Abstract
An important component of learned behaviour is the ability to forecast positive or negative outcomes based on specific sensory cues. Predictive capacity is typically manifested by appropriate behavioural patterning. However, the molecular mechanisms underlying behavioural plasticity are poorly understood. Caenorhabditis elegans displays experience-dependent behavioural responses by associating distinct environmental signals. We find that ASIC-1, a member of the degenerin/epithelial sodium channel family, which localizes at presynaptic terminals of dopaminergic neurons, is required for associative learning in C. elegans. ASIC-1 functions in these neurons to amplify normal dopaminergic signalling, necessary for associative learning. Our results reveal a novel role of DEG/ENaC ion channels in neuronal communication by enhancing the activity of dopaminergic synapses. Similar mechanisms may facilitate synaptic plasticity in vertebrates.
Keywords: behavioural plasticity, Caenorhabditis elegans, dopamine, epithelial sodium channel, learning and memory
Introduction
The nervous system processes and integrates sensory input to generate both immediate responses and long-term changes in behaviour. Associative learning, in the form of classical and operant conditioning, allows animals to capture the logical structure of their habitat and is critical for negotiating complex environments (Pavlov, 1928; Skinner, 1930). Similar to vertebrates, the soil-dwelling nematode Caenorhabditis elegans integrates sensory input to predict vital field conditions, such as food availability or the presence of predators (Mohri et al, 2005; Zhang et al, 2005). Several paradigms of experience-dependent behavioural plasticity have been described in C. elegans (Nuttley et al, 2002; Hobert, 2003; Rankin, 2004; Bargmann, 2005). Worms display associative learning in the form of conditioning to chemical, thermal and mechanical cues (Gomez et al, 2001; Saeki et al, 2001; Law et al, 2004; Mori et al, 2008).
Modulation of synaptic transmission by specific ion channels localized at both pre- and postsynaptic termini facilitates synaptic plasticity, associated with learned patterns of behaviour (Hebb, 1949; Abbott and Nelson, 2000; Voglis and Tavernarakis, 2006). Acid-sensing ion channels (ASICs) have been implicated neurotransmission in the central nervous system (Wemmie et al, 2006). ASICs are gated by protons and belong to the family of degenerin/epithelial sodium channels (DEG/ENaC), a large group of ion channels that are sensitive to the diuretic amiloride (Kellenberger and Schild, 2002). ASICs have been identified in diverse organisms ranging from nematodes to mammals, including humans and share a high degree of sequence and overall structure similarity (Krishtal, 2003). ASIC-1, a member of the mammalian ASIC subfamily has been identified in high synaptic density regions of the hippocampus, where it localizes at both pre- and postsynaptic membranes of synaptosomes. ASIC-1 deficiency impairs long-term potentiation (LTP) in the hippocampus, indicating that ASIC activity is critical for synaptic plasticity (Wemmie et al, 2002). In addition to the hippocampus, ASIC expression has also been detected in the amygdala, one of the key brain structures involved in fear conditioning (Wemmie et al, 2003). Disruption of ASIC-1 attenuates learned fear behaviour, whereas overexpression of ASIC-1 in the brain enhances fear conditioning (Wemmie et al, 2004).
These findings indicate that in mammals ASICs modulate synaptic plasticity, probably by facilitating proton-evoked sodium currents at postsynaptic termini (Wemmie et al, 2006). However, the underlying mechanisms linking ASIC function to learning and memory remain poorly understood. In addition, the function of ASICs at presynaptic terminals is unclear. Here, we present evidence that ASIC-1, a new member of the DEG/ENaC family, contributes to sustain enhanced dopaminergic neurotransmission, necessary for associative learning in C. elegans. We found that ASIC-1 localizes at presynaptic terminals of dopaminergic neurons. Animals lacking ASIC-1 are defective in associative learning, although they show normal sensory perception. Synaptic release at dopaminergic neurons is compromised in the absence of ASIC-1 activity, suggesting that modulation of dopaminergic signalling by ASIC-1 mediates associative learning in C. elegans. Given that ASIC ion channels are highly conserved across phyla (Krishtal, 2003), similar mechanisms may facilitate synaptic plasticity in vertebrates.
Results
ASIC-1 is required for associative learning in C. elegans
To assay associative learning capacity in the nematode, we utilize two robust assays, chemosensory conditioning and feeding-dependent thermotaxis, each based on different sets of sensory inputs. In chemosensory conditioning, animals that are normally attracted to certain water-soluble or volatile compounds avoid these chemoattractants after being subjected to starvation in their presence (Bargmann and Horvitz, 1991; Colbert and Bargmann, 1995; Saeki et al, 2001). In feeding-dependent thermotaxis, animals challenged with a radial thermal gradient track the isothermal curve corresponding to their prior cultivation temperature, under ad libitum feeding conditions (Hedgecock and Russell, 1975; Mori, 1999; Gomez et al, 2001; Mori et al, 2008). We found that punishment-induced aversion towards starvation-paired chemosensory stimuli is significantly impaired in animals carrying a genetic lesion (deletion allele ok415; C. elegans Gene Knockout Consortium) in the asic-1 gene, which encodes a member of the acid-sensing family of ion channels (Supplementary Figures 1–3). Mutant animals perform normal chemotaxis towards the compounds used, indicating that learning defects are not due to sensory perception deficiency (Figure 1A–D). We have also examined the capacity of asic-1(ok415) mutants for chemosensory adaptation (Bernhard and van der Kooy, 2000). We found that these animals display normal adaptation behaviour (Supplementary Figure 8). Furthermore, the feeding-dependent thermotaxis behaviour of asic-1(ok415) mutants is compromised (Figure 1E). Finally, although wild-type animals show enhanced chemotaxis towards soluble and volatile compounds after conditioning in the presence of food (Saeki et al, 2001), asic-1(ok415) mutants remain unaffected (Figure 2A and B). RNAi-mediated knock down of ASIC-1 recapitulated the phenotypes of the mutant, albeit with low penetrance because of the low efficiency of RNAi in the C. elegans nervous system (Tavernarakis et al, 2000). Other behaviours and anatomical parameters that could interfere with learning assays (locomotion, speed, mechanosensory and thermosensory responses, body size and others) are unaffected in these animals (Table I; Supplementary Figure 5 and data not shown). Thus, ASIC-1 deficiency specifically disrupts at least two different forms of associative learning in C. elegans.
Figure 1.
ASIC-1 is required for associative learning in C. elegans. (A–D) Conditioning to both gustatory and olfactory cues in the absence of food is impaired in animals carrying a deletion in the asic-1 gene. Bars depict chemotaxis indices towards the indicated water-soluble (A–C) or volatile compounds (D), calculated for either naive or conditioned wild-type (white) and asic-1(ok415) mutant animals (black). Error bars denote standard error of the mean (s.e.m.) values (n=750 in four experiments; ***P<0.001, unpaired t-test). (E) Isothermal tracking performance of asic-1(ok415) mutant animals (black bar) is significantly reduced compared with wild type (white bar). Bars depict the percentage of animals that successfully track an isothermal line corresponding to their prior cultivation temperature, in a thermal gradient. Error bars denote s.e.m. values (n=200 in eight experiments; ***P<0.001, unpaired t-test).
