Abstract
Layer 4 of the mouse somatosensory (barrel) cortex has a diversity of interneuron cell types. Tonic inhibition in other regions is cell type-specific and mediated, in part, by δ-subunit containing, extrasynaptic, GABAA receptors. We have investigated tonic inhibition in LTS cells, a major type of inhibitory neuron, and excitatory cells in layer 4 of the mouse barrel cortex using 4,5,6,7-tetrahydroisothiazolo-[5,4-c]pyridine-3-ol (THIP), a superagonist of these receptors. Bath application of 20 µM THIP produced baseline shifts, which indicates activation of tonic inhibition of both excitatory and LTS cells. The baseline shift was significantly larger in LTS cells. This finding of greater induced current in LTS cells was paralleled by a significantly greater increase in conductance with THIP application in LTS cells. The increase in conductance resulted in LTS cells requiring more current to reach threshold. Because of the differential effects of tonic inhibition on LTS cells and excitatory cells, bath application of THIP increased the network excitability, measured by multi-unit recordings. Thus, the network effect of tonic inhibition in horizontal layer 4 circuits is a paradoxical increase in excitation.
Keywords: barrel, somatosensory, THIP, tonic inhibition, interneurons
INTRODUCTION
The mouse barrel cortex is notable for the visible barrel structures in layer 4. Each barrel corresponds to a principal whisker on the contralateral face. Large bundles of fibers from the bar-reloids in the ventral posterior medial nucleus of the thalamus project to individual barrels in layer 4 (Woolsey and Van der Loos, 1970). These thalamocortical fibers synapse onto excitatory neurons and onto a type of inhibitory interneuron neuron (White and Keller, 1987) called fast-spiking (FS) cells that mediate feed-forward inhibition (Porter et al., 2001; Swadlow, 2003). Thus, inhibition is recruited at a very early stage in sensory processing. Low-threshold spiking (LTS) cells are another major type of inhibitory interneuron in layer 4 of the barrel cortex. LTS cells are distinct from FS cells because they are not primary recipients of thalamocortical input and therefore do not mediate feed-forward inhibition (Beierlein et al., 2003). LTS cells are additionally distinguished from other interneuron cell types by their morphology, biochemistry and action potential-firing patterns. LTS cells form electrical synapses with each other, which allows them to act as a synchronous inhibitory network (Beierlein et al., 2000). In addition, LTS cells have a unique form of self-inhibition through self-generated endocannabinoids (Bacci et al., 2004).
Another potential mechanism of self-regulation is through tonic inhibition. Tonic inhibition is a long-lasting inhibition that is mediated through extrasynaptic receptors (rather than fast, phasic, synaptic inhibition). These receptors respond to ambient levels of GABA and, therefore, require high sensitivity (Brickley et al., 1996; Nusser et al., 1998). Additionally, because this is a long-lasting, tonic form of inhibition, these receptors have a low rate of desensitization. Although the exact stoichiometry is unknown, tonic inhibition in some regions is mediated, in part, by δ-containing GABAA receptors (Semyanov et al., 2004). At the level of individual cells, tonic inhibition shunts a cell by increasing conductance, alters the membrane time constant, and modulates the input-output function (gain) of a cell. The effects of tonic inhibition at the network level depend on cell type. Cell-type differences have been noted in the hippocampus, with inhibitory cells having relatively large amounts of tonic inhibition. Appropriately, selectively blocking tonic inhibition in these regions increases the frequency of spontaneous inhibitory post-synaptic currents onto pyramidal cells (Semyanov et al., 2004). The expression of tonic inhibition in the complex inhibitory circuits of the cortex is largely unknown. Here we investigated the cell-type specificity of δ-mediated tonic inhibition in layer 4 of the mouse barrel cortex using 4,5,6,7-tetrahydroisothiazolo-[5,4-c]pyridine-3-ol (THIP), which is a superagonist at these receptors (Sundstrom-Poromaa et al., 2002). We looked specifically at tonic inhibition in excitatory cells and LTS cells.
OBJECTIVE
The first goal of the present study is to investigate the presence of GABAA-receptor mediated tonic conductance in neurons in layer 4 of the barrel cortex. The second goal is to confirm that this powerful form of inhibition has a greater impact on inhibitory neurons than on excitatory neurons.
METHODS
All experiments were carried out in accordance with approved procedures (Protocol #02-064) established by the Georgetown University Animal Care and Use Committee.
