Abstract
Visual attention problems have been reported in association with prenatal alcohol exposure (PAE). With related behavioral data documented in literature, further investigation of this PAE effect would benefit from integrating functional and anatomical imaging data to ascertain its neurobiological basis. The current study investigated the possible functional and anatomical bases for the PAE-related visual sustained attention deficit. Functional magnetic resonance imaging (fMRI) data were collected while the subjects performed a sustained visual attention task. High resolution, three dimensional anatomical images were also collected for morphometric evaluation. In the alcohol-affected subjects, we observed a significant white and gray matter volume reduction in the occipital-temporal area. Meanwhile, their fMRI activations in the same region resided more superiorly than that of the controls resulting in reduced activation in the ventral occipital-temporal area. The location of this PAE functional abnormality approximately matches that of the significant structural reduction. In addition to the well documented corpus callosum abnormalities observed in PAE subjects, the present results reveal a teratogenic effect on the occipital-temporal area. Furthermore, as the occipital-temporal area plays an important role in visual attention, the current observation suggests a neurobiological underpinning for the PAE related deficit in sustained visual attention.
Keywords: Fetal alcohol exposure, Sustained attention, Functional magnetic resonance imaging, Structural imaging
Introduction
The most important aspect of the teratological effect of prenatal alcohol exposure is the impact on brain development and function and the associated effects on neurocognition and behavior. Current research on fetal alcohol syndrome (FAS) and the broader range of prenatal alcohol effects, which are referred to as fetal alcohol spectrum disorders (FASD), include two types of studies. The first are behavioral/cognitive studies comparing the behavioral performance of exposed to nonexposed individuals on various neuropsychological tasks with the goal of understanding the neurocognitive phenotype associated with exposure. While many differences in functioning, including attention (Coles et al. 1997; Nanson and Hiscock 1990), memory (Mattson et al. 1996), verbal ability (Schonfeld et al. 2001), and executive function (Connor et al. 2000), have been identified as impaired in alcohol-exposed individuals, inconsistencies remain, however, between studies and across developmental stages. Specific behavioral markers (if there are any) have not been delineated as yet. The second group of studies investigates structural or neuroanatomical abnormalities. Microcephaly has been the most consistent finding with the alcohol-exposed subjects showing a general reduction in brain volume relative to control groups (Archibald et al. 2001; Bhatara et al. 2002; Riley et al. 1995; Sowell et al. 2001a; Sowell et al. 2001b). More specifically, volume and density abnormalities in both the white matter and gray matter have been noted in various brain regions including the corpus callosum, the temporoparietal area, the frontal area, and the basal ganglia. (Archibald et al. 2001; Riikonen et al. 1999; Sowell et al. 2001b; Sowell et al. 2002). By using the diffusion tensor imaging (DTI) technique, we recently also found significant difference in the diffusion fractional anisotropy and apparent diffusion coefficient in the corpus callosum of alcohol-affected young adults compared with the non-exposed controls (Ma et al. 2005).
Despite the large amount of data related respectively to the behavioral and structural impact of PAE, neuroimaging studies, which link pathological alterations in both the functional and structural aspects, are relatively rare. Our knowledge as to whether there is a more direct linkage between a specific functional deficit and a regional anatomy abnormality is therefore quite limited. Like the study of Bookstein and colleagues, which measured the linkage between the shape of the corpus callosum and executive functioning and motor functioning (Bookstein et al. 2002), more progress in understanding PAE teratogenic effect is expected to benefit from the integration of neuroimaging findings from both the functional and the structural domains (Bookheimer and Sowell 2005).
Selective attention is an appropriate behavior to examine in alcohol-affected individuals for several reasons. Many studies provided evidence that prenatal alcohol exposure impacts aspects of attention (Coles 2001; Coles et al. 2002; Connor et al. 1999; Kopera-Frye et al. 1997; Mattson et al. 2006; Nanson and Hiscock 1990). These studies support the idea that there is a behavioral impairment in the ability to maintain a focused alertness in perceiving a signal (the sustained function identified by Mirsky 1996) and in the ability to allocate attentional resource, or the ability to shift the attention from one task to another when appropriate (the shift function identified by Mirsky 1996). More specifically, on a “continuous performance” task, we (Coles et al. 2002 and Mattson et al. 2006) both found that sustained attention was particularly affected when the stimuli were presented visually rather than aurally. As this well-known task was found useful to differentiate alcohol exposed and nonexposed individuals, it would be appropriate to adopt it in further studies in understanding the relationship between functional and neuroanatomical effects of prenatal alcohol exposure. The same continuous performance paradigm had already been used in one of our previous fMRI studies on normal subjects (Le et al. 1998) which revealed activation in the occipital and inferior temporal regions for the sustained attention task. Given the behavioral characteristics of the PAE subject and the brain activation characteristics in the normals reported in previous investigations, the present study compares both functional and structural imaging measures between exposed and control groups to gain more insight into the neural basis of the PAE related attention deficit. In particular, we are interested in examining whether there are structural and functional abnormalities in the same brain region of PAE subjects that could suggest a neurobiology-behavior association.
Materials and methods
Participants
Fourteen young adults (7 PAE subjects vs. 7 controls) were recruited from a cohort enrolled in a longitudinal study of effects of prenatal alcohol exposure on subsequent development.