Figure 2.
Defective conditioning behaviour of asic-1(ok415) mutants is restored by the wild-type asic-1 gene. (A, B) Chemotaxis to both soluble and volatile chemicals after conditioning in the presence of food. Bars depict chemotaxis indices towards NaCl (A) or isoamyl alcohol (B), calculated for either naive or conditioned wild-type (white) and asic-1(ok415) mutant animals (black). Error bars denote s.e.m. values (n=300 in two experiments; **P<0.01, unpaired t-test). (C, D) Complementation of the associative learning deficiency of asic-1(ok415) mutant animals by introduction of the wild-type asic-1 gene. Bars depict chemotaxis indices calculated for animals of the indicated genetic background, after conditioning in the absence of food to NaCl (C) or isoamyl alcohol (D). Error bars denote s.e.m. values (n=900 in five experiments; ***P<0.001, unpaired t-test).
Table 1.
Phenotypic characterization of asic-1(ok415) mutant animals
| Pharyngeal pumpinga | Defecationb | Sinusoidal locomotion wavelengthc | Sinusoidal locomotion amplitudec | Speedd | Body sizee | Gentle body touch responsef | Harsh touch responsef | Nose touch responsef | Egg layingg | Thermotactic runh | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wild type | 223±14 | 44.1±4.7 | 0.72±0.1 (0.77±0.3) | 0.31±0.2 (0.28±0.3) | 1.3±0.6 (2.0±0.8) | 1.1±0.4 | 95±3 | 98±1 | 82±2 | 2.65±0.21 | 22±2 (25±3) |
| asic-1(ok415) | 218±19 | 36.6±3.1 | 0.74±0.2 (0.79±0.4) | 0.34±0.2 (0.30±0.4) | 1.2±0.7 (2.1±0.9) | 1.0±0.2 | 91±4 | 96±3 | 79±3 | 2.72±0.53 | 21±3 (26±2) |
| aPumps per minute. | |||||||||||
| bDuration of defecation cycle in seconds. | |||||||||||
| cWavelength and amplitude of sinusoidal track in millimetres. Representative images are shown in Supplementary Figure 5. | |||||||||||
| dMillimetres per second. | |||||||||||
| eAdult body length in millimetres. | |||||||||||
| fPercentage of animals responding. | |||||||||||
| gNumber of eggs laid per worm per hour. | |||||||||||
| hRun durations in seconds of animals cultivated at 20°C and assayed at 25°C as described earlier (Ryu and Samuel, 2002). | |||||||||||
| Numbers in parentheses represent measurements after conditioning under starvation. All assays were performed at 20°C unless noted otherwise. | |||||||||||
ASICs belong to the family of DEG/ENaC, a large group of ion channels that are sensitive to the diuretic amiloride (Kellenberger and Schild, 2002) (Supplementary Figure 1). ASICs have been identified in diverse organisms ranging from nematodes to mammals, including humans and share a high degree of sequence and overall structure similarity (Krishtal, 2003) (Supplementary Figures 2 and 3). The strong conservation across species suggests that ASICs shared a common ancestor relatively early in evolution. Members of the mammalian ASIC subfamily are gated by protons. Expression of these channels in heterologous cells generates transient, H+ gated, amiloride-sensitive, sodium-selective currents (Waldmann et al, 1997). C. elegans ASIC-1 shares extensive sequence similarity with vertebrate ASICs (Supplementary Figure 3). The deletion in the ok415 allele removes a highly conserved extracellular, cysteine-rich domain of the protein that is essential for function (Supplementary Figures 3 and 4). Interestingly, part of the deleted region corresponds to the proton-sensing domain of vertebrate ASICs (Chen et al, 2007; Jasti et al, 2007; Paukert et al, 2008). Introduction of the wild-type asic-1 gene in asic-1(ok415) mutants restores learning capacity in these animals (Figure 2C and D).
ASIC-1 functions in dopaminergic neurons to mediate associative learning
asic-1 is expressed in eight neurons comprising the C. elegans dopaminergic system (Figure 3A; the four cephalic neurons; two anterior deirid neurons and two posterior deirid neurons; CEP, ADE, PDE, respectively) (Chase et al, 2004; Kindt et al, 2007). Expression is also detected in four additional neurons in the tail, two of which are the bilaterally symmetric PVQ interneurons (Figure 3A). We examined the subcellular localization of ASIC-1 in these neurons by generating two GFP reporter fusions. The first is a full-length ASIC-1∷GFP reporter fusion encompassing the complete ASIC-1 protein. To generate the second reporter, we fused GFP with the intracellular amino-terminal region of ASIC-1, which contains amino-acid sequence motifs sufficient for proper localization of these ion channels in C. elegans (ASIC-1N∷GFP) (Tavernarakis et al, 2001). Both the full-length ASIC-1∷GFP and amino-terminal ASIC-1N∷GFP chimaeras showed prominent punctuate distribution along the processes of dopaminergic neurons, in synapse-rich areas (Figure 3B). A DsRED–synaptobrevin fusion colocalizes with both ASIC-1∷GFP and ASIC-1N∷GFP in these puncta (Figure 3C and D). Thus, ASIC-1 localizes at presynaptic termini of dopaminergic neurons.
Figure 3.
Synaptic localization of ASIC-1 in dopaminergic neurons. (A) Images of transgenic animals carrying a pasic-1GFP reporter fusion. The asic-1 gene is expressed in six head neurons shown in the two left panels, the four cephalic (CEP dorsal, ventral/left, right) and two deirid neurons (ADE left, right). The head amphid sensory neurons labelled with the lipophilic carbocyanine tracer DiI (Hedgecock et al, 1985) are shown for reference (top left panel). Two additional deirid dopaminergic neurons in the mid-body section (PDE left, right) express asic-1 (top right panel). In addition to the eight dopaminergic neurons, asic-1 is expressed in four tail neurons (PVQ left/right and two more unidentified neurons). The location of phasmid neurons labelled with DiI is shown for reference (bottom right panel). White bars denote 10 μm. (B) Images of transgenic animals carrying a full-length pasic-1ASIC-1∷GFP reporter fusion and an amino-terminal pasic-1ASIC-1N∷GFP, which includes the complete intracellular amino terminus of ASIC-1, containing the subcellular localization motifs. The punctate distribution of GFP in synaptic areas of head dopaminergic neuron processes is indicated by arrows. White bars denote 10 μm. (C) ASIC-1∷GFP colocalizes with a DsRED-synaptobrevin fusion, which labels synapses (indicated by arrows), driven by the asic-1 promoter (pasic-1DsRED∷SNB-1) in double-transgenic animals carrying both reporter fusions. White bars denote 20 μm. (D) Images of double-transgenic animals carrying the amino-terminal pasic-1ASIC-1N∷GFP reporter together with a pasic-1DsRED∷SNB-1 fusion. ASIC-1N∷GFP (top panels) and DsRED∷SNB-1 (middle panels) colocalize in puncta across dopaminergic neuron processes (bottom panel, arrows). White bars denote 20 μm.
