintaining the steady state level of WC-1 and the function of both proteins in circadian clock and light responses of Neurospora. Mol Cell Biol 22: 517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Yang Y, Heintzen C, Liu Y (2001a) Coiled-coil domain mediated FRQ-FRQ interaction is essential for its circadian clock function in Neurospora. EMBO J 20: 101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Yang Y, Liu Y (2001b) Interlocked feedback loops contribute to the robustness of the Neurospora circadian clock. Proc Natl Acad Sci USA 98: 7408–7413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Yang Y, Wang L, He Q, Liu Y (2003) WHITE COLLAR-1, a multifunctional Neurospora protein involved in the circadian feedback loops, light sensing, and transcription repression of wc-2. J Biol Chem 278: 3801–3808 [DOI] [PubMed] [Google Scholar]
- Cohen PT, Philp A, Vazquez-Martin C (2005) Protein phosphatase 4—from obscurity to vital functions. FEBS Lett 579: 3278–3286 [DOI] [PubMed] [Google Scholar]
- Crosthwaite SK, Dunlap JC, Loros JJ (1997) Neurospora wc-1 and wc-2: transcription, photoresponses, and the origins of circadian rhythmicity. Science 276: 763–769 [DOI] [PubMed] [Google Scholar]
- Denault DL, Loros JJ, Dunlap JC (2001) WC-2 mediates WC-1–FRQ interaction within the PAS protein-linked circadian feedback loop of Neurospora. EMBO J 20: 109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap JC (1999) Molecular bases for circadian clocks. Cell 96: 271–290 [DOI] [PubMed] [Google Scholar]
- Eide EJ, Woolf MF, Kang H, Woolf P, Hurst W, Camacho F, Vielhaber EL, Giovanni A, Virshup DM (2005) Control of mammalian circadian rhythm. Mol Cell Biol 25: 2795–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Sathyanarayanan S, Sehgal A (2007) Post-translational regulation of the Drosophila circadian clock requires protein phosphatase 1 (PP1). Genes Dev 21: 1506–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich AC, Loros JJ, Dunlap JC (2003) Rhythmic binding of a WHITE COLLAR-containing complex to the frequency promoter is inhibited by FREQUENCY. Proc Natl Acad Sci USA 100: 5914–5919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garceau N, Liu Y, Loros JJ, Dunlap JC (1997) Alternative initiation of translation and time specific phosphorylation yield multiple forms of the essential clock protein FREQUENCY. Cell 89: 469–476 [DOI] [PubMed] [Google Scholar]
- Gorl M, Merrow M, Huttner B, Johnson J, Roenneberg T, Brunner M (2001) A PEST-like element in FREQUENCY determines the length of the circadian period in Neurospora crassa. EMBO J 20: 7074–7084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grima B, Lamouroux A, Chelot E, Papin C, Limbourg-Bouchon B, Rouyer F (2002) The F-box protein slimb controls the levels of clock proteins period and timeless. Nature 420: 178–182 [DOI] [PubMed] [Google Scholar]
- He Q, Cha J, He Q, Lee H, Yang Y, Liu Y (2006) CKI and CKII mediate the FREQUENCYdependent phosphorylation of the WHITE COLLAR complex to close the Neurospora circadian negative feedback loop. Genes Dev 20: 2552–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Cheng P, He Q, Liu Y (2005a) The COP9 signalosome regulates the Neurospora circadian clock by controlling the stability of the SCFFWD-1 complex. Genes Dev 19: 1518–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Cheng P, Yang Y, He Q, Yu H, Liu Y (2003) FWD1-mediated degradation of FREQUENCY in Neurospora establishes a conserved mechanism for circadian clock regulation. EMBO J 22: 4421–4430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Liu Y (2005) Molecular mechanism of light responses in Neurospora: from light-induced transcription to photoadaptation. Genes Dev 19: 2888–2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Shu H, Cheng P, Chen S, Wang L, Liu Y (2005b) Light-independent phosphorylation of WHITE COLLAR-1 regulates its function in the Neurospora circadian negative feedback loop. J Biol Chem 280: 17526–17532 [DOI] [PubMed] [Google Scholar]
- Heintzen C, Liu Y (2007) The Neurospora crassa circadian clock. Adv Genet 58: 25–66 [DOI] [PubMed] [Google Scholar]
- Hong C, Ruoff P, Loros JJ, Dunlap JC. Closing the circadian negative feedback loop: FRQ dependent clearance of WC-1 from the nucleus. Genes Dev (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G, Chen S, Li S, Cha J, Long C, Li L, He Q, Liu Y (2007) Protein kinase A and casein kinases mediate sequential phosphorylation events in the circadian negative feedback loop. Genes Dev 21: 3283–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Edery I (2006) Balance between DBT/CKI{varepsilon} kinase and protein phosphatase activities regulate phosphorylation and stability. Proc Natl Acad Sci USA 103: 6178–6183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Ko HW, Yu W, Hardin PE, Edery I (2007) A DOUBLETIME kinase binding domain on the Drosophila PERIOD protein is essential for its hyperphosphorylation, transcriptional repression, and circadian clock function. Mol Cell Biol 27: 5014–5028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HW, Jiang J, Edery I (2002) Role for Slimb in the degradation of Drosophila period protein phosphorylated by Doubletime. Nature 420: 673–678 [DOI] [PubMed] [Google Scholar]