The original sample was recruited prenatally from an Atlanta urban hospital between 1978 and 1986. Following a screening procedure, women who reported using more than or equal to two drinks or one ounce of absolute alcohol per week (oz AA/wk) during pregnancy and women who did not use alcohol at all were asked to participate. Self-reported alcohol use by the mothers of our subjects during pregnancy is listed in Table 1. Members of the cohort have been evaluated periodically since birth (Brown et al. 1991; Coles et al. 2002; Coles et al. 1985). The sample is predominantly of low socioeconomic status and African-American. Participants in the current study had all been seen as part of a Teen Assessment Study between 1996 and 2000, and information was available concerning maternal characteristics, prenatal exposure to alcohol and other drugs, physical characteristics associated with alcohol exposure (dysmorphia scores), and results of neurocognitive testing during adolescence. This information was used to classify participants as to alcohol exposure and effects and provide the information in Table 1.
Table 1.
Demographic characteristics of alcohol exposed and control groups
| Group | Age | Gender | Ethnicity | AAPWDP | HDS | FSIQS | PSS |
|---|---|---|---|---|---|---|---|
| AAPWDP: average number of ounces of absolute alcohol used per week during pregnancy, HDS: highest of the dysmorphia scores (may be chosen from among infant, teen and adult scores), FSIQS: Full Scale IQ Score, PSS: Processing Speed Score | |||||||
| PAE | 18 | F | African American | 10 | 20 | 48 | 54 |
| 20 | F | African American | 2 | 9 | 52 | 64 | |
| 22 | M | African American | 10.4 | 27 | 44 | 50 | |
| 24 | F | Caucasian | 40 | 15 | 74 | not collected | |
| 18 | M | African American | 4 | 14 | 60 | 67 | |
| 20 | M | African American | 30 | 8 | 65 | 72 | |
| 21 | M | African American | 6 | 27 | 53 | 67 | |
| Control | 21 | M | African American | 0 | 2 | 80 | 101 |
| 21 | F | African American | 0 | 4 | 87 | 122 | |
| 22 | M | African American | 0 | 3 | 72 | 77 | |
| 22 | F | African American | 0 | 8 | 82 | 99 | |
| 18 | M | African American | 0 | 5 | 86 | 99 | |
| 23 | F | African American | 0 | 3 | 86 | 96 | |
| 22 | M | African American | 0 | 1 | 71 | 75 | |
For the present study, the seven young alcohol-affected adults were selected based on their scores on a rating of physical dysmorphia and evidence of cognitive deficits associated with prenatal alcohol exposure. Both physical features and cognitive deficit were included in selecting the participants as both are known to be associated with the impact of prenatal alcohol exposure. In the longitudinal cohort, these characteristics have been highly correlated. Physical dysmorphia was assessed at evaluations during infancy, childhood, and adolescence and the measure used here was the highest (most severe) dysmorphia score ever assigned to the individual. The seven controls were recruited from the nonexposed contrast group. Potential participants were contacted by study outreach workers and told about the study. If they were interested in participating, a short screening interview was completed to determine whether they were eligible to have an MRI. Exclusionary criteria included having implanted medical devices or metal in the body (e.g., pacemaker, aneurysm clips, hearing aids or cochlear implants, pins, screws, braces on teeth, plates, shrapnel, etc.), pregnancy, non-removable jewelry or body piercing, claustrophobia, extreme obesity, and vision problems not correctable with contact lenses. If participants were interested and eligible, outreach workers completed the consent procedure and asked participants to sign the consent forms approved by the Emory University Institutional Review Board. Parents or guardians were asked to consent for participants not cognitively competent to give consent.
Task paradigm
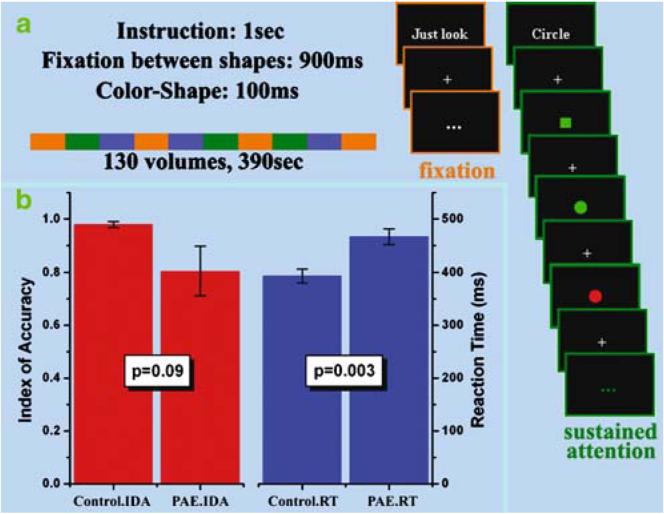
Figure 1a provides a schematic diagram of the task paradigm.
Fig. 1.
a The experimental paradigm. The three types of task blocks (fixation, indicated by orange; sustained attention, indicated by green; switch attention, indicated by blue) were arranged in a pseudo-random order in one fMRI scan. In the baseline blocks, subjects passively gaze at the fixation cross displayed at the center of the screen. In the sustained attention blocks, subjects immediately press the button whenever a circle appears (i.e. response on the 5th and 7th slide shown in the example). b The behavioral performance of the sustained attention task. The group difference significance is shown by the p values for both the comparisons of the index of accuracy (IDA, the red bars) and the reaction time (RT, the blue bars). The error bars represent the standard error
We adopted a block-design fMRI paradigm in the current study to contrast the brain activity between visual fixation and sustained attention. In the same experiment, a switching attention task was also attempted as part of a separate study and will not be discussed further in this paper. The visual fixation task served as the baseline condition by requiring the subjects to focus on a small cross (approx. 1.5°×1.5° in viewing angle) in the center of the screen. The sustained attention blocks consisted of serially presented circles and squares (5°×5°, randomly in red or green), during which time subjects were required to maintain attention on the shape of the presented objects. They were instructed to immediately press a button with the dominant index finger whenever a circle (whatever color it was) appeared on the screen.