Preparation of tangential slices
Tangential slices of layer 4 mouse primary somatosensory (barrel) cortex were obtained from adult Black 6 mice (C57BL/6; Jackson Labs) of either sex ranging in age from postnatal day 21 (P21) to P49. Mice were anaesthetized deeply with CO2 and decapitated. The brains were removed, blocked and placed for 2 – 3 min in an ice-cold, sucrose-slicing solution (mM): 234 sucrose, 11 glucose, 24 NaHCO3, 2.5 KCl, 1.25 NaH2PO4H2O, 10 MgSO4 and 0.5 CaCl2, gassed with 95% O2:5% CO2. The brain was positioned on the caudal surface on a prepared angle indicator constructed to accurately block the brain at simultaneous angles. Using a razor blade the brain was sliced with a simultaneous 30° cut in the horizontal plane and a 10° cut in the anterior – posterior plane (Fleidervish et al., 1998). The brain was then glued (cut-side down) on a vibratome (Leica) stage and immersed in cold sucrose-slicing solution. Once the surface of the brain was established, a slice (220 µm) was made and discarded. The subsequent 275-µm slice (layer 4) was incubated for at least 1 hour before recording in preheated (32°C), oxygen-equilibrated artificial cerebral spinal fluid (ACSF) (mM): 126 NaCl, 26 NaHCO3, 10 glucose, 2.5 KCl, 1.25 NaH2 PO4·H2 O, 2 MgCl2·6H2 O and 2 CaCl2·2H2 O (pH 7.4). The ACSF used during multi-unit, extracellular recording experiments (Fig. 3) contained the following (mM): 126 NaCl, 26 NaHCO3, 10 glucose, 3.5 KCl, 1.25 NaH2 PO4·H2 O, 0.5 MgCl2·6H2 O and 1 CaCl2·2H2 O. Slices were visualized with a fixed-stage upright microscope (E600 FN; Nikon) equipped with a 4× objective and a 60× insulated objective, infrared (IR) illumination, Nomarski optics and an IR-sensitive video camera (COHU).
Fig. 3. Paradoxical increase in excitability due to activation of tonic inhibition in LTS cells.
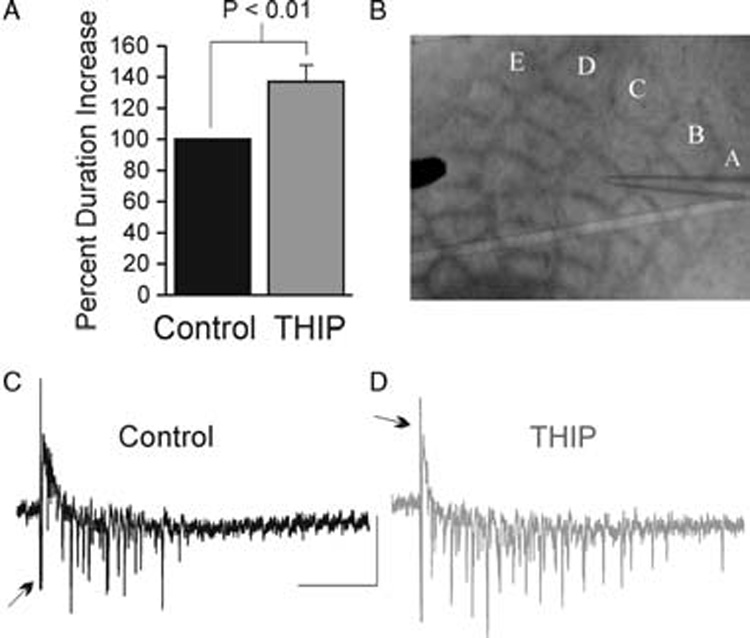
A. Multiunit responses were recorded with a low resistance glass pipette in tangential slices in mouse barrel cortex. A,B,C,D and E represent the rows of barrels of the PMBSF. B. Histogram of mean values for the duration increase from control sweeps (black) and sweeps during bath application of 20 µM THIP (grey). C,D. Raw traces of multiunit extracellular responses in control (C, black) and during application of THIP (D, grey). The duration was measured from the start of first event to the last event. Stimulus artifacts are truncated in each trace (arrows). Scale bars: 0.2 mV vertical, 100 msec horizontal
Electrophysiology
Whole-cell patch-clamp recordings from layer 4 barrel cortex were performed at room temperature (21 – 23°C) in a chamber continuously perfused with ACSF (2 ml min−1). Brief suction pulses (120 psi at 20 – 50ms) generated from a solenoid-controlled vacuum transducer were applied to break into the cell and establish the whole-cell configuration. To vary the equilibrium potential for chloride (ECl), two intracellular pipette solutions were used.