To determine the requirement for ASIC-1 function in specific subsets of neurons, we examined populations of mosaic transgenic animals for conditioning to isoamyl alcohol. We found that expression of asic-1 in all eight dopaminergic neurons is both necessary and sufficient to restore normal learning capacity in asic-1(ok415) mutants (Table II). Partial expression in the two ADEs or the four CEPs or the two PDEs (the three different classes of nematode dopaminergic neurons) results in intermediate rescue. We conclude that ASIC-1 function in all dopaminergic neurons contributes to normal conditioning behaviour.
Table 2.
Mosaic analysis of asic-1(ok415) mutants, expressing the wild-type asic-1 gene in specific subsets of neurons
| Expressing neuronsa | Chemotaxis index after conditioningb |
|---|---|
| All 12 asic-1-expressing neurons | −2.7±4.8 |
| All 8 dopaminergic neurons | −0.9±5.3 |
| 4 non-dopaminergic tail neurons | 49.8±4.1 |
| 2 ADE dopaminergic neurons | 9.8±5.7 |
| 4 CEP dopaminergic neurons | 20.4±6.2 |
| 2 PDE dopaminergic neurons | 7.0±4.9 |
| aMosaic transgenic asic-1(ok415)Ex[pasic-1GFPpasic-1ASIC-1] animals showing GFP expression in the indicated neurons were grouped and assayed. | |
| bChemotaxis index (±s.e.m.) towards isoamyl alcohol after conditioning to isoamyl alcohol in the absence of food (n=70 for each mosaic subtype, three independent experiments). | |
Dopaminergic signalling is required for conditioning
ASICs are widely distributed in the mammalian brain, where they have been implicated in the phenomena of synaptic plasticity at postsynaptic ends, and learning and memory (Wemmie et al, 2002, 2006). What function does ASIC-1 serve at presynaptic termini of dopaminergic neurons to facilitate associative learning in C. elegans? The dopaminergic system is involved in a wide array of nematode behaviours, including locomotion, egg laying, habituation, defecation and the food-encounter response (basal slowing) (Sawin et al, 2000; McDonald et al, 2006). None of these behaviours is affected by ASIC-1 deficiency (Table I; Figure 4A and B). In addition, dopamine biosynthesis in dopaminergic neurons of asic-1 (ok415) mutants appears normal (Figure 5). Nonetheless, we found that exogenous dopamine alleviates the learning deficit of these animals (Figure 4C; conditioning to isoamyl alcohol). Conversely, eliminating dopaminergic signalling, either by blocking dopamine biosynthesis or by genetic ablation of dopaminergic neurons (Supplementary Figure 6) recapitulates the associative learning defects of asic-1(ok415) mutants (Figure 4C and D; Supplementary Table 1). In both cases, exogenous dopamine supply restores associative learning (Figure 4C and D). Dopaminergic neurons were ablated through ectopic expression of the dominant (d), neurotoxic mec-4(u231) allele under the control of the asic-1 promoter (pasic-1mec-4(d)), specifically in these cells (Harbinder et al, 1997).
Figure 4.
Dopaminergic signalling is required for associative learning in C. elegans. (A) asic-1(ok415) mutant animals show normal basal slowing behaviour when encountering either food or sepharose beads. cat-2(e1112) mutants deficient in dopamine biosynthesis do not slow down upon encountering bacterial or sepharose lawns and are included as controls (Sawin et al, 2000). Animals harbouring a lesion in the plasma membrane dopamine transporter gene dat-1 show normal basal slowing behaviour as do asic-1(ok415);dat-1(tm903) double mutants. Error bars denote s.e.m. values (n=20 in five experiments). (B) Conditioning in the presence of sepharose beads compromises associative learning in wild-type animals. asic-1(ok415) mutants are unaffected. Bars depict chemotaxis indices calculated for animals of the indicated genetic background, after conditioning to isoamyl alcohol. Error bars denote s.e.m. values (n=500 in three experiments; **P<0.01, unpaired t-test). (C) Exogenous addition of dopamine (DA, 5 mM) restores the capacity for associative learning in asic-1(ok415) mutants. Defective dopamine biosynthesis in cat-2(e1112) mutant animals impairs associative learning, similarly to asic-1 deficiency. Exogenous addition of dopamine restores associative learning in cat-2(e1112) mutants. (D) Genetic ablation of dopaminergic neurons results in associative learning defects that are reversed by exogenous addition of dopamine (DA, 5 mM). Animals with at least five ablated dopaminergic neurons, including all CEP neurons were grouped and assayed (see also Supplementary Table 1). For (C, D), bars depict chemotaxis indices towards isoamyl alcohol, calculated for either naive (white bars) or conditioned animals, without (black bars) or with (gray bars) addition of dopamine (DA) in the conditioning medium. Error bars denote s.e.m. values (n=950 in five experiments; ***P<0.001, unpaired t-test). (E) Associative learning in animals lacking specific dopamine receptors. Bars depict chemotaxis indices calculated for animals of the indicated genetic background, before (white) or after (black) conditioning to isoamyl alcohol. Error bars denote s.e.m. values (n=700 in four experiments; *P<0.05; **P<0.01, unpaired t-test).
Figure 5.
Dopamine biosynthesis is not compromised in asic-1(ok415) mutant animals. (A) Dopamine-producing cephalic neurons are visualized by formaldehyde-induced fluorescence in both wt and asic-1(ok415) mutants. No fluorescence is observed in cat-2(e1112) mutants deficient in dopamine biosynthesis, which are included for control. (B) Quantification of formaldehyde-induced fluorescence wt, asic-1(ok415) and cat-2(e1112) animals. Error bars denote s.e.m. values (n=50 in three experiments for each genetic background; ***P<0.001; NS: difference not significant; unpaired t-test, compared with wt).
Interestingly, previously characterized dopamine-mediated behaviours such as basal slowing (Sawin et al, 2000) are less sensitive to mild reduction of dopamine, compared with associative learning. By treating cat-2(e1112) mutant animals, defective in dopamine biosynthesis, with increasing concentrations of exogenously supplied dopamine, we found that normal conditioning behaviour is restored in cat-2 mutants at lower concentrations of exogenous dopamine before basal slowing behaviour is back to normal (Supplementary Figure 7). In addition, animals carrying lesions in both D1- and D2-like dopamine receptors (Chase et al, 2004; Suo et al, 2004) show reduced capacity for associative learning (Figure 4E). We obtained similar results with other soluble and volatile chemical cues (not shown). Thus, dopaminergic signalling is required for associative learning in C. elegans.