At the beginning of each block, a 1-second instruction was displayed telling the subjects which task the following block would be (“Just look” for fixation, “Circle” for sustained attention). In the visual fixation blocks, the fixation cross remained on the screen. In the sustained attention blocks, each object was displayed for 100 ms with a 900 ms inter-stimuli interval filled by the fixation cross. Each subject completed four fixation blocks and three sustained attention blocks during one fMRI scan with each block lasting 39 s (including the beginning instruction). The presentation order of the task blocks is shown in Fig. 1a. There were a total of 114 objects presented in the sustained attention blocks with circles and squares appearing randomly with equal probability. As they were randomly generated by the PRESENTATION program (Neurobehavioral Systems, http://www.neurobs.com) individually for each participant, the total number of targets (circle) varied slightly across subjects (36±5).
Behavioral performance calculation
The shape detection accuracy and reaction time were both obtained for each subject. For the reaction time calculation, only “correct” responses were included. For the response accuracy, we calculated an index of accuracy (IDA), which considered both the responses that were true “hits” and the responses that were “false alarms”.
Index of Accuracy (IDA) = THF × (1 - FAF), where
THF provides a measure of detection sensitivity and the FAF provides a measure of response specificity (Metz 1978). The IDA yields an overall measure of performance by combining both sensitivity and specificity factors (Le et al. 1998).
Imaging data acquisition
Functional and anatomical data were collected with a Siemens 3T Trio scanner. The “EPI-BOLD” functional scan was composed of 130 volume measurements (34 axial slices, slice thickness=3 mm, slice gap=0 mm, FOV= 220 mm, matrix=64×64, bandwidth=2,240 Hz/Pixel, TE= 32 ms, TR=3 s, flip angle=90°). The high resolution T1-weighted 3D anatomical images were acquired with an “MPRAGE” sequence (176 sagittal slices, TR/TE=2,600/ 3.93 ms, flip angle=8°). The anatomical scan protocol had a 3D FOV=256×224×176 mm3 and a 3D matrix=256×224×176 resulting in an isotropic resolution of 1 mm.
Imaging data analysis
AFNI (http://afni.nimh.nih.gov) was used to perform fMRI data analysis. After the data preprocessing steps, which included slice timing correction, volume registration and 5 mm FWHM Gaussian blur, activation maps were derived for each subject with a multiple regression analysis. The regressors were generated by convolving the boxcar stimulation functions (the sustained and switching attention task) with an impulse response function [y=t^b×exp(-t/c), b and c are constants] (Cohen 1997). In addition, the six rigid body head motion parameters (X, Y, Z displacements and roll, pitch, yaw rotations) were also included as regressors to get a better model of the motion-related signal noise. In the statistical parametric maps, the activated brain areas were defined as those voxels with a significant sustained attention contribution (partial F-statistic) in explaining the BOLD signal variation. For each group, activation maps of individual subjects were averaged after transforming the data set into the Talairach space (Talairach and Tournoux 1988) and normalizing the F values to Z-scores. A voxel-wise threshold p<0.01 and a cluster threshold of 665 mm3 were applied inducing a combined threshold corresponding to multiple comparison corrected significance level of p=0.01 in the final activation map.
As we intended to examine the potential functional and structural associations in the PAE related alteration, the analysis of the anatomical data was performed post hoc. Because a between-group functional activation difference could indicate a possible existence of PAE related structural change in the same brain area, we used the fMRI activation to guide the anatomical data analysis. Namely, region of interest (ROI) was identified based on the brain activity difference and used for examining any anatomical differences. Details regarding this part of the analysis are described below.
Results
As graphically shown in Fig. 1b, the IDA of the controls (Mean=98%, SE=1%) in the sustained attention task was higher (nearly significant: group t-test, t=1.87, p=0.09) than that of the PAE subjects (Mean=80%, SE=9%). In addition, the reaction time was significantly (t=3.66, p= 0.003) shorter for the controls (Mean=393 ms, SE=13 ms) compared to the PAE subjects (Mean=466 ms, SE=15 ms). The relatively poorer performances in the PAE group replicated the previous observations (Coles 2001; Coles et al. 2002; Mattson et al. 2006) of the negative impact of the fetal alcohol exposure on sustained visual attention.
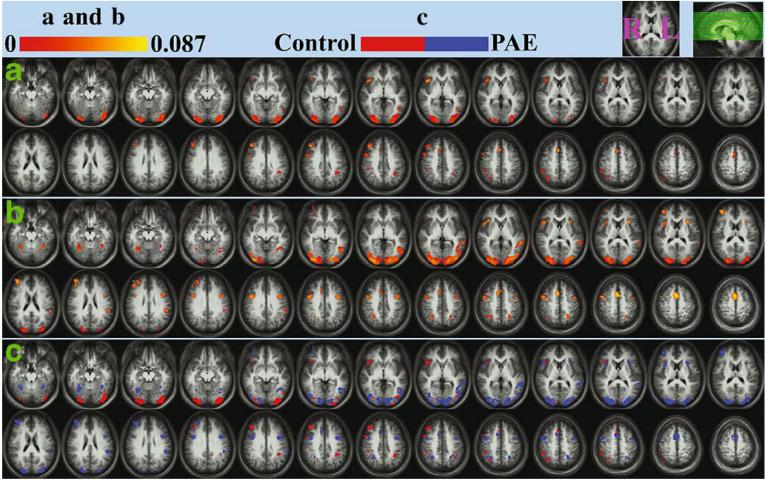
The brain networks activated during the sustained attention task were generally more widespread in the PAE participants than in the controls (Fig. 2). Besides the activations in the frontal and the parietal lobes, the most significant brain activity in both groups occurred in the occipital-temporal area. This extrastriatal cortical activation (Brodmann areas 18, 19 and 37) replicated our previous fMRI observation with the same task (Le et al. 1998). Our new finding here was a clear difference between the PAE and the control group. As shown in the CONTROL minus PAE contrast map (Fig. 2c), the group difference in the occipital-temporal activation was its location in the Z (inferior-superior) direction. The activation of the PAE group was higher than that of the controls.