Baseline shift experiments (Fig. 1) were performed in voltage-clamp mode (held at −60 mV) using a high chloride intracellular solution similar to that used in previous studies of tonic GABAA receptor-mediated inhibition (mM): 70 K-gluconate, 70 KCl, 2 NaCl, 10 HEPES, 4 EGTA (pH 7.3) corrected with KOH; 290 mOsM; ECl ~ −16 mV. The second intracellular solution was used to test excitability where a physiological chloride equilibrium potential was desired (Fig. 2) (mM): 130 K-gluconate, 10 KCl, 10 HEPES, 10 EGTA and 2 MgCl2, ECl ~ −60 mV. Glass pipettes were pulled (non-filament borosilicate glass; Garner Glass Company) in five stages with a Flaming/Brown Micropipette Puller (Model, P-97; Sutter Instruments) to obtain electrodes with resistance of 2.5 – 3.5 MΩ when filled with intracellular solution. Drugs were applied by bath application. In experiments to examine baseline shift (Fig. 1), glutamate-receptor blockers 6,7-Dinitroquinoxaline-2,3-dione (DNQX; 20 µM final; Tocris) and (+/−)-2-Amino-5-phosphonopentanoic acid (APV; 100 µM final; Tocris) were used to isolate GABAA receptor-mediated inhibition from excitatory input. The δ-containing GABA receptor superagonist (Brown et al., 2002; Farrant and Nusser, 2005) THIP (Gaboxadol 20 µM; Tocris) was used to activate tonic GABAA currents.
Fig. 1. THIP-induced tonic currents.
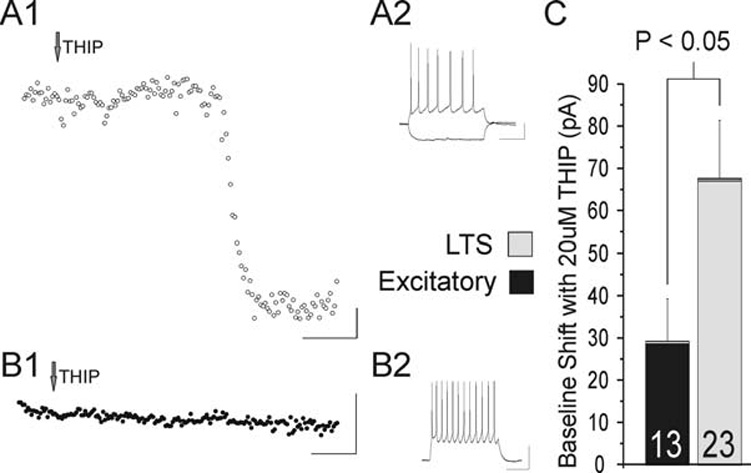
A1. THIP produces a large baseline shift (136 pA) in holding current in this LTS cell. This is seen as a downward shift because of the high Cl− intracellular solution. A2. Current clamp recording for this LTS cell. B1. Activation of tonic inhibition with 20 µM THIP produces little change in holding current in an excitatory cell. B2. Current-clamp recording for this excitatory cell. C. Summary chart of the mean baseline shifts in pA in response to 20 µM THIP by cell type. Note that although both excitatory cells (n = 13) and LTS cells (n = 23) show a significant response (P < 0.05 and P < 0.0001, respectively), the shift in baseline is significantly larger in LTS cells than in excitatory cells (P < 0.05). Data are mean ± sem. In A1 and B1, current values averaged over 10 msec are presented (dots) for every 2 sec. Scale bars: A1 and B1, 50 sec horizontal, 20 pA vertical; A2 and B2: 200 msec horizontal, 20mV vertical.
Fig. 2. THIP induced increases in conductance.