Dopamine release is compromised in asic-1 mutants
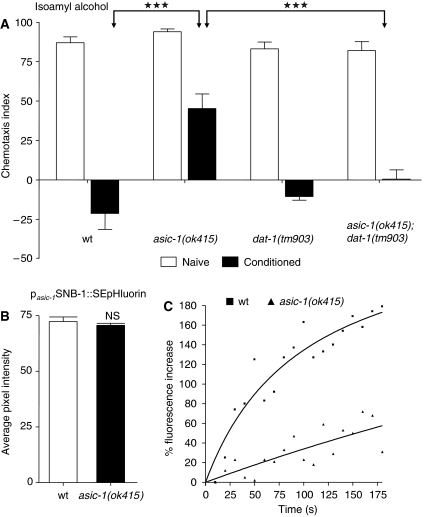
We hypothesized that ASIC-1 functions at dopaminergic synapses to facilitate dopaminergic signalling by augmenting synaptic release of dopamine. We tested this hypothesis in two ways. First, we examined the effect of removing the plasma membrane dopamine transporter (DAT) on associative learning in asic-1(ok415) mutant animals. DAT functions to terminate dopamine signalling by mediating dopamine re-uptake at presynaptic termini. After evoked release, extracellular levels of dopamine remain elevated in DAT knockout mice (Gainetdinov and Caron, 2003). In C. elegans, the sole plasma membrane DAT is encoded by the dat-1 gene (Jayanthi et al, 1998; Nass et al, 2005). Deletion of dat-1 does not alter associative learning in the nematode (Figure 6A). We found that removal of DAT-1 bypasses the requirement for ASIC-1 function in associative learning (Figure 6A). This observation indicates that ASIC-1 contributes to sustain enhanced dopaminergic neurotransmission.
Figure 6.
ASIC-1 and dopaminergic signalling. (A) Elimination of the plasma membrane DAT-1 dopamine transporter cancels the effects of ASIC-1 deficiency on conditioning behaviour. Associative learning is restored in asic-1(ok415);dat-1(tm903) double mutants, carrying lesions in both the asic-1 and dat-1 gene. DAT-1 depletion does not affect associative learning. Bars depict chemotaxis indices towards isoamyl alcohol, calculated for either naive (white) or conditioned animals (black) of the indicated genetic background. Error bars denote s.e.m. values (n=1100 in six experiments; ***P<0.001, unpaired t-test). (B) Steady-state expression of the pasic-1SNB-1∷SEpHluorin reporter fusion in wild-type and asic-1(ok415) mutant animals. Bars depict average pixel intensity calculated from images of SNB-1∷SEpHluorin-labelled synapses. Error bars denote s.e.m. values (n=150 synapses in 30 individuals per genetic background; NS: not significant difference compared with wt; unpaired t-test). (C) Synaptic release at dopaminergic neurons in compromised asic-1(ok415) mutants. The rate of fluorescence recovery after photobleaching of synapses labelled with SNB-1∷SEpHluorin, specifically in dopaminergic neurons, is markedly reduced in animals carrying a lesion in the asic-1 gene (triangles), compared with wild-type controls (squares). The percentage of fluorescence increase after photobleaching is graphed against time. Values are calculated relatively to fluorescence intensity remaining at the point of photobleaching completion.
Second, we sought to directly compare the activity of dopaminergic synapses in wild-type animals and asic-1(ok415) mutants. We monitored synaptic release in dopaminergic neurons of live transgenic animals expressing a synaptobrevin-super ecliptic pHluorin reporter fusion (SNB-1∷SEpHluorin) under the asic-1 promoter, by optical recording of fluorescence recovery after photobleaching (FRAP) (Miesenbock et al, 1998; Samuel et al, 2003). Steady-state levels of SNB-1∷SEpHluorin synapse fluorescence are similar between wt animals and asic-1(ok415) mutants (Figure 6B). However, we found that the rate of neurotransmitter release at dopaminergic synapses is reduced in asic-1(ok415) mutants compared with wild-type controls (Figure 6C). We conclude that ASIC-1 is required to attain normal levels of dopaminergic signalling, necessary for associative learning in C. elegans.
ASIC-1 is required for enhanced dopamine release following conditioning
How does training, in the form of conditioning, affect dopaminergic signalling to alter future behaviour? To address this question, we monitored dopamine release before and after conditioning, both in wild-type animals and in asic-1 mutants. We found that in wild-type animals, conditioning elicits an increase in the rate of dopamine release compared with naive controls (Figure 7A). This observation indicates that dopaminergic neuron synaptic activity is subject to modulation by training in C. elegans. We next examined the effect of conditioning on dopamine release in asic-1(ok415) mutant animals. Our analysis revealed that training-induced, enhanced synaptic activity is significantly reduced in asic-1(ok415) mutants compared with wild type (Figure 7A). Although, conditioning in these animals does increase the rate of dopamine release above that of naive individuals, synaptic activity remains considerably lower than wild type in both cases. We conclude that ASIC-1 deficiency diminishes the rate of dopamine release at dopaminergic neuron synapses, in both naive and trained animals.
Figure 7.
Modulation of dopaminergic signalling by conditioning in wild-type animals and asic-1(ok415) mutants. (A) The percentage of fluorescence increase after photobleaching for both naive and conditioned animals of the indicated genetic background is graphed against time. For conditioning, synchronous nematode populations were exposed to isoamyl alcohol in the absence of food. Values are calculated relatively to fluorescence intensity remaining at the point of photobleaching completion. Error bars denote standard deviation (n>600 synapses in 100 individuals). (B) A working model for dopamine signalling modulation at dopaminergic neuron synapses. ASIC-1 functions to facilitate a positive feedback loop that reinforces dopamine release upon activation of dopaminergic neurons. We propose that evoked dopamine release, and the consequent local pH drop, activate the dopamine autoreceptors DOP-2 and ASIC-1, respectively, which promote further dopamine release, resulting in sustained dopaminergic signalling.
Discussion
ASIC-1 function and dopamine signalling in C. elegans associative learning
In this study, we demonstrate that ASIC-1 functions in dopaminergic neurons to mediate normal dopaminergic signalling, necessary for associative learning in C. elegans. Our findings reveal a new role of DEG/ENaC ion channels in neuronal communication by regulating the activity of dopaminergic synapses. Biogenic amines such as dopamine are known to modulate behavioural output during operant conditioning in diverse organisms. For example, dopamine is required for aversive reinforcement (punishment response) in Drosophila (Riemensperger et al, 2005). In humans, dopaminergic signalling underlies appetitive reinforcement (reward prediction) and addictive behaviour (Pessiglione et al, 2006; Schultz, 2007). Recent studies further underscore the importance of dopaminergic signalling in behavioural plasticity (Day et al, 2007; Lammel et al, 2008). Similarly, in nematodes, dopamine modulates certain types of behavioural adaptation to distinct sensory cues (Sanyal et al, 2004; Kindt et al, 2007). Our observations provide insight into a novel mechanism of synaptic plasticity operating at presynaptic terminals of dopaminergic neurons, which links dopaminergic signalling and presynaptic ASICs, to facilitate associative learning in C. elegans. Recently, ASICs have been located in mammalian mid-brain dopaminergic neurons (Pidoplichko and Dani, 2006). Further studies should clarify whether these channels mediate associative learning phenomena in mammals, by modulating dopamine signalling in the brain.
ASIC-1 and dopamine modulation of behavioural plasticity
Dopamine signals emitted by the eight dopaminergic neurons of C. elegans are received by specific dopamine receptors expressed in many different cell types. Four dopamine receptors (DOP-1 to 4) have been characterized in the nematode (Chase et al, 2004; Sanyal et al, 2004; Suo et al, 2004; Sugiura et al, 2005). DOP-1 is a D1-like dopamine receptor expressed in several cells, including mechanosensory neurons, cholinergic motor neurons, interneurons, excretory gland cells, head muscles and neuronal support cells. DOP-2 is orthologous to the human D2-type dopamine receptor and is expressed in all the dopaminergic neurons and some other interneurons. DOP-3 is homologous to mammalian D2 dopamine receptors and is expressed predominantly in neurons other than the dopaminergic neurons and to a lesser extent in body wall muscles. Finally, DOP-4 is similar to D1-like dopamine receptors unique to invertebrates, distinct from mammalian D1-like receptors and is expressed in pharyngeal neurons and interneurons, in the vulva, intestine, rectal glands and rectal epithelial cells.