Fig. 2.
The “sustained attention” brain activation of the normal controls (a), PAE subjects (b) and the activation contrast between these two groups (c). The activation maps (a and b) were generated by averaging the individual data within group and applying a voxel-wise plus cluster threshold (z-score threshold p<0.01/voxel plus cluster threshold 665 mm3: p<0.01/overall). The color coding (in a and b) represents the magnitude of the regression coefficient. The contrast map (c) was generated by subtracting activated voxels of the PAE subjects from those of the controls (blue area, only activated in PAE subjects; red area, only activated in controls). The left/right (L/R) hemisphere and the slice locations are shown at the upper right corner of the graph. The anatomical underlays here are the averaged images of all the 14 participants
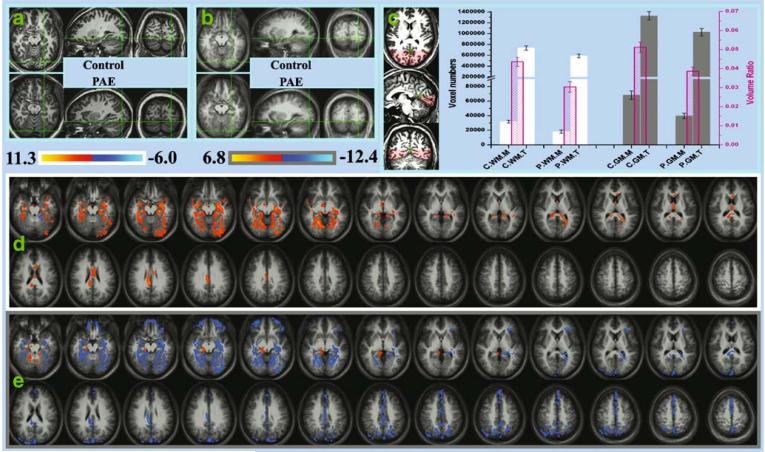
Besides this functional activation discrepancy, structural group differences in the same area were also detected. During visual inspection of the anatomical images, we found that the occipital-temporal region of the controls extended more downward (relative to the AC-PC line) than that of the PAE subjects. As shown in the Fig. 3a, when the same Talairach coordinate is selected in the occipital-temporal area on one control and one PAE participant, we can clearly see that at the same Z level, the location is almost in the cerebellum in the PAE subject, while it is still within the cerebral area in the control subject. This anatomical difference is also present in group averages shown in the Fig. 3b. At the same Talairach coordinate, while the controls can show relatively clear white matter structures, the PAE subjects primarily just have mixed gray matter from the cerebral and cerebellum cortex.
Fig. 3.
a The anatomical comparison between one control (upper row) and one PAE subject (lower row). The green cursor marked the same Talairach position in the two subjects. While the occipital-temporal cerebral structure can still clearly be seen with Z=-10 mm in the control, the PAE subject almost only show cerebellum in the same area. b The same comparison as in (a), but the images are substituted by group means. More (less) white (gray) matter at the cursor position can be seen in the controls. c The comparison of white and gray matter volume between the control and exposed group. The green cross in the anatomical images marked the intersection point of the parietal-occipital sulcus and the calcarine sulcus. The individual occipital-temporal ROI mask covers the cerebral structure lower than and posterior to this point (the shaded area). The white and gray bars represent the volumes of the white and gray matter, respectively, in units of voxel number (left axis). The pink bars (scaled by the right pink axis) represent the ROI-mask/whole-brain volume ratio (i.e., the height ratio of the two bars standing behind). The error bars represent the standard error. C: control, P: PAE, WM: white matter, GM: gray matter, M: mask ROI volume, T: total brain volume. d/e The VBM result of the white/gray matter density (denoted by a white/gray frame, respectively). The slice locations here are exactly the same as those shown in Fig. 1. The color coding represents the t value from the control-PAE group t-test. Areas with a higher density in controls/PAEs are thus marked by red-yellow/blue-cyan. The maps have a threshold of p<0.05/voxel plus 3,511 mm3 cluster (p<0.01/overall)
The between-group differences respectively seen in the functional and anatomical data are both important in explaining the PAE related behavioral deficit. While each of them could act independently, they may also be interdependent because the activation in PAE subjects appearing more superior may be solely due to the reduction in the occipital-temporal region (mainly in the Z direction). To test this hypothesis and ascertain how much of PAE-control mismatch in activation is a result of the corresponding structural difference, we quantitatively confirmed the occipital-temporal reduction observed in the visual inspection and assessed the activation mismatch after compensating for the structural difference with anatomic normalization.