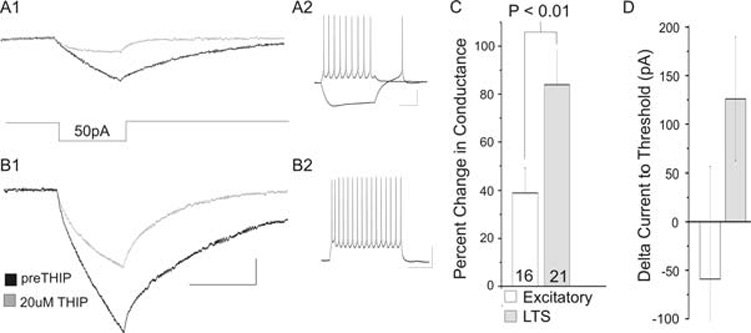
A1. The increase in conductance in an LTS cell with 20 µM THIP, seen as a decrease response to a 50 pA hyperpolarizing step. Below the traces is a schematic of the 50 pA hyperpolarizing step. A2. Current-clamp recording for this LTS cell. B1. An excitatory cell shows an increase in conductance in 20 µM THIP (grey trace). B2. Current-clamp trace of this excitatory cell. C. The mean percentage change with THIP application in excitatory (n = 16) and LTS cells (n = 21). D. The increase in conductance results in a trend towards an increase in total current to threshold in LTS cells (grey, 126 pA increase, P = 0.06, two-tailed). Because of the large variability, this increase is not significantly different from the change in excitatory cells (P = 0.17). In C and D, data are mean ± sem. Scale bars: A1 and B1, 20 msec horizontal, 2 mV vertical; A2 and B2, 200 msec horizontal, 20 mV vertical.
Cells were physiologically characterized in current clamp mode. For this, the membrane potential of the cell was adjusted to −60 mV, then hyperpolarizing and depolarizing current pulses were delivered for a duration of 600 msec in 12 consecutive sweeps to establish an ‘IV test’ for each cell (Bacci et al., 2003). We differentiated the inhibitory LTS cells from the excitatory regular spiking (RS) cells using the physiological criteria established by Beierlein et al. (Beierlein et al., 2003). In that study, based on synaptically paired RS and LTS cells, it was established that the position and shape of the after-hyperpolarization potential (AHP) were crucial in differentiating these cell types. Therefore, cells with a more depolarized first AHP compared to the last AHP in a depolarizing train of action potentials were classified as excitatory. Cells with either rounded or biphasic AHP and a less depolarized first AHP compared to last AHP were classified as LTS. An example of an LTS cell by this AHP-difference definition is seen in Fig. 1A. Additionally, all cells showing a rebound spike in response to release from a hyperpolarizing current step (a low-threshold spike) were classified as LTS cells. An example of a cell identified as LTS by this criterion can be seen in Fig. 2A.
Extracellular multiunit responses
To stimulate neurons in the slice, a concentric unipolar stimulating electrode (FHC) was placed in the barrel field. Current pulses of increasing intensity were applied every 10–20 sec using a CPI pulse generator (Carl Pisaturo, Stanford University). Field potentials were recorded with a low resistance (<1 MΩ) patch pipette filled with ACSF and positioned just above the slice (Fig. 3A). The field responses were recorded in current clamp mode (gain at 100 pA mV−1) with AC filtering not exceeding 100 Hz.
Data collection and analysis
Voltage-clamp and current-clamp recordings were obtained using a Multiclamp patch-clamp amplifier (Model 700 A; Axon Instruments) digitized with a DigiData (Model 1322A; Axon Instruments) and a Dell PC computer using SCSI interface. Data was analyzed offline using Clampfit (v. 9.2; Axon Instruments) and Microsoft Excel. All tests for statistical difference were done using two-tailed Student’s t-tests (paired and non-paired, as appropriate). Baselines (Fig. 1) were based on a 1 min time window, with values determined by taking the peak of a Gaussian fit of a histogram of current levels during this time window. Changes in conductance with THIP application were monitored by measuring the voltage change in response to a 20 ms, −50 pA step (Fig. 2). Values for each cell are based on the average of 10 sweeps.