Which dopamine receptors mediate the effects of dopamine on associative learning? DOP-2 is the dopamine autoreceptor in C. elegans and is expressed in dopaminergic neurons. Thus, DOP-2 may modulate dopamine release from these neurons by facilitating neuronal activity, similarly to ASIC-1 (Figure 7B). Consistently with this scenario, we find that DOP-2 deficiency mimics the effects of ASIC-1 deficiency on conditioning phenotypes (Figure 4E). Our results also indicate that DOP-3 functions downstream of DOP-2 because DOP-3 depletion restores normal conditioning behaviour in DOP-2-depleted animals. Interestingly, we also observed that DOP-1 deficiency cancels the effects of DOP-3 deficiency on associative learning (Figure 4E). Indeed, DOP-1 is known to antagonize DOP-3 as dop-1 mutations suppress dop-3 mutant phenotypes (Chase et al, 2004). DOP-3 and DOP-1 are co-expressed in cholinergic motor neurons and specific mechanosensory neurons (Chase et al, 2004). We hypothesize that dopamine facilitates behavioural reprogramming, following training, by modulating the activity of specific neuronal circuits through cognate dopamine receptors. Interestingly, dopamine alters habituation to sudden mechanosensory stimuli by modulating the response of specific mechanoreceptors (Kindt et al, 2007). In the absence of food, animals habituate more rapidly than in the presence of food. Such contextual information modulates behaviour in several learning paradigms, in the nematode (Colbert and Bargmann, 1997; Rankin, 2000; Law et al, 2004; Kindt et al, 2007). Monitoring the activity of dopamine-targeted neurons in behaving animals should provide insight into how dopamine influences the learning competence of the nervous system in C. elegans.
A working model for the involvement of ASIC-1 in modulating dopamine signalling
How does ASIC-1 influence synaptic release in dopaminergic neurons? Our working hypothesis is that ASIC-1 promotes dopamine release at dopaminergic presynaptic termini. According to this hypothesis, ASIC-1 deficiency causes a reduction in the release of dopamine from dopaminergic neurons. We have tested this hypothesis in three ways. First, we examined whether exogenous addition of dopamine reverses the effects of ASIC-1 mutations on conditioning behaviour. Second, we eliminated the plasma membrane DAT-1, which mediates dopamine re-uptake by dopaminergic neurons. DATs function to terminate dopaminergic signalling by clearing dopaminergic synapses and reducing dopamine build-up. Thus, knockout of DAT-1 is anticipated to increase the concentration of dopamine at the synapse and could effectively compensate for ASIC-1 deficiency. Finally, we measured synaptic activity by monitoring the rate of synaptic vesicle exocytosis at dopaminergic synapses using synaptic vesicle-targeted pHluorin and FRET, before and after conditioning, in both wild-type animals and asic-1(ok415) mutants. Our findings confirm the original working hypothesis. Specifically, we observed that the rate of neurotransmitter release at dopaminergic synapses is lower in animals carrying an asic-1 deletion. Consistently, DAT-1 deficiency restores normal conditioning behaviour in ASIC-1-deficient animals and exogenous addition of dopamine reverses the effects of asic-1 deletion. Furthermore, slow dopamine release in asic-1 mutant animals can be compensated by prolonged exposure to chemoattractants in the absence of food, which reduces their chemotaxis index to wild-type levels (Supplementary Figure 7).
On the basis of the totality of our observations, we propose that ASIC-1 functions to establish a positive feedback loop that reinforces dopamine release upon activation of dopaminergic neurons (Figure 7B). Evoked synaptic vesicle discharge releases both dopamine and protons in the synaptic cleft. Concomitant localized acidification at presynaptic release sites may activate ASIC-1, further depolarizing the presynaptic membrane, thus facilitating dopamine release. This working model bears resemblance to the proposed role of ASICs in LTP through NMDA glutamate receptors at postsynaptic sites in the hippocampus and other brain regions expressing ASICs (Wemmie et al, 2006). Activation of the NMDA receptor or PKC circumvents LTP defects associated with ASIC deficiency, indicating that probably ASIC-1 functions upstream of and normally stimulates NMDA receptor activity (Wemmie et al, 2002). Proton-evoked sodium currents generated by ASIC at postsynaptic terminals are likely to contribute to membrane depolarization and relief of the Mg2+ ions blocking NMDA receptors (Wemmie et al, 2002; Gao et al, 2005). Release of protons encapsulated together with neurotransmitters within synaptic vesicles may generate pH fluctuations at synapse micro-domains during synaptic transmission. Therefore, acidic pH conditions that develop at the synapse as a consequence of synaptic transmission elicit ASIC activation at both pre- and postsynaptic termini, potentiating neuronal communication. Interestingly, ASICs also appear to conduct calcium (Xiong et al, 2004; Yermolaieva et al, 2004). Calcium entry may, in turn, trigger signalling pathways that enhance LTP. Further studies should provide insight into how ASIC-1 activity is regulated to influence dopaminergic signalling during learning.
Materials and methods
Strains and genetics
We followed standard procedures for C. elegans strain maintenance, crosses and other genetic manipulations (Brenner, 1974). Nematode rearing temperature was kept at 20°C and the Escherichia coli strain OP50 was used as a food source. Some nematode strains were obtained by the C. elegans Knockout Consortium (Robert Barstead, Oklahoma Medical Research Foundation, USA), the Caenorhabditis Genetics Center (Theresa Stiernagle, University of Minnesota, Minneapolis, USA) and the National Bioresource Project, Japan (Shohei Mitani). The following strains were used in this study: N2: wild-type Bristol isolate; RB680: asic-1(ok415)I; CB1112: cat-2(e1112)II, dat-1(tm903)III, asic-1(ok415)I;dat-1(tm903)III; LX645: dop-1(vs100)X; LX702: dop-2(vs105)V, LX703: dop-3(vs106)X; LX705: dop-1(vs100)dop-3(vs106)X; LX706: dop-2(vs105)V, dop-1(vs100)X; LX704: dop-2(vs105)V;dop-3(vs106)X; LX734: dop-2(vs105)V;dop-1(vs100)dop-3(vs106)X, N2Ex[pasic-1GFPpRF4], N2Ex[pasic-1ASIC-1N∷GFPpRF4], N2Ex[pasic-1mec-4(u231)pmyo-2GFP] referred to in the text as pasic-1mec-4(d), N2Ex[pasic-1mec-4(u231)] Ex[pasic-1GFP], asic-1(ok415)Ex[pmyo-2GFPpasic-1ASIC-1] referred to in the text as asic-1(ok415)Ex[asic-1], asic-1(ok415)Ex[pasic-1GFPpasic-1ASIC-1], N2Ex[pasic-1ASIC-1N∷GFPpasic-1DsRED∷SNB-1pRF4], N2Ex[pasic-1SNB-1∷SEpHluorinpRF4] and asic-1(ok415)Ex[pasic-1SNB-1∷SEpHluorinpRF4].