Post hoc analysis and results
To examine the anatomical difference in the occipital-temporal area, both voxel-based morphometry (VBM) (Ashburner and Friston 2000; Good et al. 2001) and region of interest volumetric statistical approaches were used. While the VBM, on one hand, performs a local comparison of gray/white matter content, the ROI based approach, on the other hand, provides a more global assessment of tissue volume differences between the two groups.
The VBM analysis was achieved through normalization, segmentation, smoothing, and statistical comparison. In the normalization step, all subjects' 3D anatomical data were transformed into the Talairach space. The segmentation partitioned the normalized images into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) probabilities using a method described by Ashburner and Fristion (Ashburner and Friston 2000) in the SPM2 package (http://www.fil.ion.ucl.ac.uk/spm). The GM and WM probability images were then smoothed by a 5 mm FWHM isotropic Gaussian kernel and the statistical comparison was performed on GM and WM images, respectively, using a voxel-wise t-test contrasting between the two subject groups. As shown in the Fig. 3d,e, the VBM analysis demonstrated that the control subjects have a significantly higher WM content in the ventral occipital-temporal and the corpus callosum areas. In contrast, the PAE participants show higher GM content throughout the occipital-temporal, parietal and frontal areas.
The ROI volume comparison was done based on an ROI manually drawn on each individual's 3D anatomical images. In each subject's Talairach brain, the ROI consists of the portion of the cerebrum below and posterior to the intersection point of the parietal-occipital sulcus and the calcarine sulcus (see Fig. 3c). The group comparison was based on the WM and GM volumes within the ROI. Considering that microcephaly has been the most consistent structural finding in studies of fetal alcohol exposure (Archibald et al. 2001; Bhatara et al. 2002; Riley et al. 1995; Sowell et al. 2001a; b), we also compared the normalized (by the whole brain volume) WM and GM volume to see whether the PAE participants have a disproportional reduction in the occipital-temporal region. As illustrated in the Fig. 3c, the group t-test statistically confirms our earlier observation. In addition to replicating the previously reported cerebral volume reduction in PAE subjects (WM/GM ROI volume comparison, t=4.9/4.2, p= 0.0003/0.001; total brain volume comparison, t=2.8/2.9, p= 0.02/0.01), our data also demonstrate a disproportional reduction of WM and GM volumes in the occipital-temporal region (ROI/TOTAL volume ratio comparison of WM and GM, tWM=3.7, pWM=0.003; tGM=3.6, pGM=0.004).
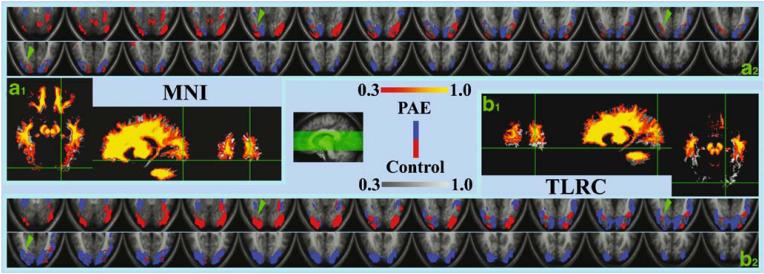
With the PAE-related occipital-temporal volume reduction validated by the VBM analysis and the ROI comparison, we further tested whether the more superior volume of activity seen in the PAE subjects were simply due to this anatomical mismatch. To this end, each subject's anatomical images were transformed (using SPM2) into the MNI (Montreal Neurological Institute) brain space with a 12-parameter affine normalization algorithm (Ashburner and Friston 1999). This “intensity-based” MNI normalization minimized the total residual squared difference between the image and the template and stretched the smaller cerebrum area in the PAE subjects to match that of the normal brain. Figure 4a1,b1 illustrates the effect of the MNI transformation. While the volume difference in occipital-temporal areas (only displayed for the WM data) were clearly seen in the Talairach (TLRC) brains, this group difference was mostly eliminated by the MNI transformation, making the two group averaged brains closely match each other. The individual MNI transformation parameters were then applied to each subject's functional activation map and the average group activation maps contrast was recalculated thereafter. The effect of this MNI compensation on the functional contrast maps are shown in the Fig. 4a2,b2. As indicated by the green arrows, the group subtraction canceled more activation in the MNI space as compared to the original TLRC result. However, the cancellation was still far from complete even after the anatomy mismatch was corrected/compensated. This indicates that the more superior activation volume seen in the PAE subjects can not be explained by the volume reduction in these subjects alone.
Fig. 4.
The control vs. PAE anatomy and activation difference based respectively on the MNI (a) and TLRC (b) normalization. The a1 and b1 are the white matter segments of the brain. By overlay the PAE images (red and yellow) on the controls (gray scale), it can be seen that the temporal-occipital displacement shown in the TLRC space (b1) is corrected/compensated by the MNI transformation (a1). The a2 and b2 are the functional activity subtraction maps as shown in Fig. 2c but the display here just focuses on the ventral part of the temporal-occipital area. For convenient comparison purpose, the original TLRC subtraction result was transformed into the MNI space and re-sliced so that the slice location in the a2 and b2 are exactly the same. Although more mutual cancellations were shown in the MNI based subtraction (e.g., compare the locations marked by the green arrows in the a2 and b2), the group functional activation differences could not completely be removed. The activations of the PAE group still appear higher then those of the controls even after the MNI correction. The slice locations in the a2 and b2 are shown at the center of the figure. In the white matter comparison (a1 and b1), the color coding (0.3-1.0; red and yellow for the PAE subjects; gray scale for the controls) represents the probability a voxel can be identified as the white matter. In the subtraction maps (a2 and b2), red/blue spots represent activations only in the control/PAE group
Discussion
The present study focused on the occipital-temporal area in terms of both the structural and functional impact of prenatal alcohol exposure. Besides the observed differences in the cerebral anatomy, functional activations in the PAE participants were found to be more superior and this activation difference between PAE and control subjects can not be attributed solely to the anatomical difference.