- Kwon I, Lee J, Chang SH, Jung NC, Lee BJ, Son GH, Kim K, Lee KH (2006) BMAL1 shuttling controls transactivation and degradation of the CLOCK/BMAL1 heterodimer. Mol Cell Biol 26: 7318–7330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Loros JJ, Dunlap JC (2000) Interconnected feedback loops in the Neurospora circadian system. Science 289: 107–110 [DOI] [PubMed] [Google Scholar]
- Lin JM, Kilman VL, Keegan K, Paddock B, Emery-Le M, Rosbash M, Allada R (2002) A role for casein kinase 2alpha in the Drosophila circadian clock. Nature 420: 816–820 [DOI] [PubMed] [Google Scholar]
- Liu Y (2005) Analysis of posttranslational regulations in the Neurospora circadian clock. Meth Enzymol 393: 379–393 [DOI] [PubMed] [Google Scholar]
- Liu Y, Bell-Pedersen D (2006) Circadian rhythms in Neurospora crassa and other filamentous fungi. Eukaryot Cell 5: 1184–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Loros J, Dunlap JC (2000) Phosphorylation of the Neurospora clock protein FREQUENCY determines its degradation rate and strongly influences the period length of the circadian clock. Proc Natl Acad Sci USA 97: 234–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loros JJ, Dunlap JC (2001) Genetic and molecular analysis of circadian rhythms in NEUROSPORA. Annu Rev Physiol 63: 757–794 [DOI] [PubMed] [Google Scholar]
- Lowrey PL, Shimomura K, Antoch MP, Yamazaki S, Zemenides PD, Ralph MR, Menaker M, Takahashi JS (2000) Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science 288: 483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Loros JJ, Dunlap JC (1998) Nuclear localization is required for function of the essential clock protein FREQUENCY. EMBO J 17: 1228–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrow M, Garceau N, Dunlap JC (1997) Dissection of a circadian oscillation into discrete domains. Proc Natl Acad Sci USA 94: 3877–3882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Williams DR, Byrne MO, Qin X, Egli M, McHaourab HS, Stewart PL, Johnson CH (2007) Elucidating the ticking of an In Vitro Circadian Clockwork. PLoS Biol 5: e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Imai K, Ito H, Nishiwaki T, Murayama Y, Iwasaki H, Oyama T, Kondo T (2005) Reconstitution of circadian oscillation of cyanobacterial KaiC phosphorylation in vitro. Science 308: 414–415 [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Suzuki K, Ishii C, Inoue H (2004) Highly efficient gene replacements in Neurospora strains deficient for nonhomologous end-joining. Proc Natl Acad Sci USA 101: 12248–12253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Blau J, Rothenfluh A, Adodeely M, Kloss B, Young MW (1998) double-time is a new Drosophila clock gene that regulates PERIOD protein accumulation. Cell 94: 83–95 [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Taylor W (2000) Automated recordings of bioluminescence with special reference to the analysis of circadian rhythms. Methods Enzymol 305: 104–119 [DOI] [PubMed] [Google Scholar]
- Sathyanarayanan S, Zheng X, Xiao R, Sehgal A (2004) Posttranslational regulation of Drosophila PERIOD protein by protein phosphatase 2A. Cell 116: 603–615 [DOI] [PubMed] [Google Scholar]
- Schafmeier T, Haase A, Kaldi K, Scholz J, Fuchs M, Brunner M (2005) Transcriptional feedback of neurospora circadian clock gene by phosphorylation-dependent inactivation of its transcription factor. Cell 122: 235–246 [DOI] [PubMed] [Google Scholar]
- Schafmeier T, Kaldi K, Diernfellner A, Mohr C, Brunner M (2006) Phosphorylation-dependent maturation of Neurospora circadian clock protein from a nuclear repressor toward a cytoplasmic activator. Genes Dev 20: 297–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwerdtfeger C, Linden H (2000) Localization and light-dependent phosphorylation of white collar 1 and 2, the two central components of blue light signaling in Neurospora crassa. Eur J Biochem 267: 414–422 [DOI] [PubMed] [Google Scholar]
- Sehgal A (2004) Molecular Biology of Circadian Rhythms. Hoboken, NJ: John Wiley & Sons [Google Scholar]
- Talora C, Franchi L, Linden H, Ballario P, Macino G (1999) Role of a white collar-1-white collar-2 complex in blue-light signal transduction. EMBO J 18: 4961–4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cheng P, He Q, Wang L, Liu Y (2003) Phosphorylation of FREQUENCY protein by casein kinase II is necessary for the function of the Neurospora circadian clock. Mol Cell Biol 23: 6221–6228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cheng P, Liu Y (2002) Regulation of the Neurospora circadian clock by casein kinase II. Genes & Dev 16: 994–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cheng P, Zhi G, Liu Y (2001) Identification of a calcium/calmodulin-dependent protein kinase that phosphorylates the Neurospora circadian clock protein FREQUENCY. J Biol Chem 276: 41064–41072 [DOI] [PubMed] [Google Scholar]
- Yang Y, He Q, Cheng P, Wrage P, Yarden O, Liu Y (2004) Distinct roles for PP1 and PP2A in the Neurospora circadian clock. Genes Dev 18: 255–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MW, Kay SA (2001) Time zones: a comparative genetics of circadian clocks. Nat Rev Genet 2: 702–715 [DOI] [PubMed] [Google Scholar]
- Yu W, Zheng H, Houl JH, Dauwalder B, Hardin PE (2006) PER-dependent rhythms in CLK phosphorylation and E-box binding regulate circadian transcription. Genes Dev 20: 723–733 [DOI] [PMC free article] [PubMed] [Google Scholar]