Behavioural assays
Chemotaxis to soluble and volatile compounds was performed at 20°C, on 9 cm agar plates as described earlier (Ward, 1973; Bargmann and Horvitz, 1991; Bargmann et al, 1993; Colbert and Bargmann, 1995, 1997; Saeki et al, 2001). The chemotaxis index was calculated by subtracting the number of animals found at the trap from the number of animals at the source of the chemical, divided by the total number of animals entered into the assay (Bargmann and Horvitz, 1991). The resulting values were expressed and graphed as percentiles. Both naive and conditioned animals were challenged for 1 h with gradients of NaCl, NH4Cl, CH3COONa and isoamyl alcohol (gradient sources: CH3COONa: 200 mM; NH4Cl: 250 mM; NaCl: 100 mM; isoamyl alcohol: 1/100 dilution in water). About 200 adult animals for each strain were assayed in each experiment. For conditioning to water-soluble chemicals, animals were exposed to 20 mM NaCl, 50 mM NH4Cl or 50 mM CH3COONa on agar plates devoid of bacterial food, for 3 h, prior to assaying chemotaxis to the respective compound. For conditioning to isoamyl alcohol, a 3-μl drop of pure isoamyl alcohol was placed on the lid of a conditioning plate without food. Animals were conditioned to isoamyl alcohol for 90 min. Conditioning to isoamyl alcohol was also performed on 6 cm assay plates containing 600 μl of 30 mg/ml Sephadex beads. For adaptation assays, animals were exposed to isoamyl alcohol for 180 min in the absence of food. Appropriate time intervals for conditioning and adaptation were determined by time course analysis of chemotaxis after exposure to soluble and volatile cues for periods ranging from 30 to 240 min, in the absence of food. The effects of dopamine on chemotactic behaviours were examined by conditioning both wild-type and mutant animals on agar plates containing 5 mM dopamine hydrochloride (Sigma-Aldrich, St Louis, MO, USA). Dopamine was also added to chemotaxis assay plates at the same concentration. Basal slowing assays with well-fed animals were performed essentially as described earlier (Sawin et al, 2000). For each experiment, 10 young adult animals were followed and their body bents were counted during a 20-s period, both in the absence of food and on a bacterial lawn. Basal slowing was also assayed on thin films of Sephadex G-200 (Sigma-Aldrich) beads on agar plates, used to create axenic substrates with texture simulating that of a bacterial lawn (Sawin et al, 2000). Single worm isothermal tracking assays were performed essentially as described earlier (Hedgecock and Russell, 1975; Mori and Ohshima, 1995; Gomez et al, 2001). In each assay, more than 20 individuals from each genetic background were tested. Thermotaxis index was calculated as the percentage of animals capable of moving for at least half of the assay time on the isothermal line that corresponds to their cultivation temperature, in eight independent assays. Area-restricted search behaviour, osmotic avoidance, nose touch and body touch were assayed as described earlier (Culotti and Russell, 1978; Chalfie and Sulston, 1981; Kaplan and Horvitz, 1993; Hills et al, 2004). We scored sinusoidal locomotion characteristics (amplitude and wavelength) using age-matched animals transferred onto fresh plates and allowed to cut tracks on the bacterial lawn for 10–20 min. The amplitude of the path and the distance between successive peaks in the path (the wavelength) were then measured on photographed tracks. For each strain, 10 trials were conducted and 50 measurements were recorded per trial (Tavernarakis et al, 1997).
Microscopy
Animals were mounted in 2% agarose pads, anaesthetized with 20 mM sodium azide and observed at room temperature. Worms were harvested and observed using a high magnification oil-immersion objective lens (63 × , Zeiss Plan-NEOFLUAR; numerical aperture1.4; Carl Zeiss, Jena, Germany), on a confocal microscope (Zeiss Axioscope with a Bio-Rad Radiance 2100 scanhead; Bio-Rad, Hercules, USA), using the Bio-Rad LaserSharp 2000 software package. Labelling of amphid and phasmid neurons with the lipophilic carbocyanine tracer DiI (dioctadecyl-tetramethylindodicarbocyanine-disulphonic acid; Invitrogen, Carlsbad, USA) was done as described earlier (Hedgecock et al, 1985). Labelling of dopaminergic neurons was performed by formaldehyde-induced fluorescence as described earlier (Sulston et al, 1975; Lints and Emmons, 1999). Briefly, young adult animals were treated for 10 min with a solution of 4% paraformaldehyde in phosphate buffer, on a glass slide, at room temperature. After removing excess liquid, animals were incubated for 10 min at 100°C. Fluorescence was observed using a band-pass 395–440 nm filter for excitation and a 470 nm, long-pass filter for the emission. Measurements of FRAP at C. elegans neuronal synapses were done as described earlier (Samuel et al, 2003), using a Zeiss-Bio-Rad Radiance 2100 confocal microscope. Specifically, animals were immobilized on 2% agarose pads with 0.01% levamisole. Individual synapses were visualized by means of Synaptobrevin∷SEpHluorin fluorescence. A laser microbeam (wavelength 476 nm, power 5 mW) was used to bleach individual fluorescently labelled synapse regions of all three types of dopaminergic neurons (ADE, CEP and PDE; three consequent photo-bleaching cycles, with duration of 5-s each). Upon completion of photobleaching, fluorescence was reduced by 50–60% of the initial intensity. Subsequently, fluorescence recovery, specifically within the photobleached area, was monitored at a wavelength of 488 nm. At least five synapses for each neuron type were analysed per animal (between 15 and 20 measurements for each individual; more than 10 animals were analysed for each condition/genetic background). Emission intensity was measured on grayscale images with a pixel depth of 8 bit (256 shades of gray). We calculated the mean and maximum pixel intensity for each animal in these images using the ImageJ software (http://rsb.info.nih.gov/ij/). For each transgenic line and condition, we processed at least 150 images over at least five independent trials. Colocalization of GFP and DsRED was assessed by merging images obtained sequentially with a 488 nm laser beam and a 515±15 band-pass filter for GFP, and a 543 nm laser beam and a 570 nm long-pass filter for DsRED. Dye filling (DiI; Invitrogen) and visualization of amphid and phasmid nematode neurons was performed essentially as described earlier (Hedgecock et al, 1985; Perkins et al, 1986).
Bioinformatics
All sequences of C. elegans genes and proteins used for phylogenetic analysis were retrieved from Wormbase (http://www.wormbase.org/; version WS159). The GenBank accession number for the C. elegans ASIC-1 amino-acid sequence is AAB93309. BLAST searches (Altschul et al, 1997) were performed with the National Center for Biotechnology Information web servers (NCBI; http://www.ncbi.nlm.nih.gov/BLAST/). Multiple sequence alignments were generated using the M-Coffee algorithm (Notredame et al, 2000; http://tcoffee.vital-it.ch) and displayed with BoxShade 3.21 (EMBNet; http://www.ch.embnet.org/software/BOX_form.html). Prosite searches for conserved motifs in protein sequences were performed with the Expert Protein Analysis System (ExPASy) proteomics web-based server at the Swiss Institute of Bioinformatics (SIB; http://www.expasy.ch/tools/scanprosite/). Transmembrane topology predictions were performed with the Phobius algorithm (Kall et al, 2004); (http://phobius.cgb.ki.se/).