The ventral occipital-temporal extrastriate region contains neurons selectively responsible for perception of object features (such as color, shape, or texture) and thus plays a critical role in object recognition in the ventral visual pathway (Fujita et al. 1992; Wilkinson and Wilson 2001). Moreover, as shown in much of the literature (Kanwisher and Wojciulik 2000), the visual activity in this brain area can be strongly modulated by behavioral relevance or attention. As the present experiment requires the subjects to continuously focus the attention on shape detection, the contributions of the structural changes in occipital-temporal area to the behavioral deficit could possibly be twofold. On one hand, the WM/GM hypoplasia could reflect a selective neuron insufficiency that leads to impaired feature (shape) detection performance. On the other hand, the WM reduction could also reflect an incomplete myelination process (Guerri and RenauPiqueras 1997). Given the importance of the myelination in normal neuronal function, the top-down attentional modulation may also suffer. The upward shifting of the functional activation in the PAE group could be driven by both of these factors. The first factor contributes to this perhaps through functional compensation or neural plasticity. As the anatomical impairment is located in the ventral portion of the occipital-temporal region, functional compensation would likely come from the more superior regions. The second factor may contribute through attentional regulation. In the sustained attention task, subjects were required to pay attention to the shape feature of each geometric figure and ignore the color (could randomly be red or green). However, the color of the object could act as a distracter in this task. Since there is imaging evidence showing that the extrastriate color discrimination area is located more superior than that of the shape area (Corbetta et al. 1991), the superiorly shifted functional activation in the exposed group may also indicate that the PAE subjects were more distracted by the color and did not concentrate on the shapes as well as the controls. This interpretation has support from previous animal (Luck et al. 1997) and human (Kastner et al. 1998) studies, which both showed that different cortical representations within the object recognition pathway interact in a competitive, suppressive fashion, so that directing attention to one of them will counteract the suppression from others. The more superior PAE activation could be due to a relatively weaker competitive suppression from the “shape area”, or a relatively weaker top-down attentional regulation on the “color area,” or both.
To test this color distraction conjecture, we performed a post hoc behavioral test on some participants (6 controls, 6 PAE subjects with dysmorphia, and 4 PAE subjects without dysmorphia) that were subsequently added into our longitudinal study cohort. We simply changed the stimuli in the sustained attention period to gray scale, and compared (within subject paired t-test) this performance with that recorded in the fMRI session with chromatic stimuli. Although none of the performance indices showed any significant difference between gray-scale and colored stimulus in any of the subject groups (control, PAE without dysmorphia and PAE with dysmorphia), the IDA slightly increased in the gray-scale condition (control, IDAcolor=0.96, IDAgray=0.98, t-test p=0.37; dysmorphia PAE, IDAcolor=0.93, IDAgray=0.94, t-test p=0.14; nondysmorphia PAE, IDAcolor=0.89, IDAgray=0.90, t-test p= 0.22). If we combine the two groups of PAE subjects together without considering the presence/absence of dysmorphia, the IDA difference in the alcohol-exposed group reached the significance level (PEA, IDAcolor=0.91, IDAgray=0.93, t-test p=0.05). While practice-related effects on these behavioral results may be present because the gray-scale task was always performed after the colored task (during fMRI scan), the IDA difference did appear along the direction predicted by the color distraction interpretation. Further studies with a counter-balanced experimental design will be needed to test this hypothesis more strictly.
Our focus on the occipital-temporal region does not necessarily mean that it was the only PAE-affected brain area we found in the current study. As shown in the Fig. 3d, e, our VBM result also shows decreased WM in the corpus callosum and increased GM at least in the ventral frontal lobe and bilateral parietal areas. These observations are all consistent with the previous findings reported by morphological studies of PAE (Archibald et al. 2001; Ma et al. 2005; Sowell et al. 2001a; Sowell et al. 2001b; Sowell et al. 2002). As generally described by Sowell and colleagues (2001b), the PAE subjects tend to have too much gray matter and too little white matter. The PAE related occipital-temporal abnormality was actually revealed previously by the “surface-based image analysis” (Sowell et al. 2002), which showed a decreased “DFC” (distance from the center) and increased GM density in this area. However, the teratogenic impact of PAE in this region did not receive much attention in the previous studies. One reason for this could be that the currently published morphological studies are still rare and mainly from the same research group (Sowell and colleagues). Though different data analysis approaches (volumetric, VBM, surface-based et al.) have been reported in different studies, the PAE subjects examined were actually from the same population (Sowell et al. 2002). While the result consistency and reliability are high when examining the same group of subjects in a series of studies, structural changes less salient in those specific subjects (but not necessarily less salient in other subjects) could be easily ignored. Given the high variance of PAE impact on different subjects (possibly due to the varied amount of alcohol exposure and the varied development period that was affected), imaging results from different research groups on different alcohol exposed cohorts are also very important. Another reason that the occipital-temporal abnormality received insufficient attention would be the lack of studies that simultaneously examined both the functional and anatomical changes. The current finding of the brain structural change was in fact guided by the observed fMRI activations. The significant extrastriate activation mismatch suggested a possible anatomical difference, which led to our subsequent VBM and volumetric comparison. Without the activation difference being observed, investigators may not pay close attention to this specific area. The fMRI activation in this case can be regarded as the thread that connects the behavioral deficits, neural activity change and anatomical alterations.
In line with previous studies (Coles et al. 2002; Mattson et al. 2006; Mirsky 1996), the brain function under current investigation is termed “sustained attention”. However, as seen in the above discussion, the study probed the functional deficit in more than attention. Because the fundamental visual processing elements (such as visual perception and feature detection) were not controlled in the “fixation” condition, the behavioral deficit and activation changes seen in the PAE group could also be due to the abnormalities in these relatively lower level visual processes. Though our previous data (Coles et al. 2002) showed that visual acuity could not account for the behavioral deficit in the PAE group, recent evidence from VEP (visual evoked potential) studies suggested that the fundamental visual functions (contrast sensitivity, grating acuity, color perception) could be impaired by prenatal exposure to organic solvents including alcohol (Till et al. 2005). To dissociate the attention and fundamental visual processing contributions in the PAE affected brain would be necessary in further investigations.
The limitations of the present study also include the small sample size and the difference in IQ between groups. Given the preliminary and exploratory nature of the investigation, we deliberately selected participants who showed physical and cognitive characteristics of their prenatal exposure to maximize the possibility of finding group differences. While the functional and structural change we observed may not be generalized due to this subject selection bias, the present data do suggest more directions for future exploration about the PAE-related alterations on the visual attention network. As more subjects are adding to our longitudinal study cohort, further evaluations of the current findings with a larger population and a better control group will be our next goal.
The first fMRI study that examined functional abnormalities in PAE subjects has just been published recently (Malisza et al. 2005). The lack of published research probably reflects the difficulties of applying functional imaging approaches in the investigation of this disorder, which on the other hand has been clinically diagnosed for more than 30 years. An important aspect of the difficulties comes from the fact that function and anatomy could both be affected by the alcohol exposure and these two effects commonly are intermixed in the imaging results. A brain activation difference between the PAE and control group would not necessarily reflect a functional alteration, but rather, an anatomical mismatch (Bookheimer and Sowell 2005). Researchers need to examine the changes from both sides and try to ascertain to what extent the observed activation differences come from each factor. We have taken a step forward in this direction by estimating these contributions in the occipital-temporal region. However, the information we obtained is still not comprehensive. As the data analysis approaches that can correlate brain activation and morphological changes in the whole brain are still lacking, the ROI based analysis limited us in examining the PAE effects to a larger extent in the visual attention network. In addition to the occipital-temporal region (which by and large is related to the target of the attentional regulation), the frontal and parietal regions (which are more related to the source of the attentional regulation) also exhibited activation differences between the PAE and control subjects. For example, the PAE group showed more activation in the bilateral frontal eye field (FEF) suggesting a more unstable attentional focus in the alcohol exposed subjects. In the current report, we stayed away from discussing these regions because possible anatomical changes in these regions are not clear. Future studies will be needed to examine the functional and structural changes in these parts of the brain to support a more general view of the PAE-related visual attention circuit alterations.
Acknowledgement
This work is supported by NIH grants RO1AA014373, RO1EB002009 and Georgia Research Alliance.
References
- Archibald SL, Fennema-Notestine C, Gamst A, Riley EP, Mattson SN, Jernigan TL. Brain dysmorphology in individuals with severe prenatal alcohol exposure. Developmental Medicine & Child Neurology. 2001;43:148–154. [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Human Brain Mapping. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry—The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Bhatara VS, Lovrein F, Kirkeby J, Swayze V, Unruh E, Johnson V. Brain function in fetal alcohol syndrome assessed by single photon emission computed tomography. South Dakota Journal of Medicine. 2002;55:59–62. [PubMed] [Google Scholar]
- Bookheimer SY, Sowell ER. Brain imaging in FAS commentary on the article by Malisza et al. Pediatric Research. 2005;58:1148–1149. doi: 10.1203/01.pdr.0000188720.59781.b3. [DOI] [PubMed] [Google Scholar]
- Bookstein FL, Streissguth AP, Sampson PD, Connor PD, Barr HM. Corpus callosum shape and neuropsychological deficits in adult males with heavy fetal alcohol exposure. Neuroimage. 2002;15:233–251. doi: 10.1006/nimg.2001.0977. [DOI] [PubMed] [Google Scholar]
- Brown RT, Coles CD, Smith IE, Platzman KA, Silverstein J, Erickson S, et al. Effects of prenatal alcohol exposure at school age. II. Attention and behavior. Neurotoxicology and Teratology. 1991;13:369–376. doi: 10.1016/0892-0362(91)90085-b. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Coles CD. Fetal alcohol exposure and attention: Moving beyond ADHD. Alcohol Research & Health. 2001;25:199–203. [PMC free article] [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Lynch ME, Freides D. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcoholism: Clinical and Experimental Research. 2002;26:263–271. [PubMed] [Google Scholar]
- Coles CD, Platzman KA, RaskindHood CL, Brown RT, Falek A, Smith IE. A comparison of children affected by prenatal alcohol exposure and attention deficit hyperactivity disorder. Alcoholism: Clinical and Experimental Research. 1997;21:150–161. [PubMed] [Google Scholar]