Statistics
Chemotaxis and thermotaxis indices, mean and standard error values were calculated from at least five independent experiments. The Student's t-test was used for two-way comparisons with a significance cutoff level of P<0.05. Analysis of variance was used for comparisons of multiple groups and was followed by Bonferroni-corrected multiple group comparison t-tests. Statistical analysis was carried out using the Microsoft Office 2003 Excel software package (Microsoft Corporation, Redmond, USA) and the Prism software package (GraphPad Software Inc., San Diego, USA).
Supplementary Material
Supplementary Information
Acknowledgments
We thank Angela Pasparaki for expert technical assistance and Manolis Vlachos for help with experiments. We thank Daphne Bazopoulou, Nikos Kourtis and Harbinder Singh for comments on the paper. Some nematode strains used in this study were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources (NCRR), the C. elegans Gene Knockout Project at OMRF (http://www.mutantfactory.ouhsc.edu/), which is part of the International C. elegans Gene Knockout Consortium and Dr Shohei Mitani (National Bioresource Project) in Japan. We thank A Fire for plasmid vectors and James Rothman for the pHluorin GFP variants. This study funded by grants from EMBO and the EU 6th Framework Programme to NT. NT is an EMBO Young Investigator.
References
- Abbott LF, Nelson SB (2000) Synaptic plasticity: taming the beast. Nat Neurosci 3 (Suppl): 1178–1183 [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bargmann C (2005) Neuroscience: comraderie and nostalgia in nematodes. Curr Biol 15: R832–R833 [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Hartwieg E, Horvitz HR (1993) Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74: 515–527 [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR (1991) Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7: 729–742 [DOI] [PubMed] [Google Scholar]
- Bernhard N, van der Kooy D (2000) A behavioral and genetic dissection of two forms of olfactory plasticity in Caenorhabditis elegans: adaptation and habituation. Learn Mem 7: 199–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Sulston J (1981) Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev Biol 82: 358–370 [DOI] [PubMed] [Google Scholar]
- Chase DL, Pepper JS, Koelle MR (2004) Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat Neurosci 7: 1096–1103 [DOI] [PubMed] [Google Scholar]
- Chen X, Polleichtner G, Kadurin I, Grunder S (2007) Zebrafish acid-sensing ion channel (ASIC) 4, characterization of homo- and heteromeric channels, and identification of regions important for activation by H+. J Biol Chem 282: 30406–30413 [DOI] [PubMed] [Google Scholar]
- Colbert HA, Bargmann CI (1995) Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron 14: 803–812 [DOI] [PubMed] [Google Scholar]
- Colbert HA, Bargmann CI (1997) Environmental signals modulate olfactory acuity, discrimination, and memory in Caenorhabditis elegans. Learn Mem 4: 179–191 [DOI] [PubMed] [Google Scholar]
- Culotti JG, Russell RL (1978) Osmotic avoidance defective mutants of the nematode Caenorhabditis elegans. Genetics 90: 243–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM (2007) Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci 10: 1020–1028 [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Caron MG (2003) Monoamine transporters: from genes to behavior. Annu Rev Pharmacol Toxicol 43: 261–284 [DOI] [PubMed] [Google Scholar]
- Gao J, Duan B, Wang DG, Deng XH, Zhang GY, Xu L, Xu TL (2005) Coupling between NMDA receptor and acid-sensing ion channel contributes to ischemic neuronal death. Neuron 48: 635–646 [DOI] [PubMed] [Google Scholar]
- Gomez M, De Castro E, Guarin E, Sasakura H, Kuhara A, Mori I, Bartfai T, Bargmann CI, Nef P (2001) Ca2+ signaling via the neuronal calcium sensor-1 regulates associative learning and memory in C. elegans. Neuron 30: 241–248 [DOI] [PubMed] [Google Scholar]
- Harbinder S, Tavernarakis N, Herndon LA, Kinnell M, Xu SQ, Fire A, Driscoll M (1997) Genetically targeted cell disruption in Caenorhabditis elegans. Proc Natl Acad Sci USA 94: 13128–13133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb DO (1949) The Organization of Behavior; A Neuropsychological Theory. New York: Wiley [Google Scholar]
- Hedgecock EM, Culotti JG, Thomson JN, Perkins LA (1985) Axonal guidance mutants of Caenorhabditis elegans identified by filling sensory neurons with fluorescein dyes. Dev Biol 111: 158–170 [DOI] [PubMed] [Google Scholar]
- Hedgecock EM, Russell RL (1975) Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA 72: 4061–4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills T, Brockie PJ, Maricq AV (2004) Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J Neurosci 24: 1217–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O (2003) Behavioral plasticity in C. elegans: paradigms, circuits, genes. J Neurobiol 54: 203–223 [DOI] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E (2007) Structure of acid-sensing ion channel 1 at 1.9 Å resolution and low pH. Nature 449: 316–323 [DOI] [PubMed] [Google Scholar]
- Jayanthi LD, Apparsundaram S, Malone MD, Ward E, Miller DM, Eppler M, Blakely RD (1998) The Caenorhabditis elegans gene T23G5.5 encodes an antidepressant- and cocaine-sensitive dopamine transporter. Mol Pharmacol 54: 601–609 [PubMed] [Google Scholar]
- Kall L, Krogh A, Sonnhammer EL (2004) A combined transmembrane topology and signal peptide prediction method. J Mol Biol 338: 1027–1036 [DOI] [PubMed] [Google Scholar]
- Kaplan JM, Horvitz HR (1993) A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc Natl Acad Sci USA 90: 2227–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Schild L (2002) Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev 82: 735–767 [DOI] [PubMed] [Google Scholar]
- Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, Rankin CH, Schafer WR (2007) Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans. Neuron 55: 662–676 [DOI] [PubMed] [Google Scholar]
- Krishtal O (2003) The ASICs: signaling molecules? Modulators? Trends Neurosci 26: 477–483 [DOI] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Hackel O, Jones I, Liss B, Roeper J (2008) Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron 57: 760–773 [DOI] [PubMed] [Google Scholar]
- Law E, Nuttley WM, van der Kooy D (2004) Contextual taste cues modulate olfactory learning in C. elegans by an occasion-setting mechanism. Curr Biol 14: 1303–1308 [DOI] [PubMed] [Google Scholar]
- Lints R, Emmons SW (1999) Patterning of dopaminergic neurotransmitter identity among Caenorhabditis elegans ray sensory neurons by a TGFbeta family signaling pathway and a Hox gene. Development 126: 5819–5831 [DOI] [PubMed] [Google Scholar]
- McDonald PW, Jessen T, Field JR, Blakely RD (2006) Dopamine signaling architecture in Caenorhabditis elegans. Cell Mol Neurobiol 26: 593–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesenbock G, De Angelis DA, Rothman JE (1998) Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 394: 192–195 [DOI] [PubMed] [Google Scholar]