- Coles CD, Smith I, Fernhoff PM, Falek A. Neonatal neurobehavioral characteristics as correlates of maternal alcohol use during gestation. Alcoholism: Clinical and Experimental Research. 1985;9:454–460. doi: 10.1111/j.1530-0277.1985.tb05582.x. [DOI] [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Direct and indirect effects of prenatal alcohol damage on executive function. Developmental neuropsychology. 2000;18:331–354. doi: 10.1207/S1532694204Connor. [DOI] [PubMed] [Google Scholar]
- Connor PD, Streissguth AP, Sampson PD, Bookstein FL, Barr HM. Individual differences in auditory and visual attention among fetal alcohol-affected adults. Alcoholism: Clinical and Experimental Research. 1999;23:1395–1402. [PubMed] [Google Scholar]
- Corbetta M, Miezin FM, Dobmeyer S, Shulman GL, Petersen SE. Selective and divided attention during visual discriminations of shape, color, and speed: Functional anatomy by positron emission tomography. Journal of Neuroscience. 1991;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita I, Tanaka K, Ito M, Cheng K. Columns for visual features of objects in monkey inferotemporal cortex. Nature. 1992;360:343–346. doi: 10.1038/360343a0. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. A voxel-based morphometric study of aging in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Guerri C, RenauPiqueras J. Alcohol, astroglia, and brain development. Molecular Neurobiology. 1997;15:65–81. doi: 10.1007/BF02740616. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Wojciulik E. Visual attention: Insights from brain imaging. Nature Reviews. Neuroscience. 2000;1:91–100. doi: 10.1038/35039043. [DOI] [PubMed] [Google Scholar]
- Kastner S, Weerd PD, Desimone R, Ungerleider LG. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- Kopera-Frye K, Olson HC, Streissguth AP. Teratogenic effects of alcohol on attention. In: BurackEnns JAJT, editor. Attention, development and psychopathology. Guilford; New York: 1997. pp. 171–204. [Google Scholar]
- Le TH, Pardo JV, Hu X. 4T-fMRI study of nonspatial shifting of selective attention: Cerebellar and parietal contributions. Journal of Neurophysiology. 1998;79:1535–1548. doi: 10.1152/jn.1998.79.3.1535. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. Journal of Neurophysiology. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Ma X, Coles CD, Lynch ME, LaConte SM, Zurkiya O, Wang D, et al. Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcoholism: Clinical and Experimental Research. 2005;29:1214–1222. doi: 10.1097/01.alc.0000171934.22755.6d. [DOI] [PubMed] [Google Scholar]
- Malisza KL, Allman A-A, Shiloff D, Jakobson L, Longstaffe S, Chudley AE. Evaluation of spatial working memory function in children and adults with fetal alcohol spectrum disorders: A functional magnetic resonance imaging study. Pediatric Research. 2005;58:1150–1157. doi: 10.1203/01.pdr.0000185479.92484.a1. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Calarco KE, Lang AR. Focused and shifting attention in children with heavy prenatal alcohol exposure. Neuropsychology. 2006;20:361–369. doi: 10.1037/0894-4105.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Riley EP, Delis DC, Stern C, Jones KL. Verbal learning and memory in children with fetal alcohol syndrome. Alcoholism: Clinical and Experimental Research. 1996;20:810–816. doi: 10.1111/j.1530-0277.1996.tb05256.x. [DOI] [PubMed] [Google Scholar]
- Metz CE. Basic principles of ROC analysis. Seminars in Nuclear Medicine. 1978;8:283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- Mirsky AF. Disorders of attention: a neuropsychological perspective. In: LyonKrasnegor GRNA, editor. Attention, memory and executive function. Paul H Brookes; Baltimore: 1996. pp. 71–95. [Google Scholar]
- Nanson JL, Hiscock M. Attention deficits in children exposed to alcohol prenatally. Alcoholism: Clinical and Experimental Research. 1990;14:656–661. doi: 10.1111/j.1530-0277.1990.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Riikonen R, Salonen I, Partanen K, verho S. Brain perfusion SPECT and MRI in foetal alcohol syndrome. Developmental Medicine & Child Neurology. 1999;41:652–659. doi: 10.1017/s0012162299001358. [DOI] [PubMed] [Google Scholar]
- Riley EP, Mattson SN, Sowell ER, Jernigan TL, Sobel DF, Jones KL. Abnormalities of the corpus callosum in children prenatally exposed to alcohol. Alcoholism: Clinical and Experimental Research. 1995;19:1198–1202. doi: 10.1111/j.1530-0277.1995.tb01600.x. [DOI] [PubMed] [Google Scholar]
- Schonfeld AM, Mattson SN, Lang AR, Delis DC, Riley EP. Verbal and nonverbal fluency in children with heavy prenatal alcohol exposure. Journal of Studies on Alcohol. 2001;62:239–246. doi: 10.15288/jsa.2001.62.239. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Mattson SN, Thompson PM, Jernigan TL, Riley EP, Toga AW. Mapping callosal morphology and cognitive correlates: Effect of heavy prenatal alcohol exposure. Neurology. 2001a;57:235–244. doi: 10.1212/wnl.57.2.235. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, et al. Voxel-based morphometric analysis of the brain in children and adolescents prenatally exposed to alcohol. Neuroreport. 2001b;12:515–523. doi: 10.1097/00001756-200103050-00018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, et al. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cerebral Cortex. 2002;12:856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme Medical Publishers, Inc.; New York: 1988. [Google Scholar]
- Till C, Westall CA, Koren G, Nulman I, Rovet JF. Vision abnormalities in young children exposed prenatally to organic solvents. Neurotoxicology. 2005;26(4):599–613. doi: 10.1016/j.neuro.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Wilkinson F, Wilson HR. Global processes in from vision and their relationship to spatial attention. In: JenkinHarris ML, editor. Vision and attention. Springer; New York: 2001. pp. 63–81. [Google Scholar]