- Mohri A, Kodama E, Kimura KD, Koike M, Mizuno T, Mori I (2005) Genetic control of temperature preference in the nematode Caenorhabditis elegans. Genetics 169: 1437–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I (1999) Genetics of chemotaxis and thermotaxis in the nematode Caenorhabditis elegans. Annu Rev Genet 33: 399–422 [DOI] [PubMed] [Google Scholar]
- Mori I, Ohshima Y (1995) Neural regulation of thermotaxis in Caenorhabditis elegans. Nature 376: 344–348 [DOI] [PubMed] [Google Scholar]
- Mori I, Sasakura H, Kuhara A (2008) Worm thermotaxis: a model system for analyzing thermosensation and neural plasticity. Curr Opin Neurobiol 17: 712–719 [DOI] [PubMed] [Google Scholar]
- Nass R, Hahn MK, Jessen T, McDonald PW, Carvelli L, Blakely RD (2005) A genetic screen in Caenorhabditis elegans for dopamine neuron insensitivity to 6-hydroxydopamine identifies dopamine transporter mutants impacting transporter biosynthesis and trafficking. J Neurochem 94: 774–785 [DOI] [PubMed] [Google Scholar]
- Notredame C, Higgins DG, Heringa J (2000) T-Coffee: a novel method for fast and accurate multiple sequence alignment. J Mol Biol 302: 205–217 [DOI] [PubMed] [Google Scholar]
- Nuttley WM, Atkinson-Leadbeater KP, Van Der Kooy D (2002) Serotonin mediates food-odor associative learning in the nematode Caenorhabditis elegans. Proc Natl Acad Sci USA 99: 12449–12454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paukert M, Chen X, Polleichtner G, Schindelin H, Grunder S (2008) Candidate amino acids involved in H+ gating of acid-sensing ion channel 1a. J Biol Chem 283: 572–581 [DOI] [PubMed] [Google Scholar]
- Pavlov IP (1928) Lectures on Conditioned Reflexes. New York: International Publishers [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG (1986) Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol 117: 456–487 [DOI] [PubMed] [Google Scholar]
- Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD (2006) Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature 442: 1042–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoplichko VI, Dani JA (2006) Acid-sensitive ionic channels in midbrain dopamine neurons are sensitive to ammonium, which may contribute to hyperammonemia damage. Proc Natl Acad Sci USA 103: 11376–11380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin CH (2000) Context conditioning in habituation in the nematode Caenorhabditis elegans. Behav Neurosci 114: 496–505 [PubMed] [Google Scholar]
- Rankin CH (2004) Invertebrate learning: what can't a worm learn? Curr Biol 14: R617–R618 [DOI] [PubMed] [Google Scholar]
- Riemensperger T, Voller T, Stock P, Buchner E, Fiala A (2005) Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol 15: 1953–1960 [DOI] [PubMed] [Google Scholar]
- Ryu WS, Samuel AD (2002) Thermotaxis in Caenorhabditis elegans analyzed by measuring responses to defined thermal stimuli. J Neurosci 22: 5727–5733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki S, Yamamoto M, Iino Y (2001) Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J Exp Biol 204: 1757–1764 [DOI] [PubMed] [Google Scholar]
- Samuel AD, Silva RA, Murthy VN (2003) Synaptic activity of the AFD neuron in Caenorhabditis elegans correlates with thermotactic memory. J Neurosci 23: 373–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal S, Wintle RF, Kindt KS, Nuttley WM, Arvan R, Fitzmaurice P, Bigras E, Merz DC, Hebert TE, van der Kooy D, Schafer WR, Culotti JG, Van Tol HH (2004) Dopamine modulates the plasticity of mechanosensory responses in Caenorhabditis elegans. EMBO J 23: 473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR (2000) C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 26: 619–631 [DOI] [PubMed] [Google Scholar]
- Schultz W (2007) Behavioral dopamine signals. Trends Neurosci 30: 203–210 [DOI] [PubMed] [Google Scholar]
- Skinner BF (1930) On the conditions of elicitation of certain eating reflexes. Proc Natl Acad Sci USA 16: 433–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura M, Fuke S, Suo S, Sasagawa N, Van Tol HH, Ishiura S (2005) Characterization of a novel D2-like dopamine receptor with a truncated splice variant and a D1-like dopamine receptor unique to invertebrates from Caenorhabditis elegans. J Neurochem 94: 1146–1157 [DOI] [PubMed] [Google Scholar]
- Sulston J, Dew M, Brenner S (1975) Dopaminergic neurons in the nematode Caenorhabditis elegans. J Comp Neurol 163: 215–226 [DOI] [PubMed] [Google Scholar]
- Suo S, Ishiura S, Van Tol HH (2004) Dopamine receptors in C. elegans. Eur J Pharmacol 500: 159–166 [DOI] [PubMed] [Google Scholar]
- Tavernarakis N, Everett JK, Kyrpides NC, Driscoll M (2001) Structural and functional features of the intracellular amino terminus of DEG/ENaC ion channels. Curr Biol 11: R205–R208 [DOI] [PubMed] [Google Scholar]
- Tavernarakis N, Shreffler W, Wang S, Driscoll M (1997) unc-8, a DEG/ENaC family member, encodes a subunit of a candidate mechanically gated channel that modulates C. elegans locomotion. Neuron 18: 107–119 [DOI] [PubMed] [Google Scholar]
- Tavernarakis N, Wang SL, Dorovkov M, Ryazanov A, Driscoll M (2000) Heritable and inducible genetic interference by double-stranded RNA encoded by transgenes. Nat Genet 24: 180–183 [DOI] [PubMed] [Google Scholar]
- Voglis G, Tavernarakis N (2006) The role of synaptic ion channels in synaptic plasticity. EMBO Rep 7: 1104–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M (1997) A proton-gated cation channel involved in acid-sensing. Nature 386: 173–177 [DOI] [PubMed] [Google Scholar]
- Ward S (1973) Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proc Natl Acad Sci USA 70: 817–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Askwith CC, Lamani E, Cassell MD, Freeman JH Jr, Welsh MJ (2003) Acid-sensing ion channel 1 is localized in brain regions with high synaptic density and contributes to fear conditioning. J Neurosci 23: 5496–5502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Chen J, Askwith CC, Hruska-Hageman AM, Price MP, Nolan BC, Yoder PG, Lamani E, Hoshi T, Freeman JH Jr, Welsh MJ (2002) The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron 34: 463–477 [DOI] [PubMed] [Google Scholar]
- Wemmie JA, Coryell MW, Askwith CC, Lamani E, Leonard AS, Sigmund CD, Welsh MJ (2004) Overexpression of acid-sensing ion channel 1a in transgenic mice increases acquired fear-related behavior. Proc Natl Acad Sci USA 101: 3621–3626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wemmie JA, Price MP, Welsh MJ (2006) Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci 29: 578–586 [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, Minami M, Hey J, Wei WL, MacDonald JF, Wemmie JA, Price MP, Welsh MJ, Simon RP (2004) Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell 118: 687–698 [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Leonard AS, Schnizler MK, Abboud FM, Welsh MJ (2004) Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci USA 101: 6752–6757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lu H, Bargmann CI (2005) Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 438: 179–184 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information