RESULTS
The GABA analog THIP induces a tonic current in cortical neurons
Recently, others have shown that low concentrations of the GABA superagonist THIP selectively enhances tonic inhibition through extrasynaptic GABAA receptors (Drasbek and Jensen, 2006; Ortinski et al., 2006). There is also evidence that the GABAA receptor-mediated tonic current is cell-type selective (Semyanov et al., 2003). We address the question of cell-type specificity of tonic inhibition in the cortex by examining two major types of neurons in layer 4 of mouse barrel cortex. By examining both LTS cells and excitatory cells, we are better able to investigate the more global effect of tonic inhibition on the network. In this study, whole-cell patch clamp recordings were made from neurons located in the posteromedial barrel subfield (PMBSF) of the mouse barrel cortex. This slice isolates layer 4 and maintains the horizontal circuitry of the barrel cortex. For these experiments, recordings are performed at room temperature using high chloride, intracellular electrodes (see Methods). First, cell identity is determined in current-clamp mode by injecting depolarizing and hyperpolarizing current steps. We then examine the induction of a δ-subunit mediated tonic conductance in voltage clamp mode via bath application of THIP (20 µM). In these experiments, the amplifier is switched to voltage clamp and current is injected to hold or clamp the membrane potential at −60 mV. Because of the high intracellular chloride concentration, activation of tonic inhibition by THIP is seen as a downward shift in the baseline holding current (Fig. 1A, B). Following a complete wash-in of THIP, LTS cells show a large increase in tonic inhibition with an average baseline shift of 67 ± 14 pA (n = 13, P < 0.0001) (Fig. 1A). In excitatory cells the shift is less than half of this value (29 ± 14 pA, n = 23, P < 0.05) (Fig. 1B). This difference in baseline shifts between LTS and excitatory cells is statistically significant (P < 0.05) (Fig. 1C).
THIP-induced increases in conductance
To measure tonic conductance in LTS and excitatory cells we measured THIP-induced increases in conductance using physiological intracellular chloride concentrations (Fig. 2). In each case the membrane potential is first adjusted to −60 mV in current-clamp mode. We then apply a small (20 msec, 50 pA) hyperpolarizing current pulse (Fig. 2A, B). The conductance is calculated as the reciprocal of the resistance. In the presence of THIP, we observe an 84 ± 14% increase in conductance (7.8 ± 1.6 nS, n = 9, P = 0.0001) in LTS cells (Fig. 2A), seen as a decreased change in membrane potential in response to the 50 pA current step. Similar to voltage-clamp experiments, excitatory cells exhibit a much smaller response to THIP, showing a 39 ± 10% increase in conductance (3.9 ± 1.3 nS, n = 16, P < 0.01) (Fig. 2B). As expected, comparing LTS to excitatory cells, LTS cells show a statistically significant larger percentage increase in conductance with THIP application than excitatory cells (P < 0.01) (Fig. 2C).
In LTS cells, this increase in conductance produces a trend toward an increase in the total amount of current to threshold. In these experiments, total current to threshold is the current injected to hold the cell ~ −60 mV, plus the current (applied as a 1 msec step) required to elicit an action potential. With THIP application, LTS cells show a 130 ± 60 pA increase in total current to threshold (from 1530 pA to 1660 pA, P = 0.06) (Fig. 2D). In excitatory cells there is no statistically significant difference in total current to threshold from predrug conditions. Indeed, the trend for excitatory cells is in the opposite direction. Excitatory cells show an average decrease in total current to threshold of 60 ± 115 pA, P > 0.6, from 1880 pA to 1820 pA. Because of the large variability in this measure, the difference between excitatory cells and LTS cells is not statistically significant (P = 0.17).
The effects of THIP on network activity
We observe a greater THIP-activated baseline shift and increase in conductance in LTS cells than in excitatory neurons. In order to determine the net impact of an inhibitory neuron selective tonic conductance in cortical networks, we measure multiunit responses following bath application of THIP. Normally, cortical slices are ‘quiescent’; therefore, for these experiments, the slices are bathed in low Mg2+ ACSF containing 0.5mM Mg2+ to promote cell firing (Schwartzkroin and Prince, 1978; Mody et al., 1987). Extracellular potentials are evoked with a brief (200 µs, 1 mA) stimulus and recorded in current-clamp mode with a low-resistance glass pipette (<1 MΩ) filled with ACSF. The stimulating electrode is placed in the PMBSF, and multiunit responses are typically recorded one to two barrels away (Fig. 3A). Here we show that THIP (at concentrations that activate extrasynaptic δ-subunit containing GABAA receptors) increases the duration of these events by 36 ± 10.7%, P = 0.01, n = 8 (Fig. 3B). Bath application of 100 µM GABA (n = 2) and 1 mM GABA (n = 4) eliminates this activity (data not shown). Thus, these data indicate that, because THIP is selective for tonic inhibition and tonic inhibition is selective to inhibitory neurons, THIP excites the network.
CONCLUSIONS
In whole-cell voltage-clamp recordings, the δ-subunit containing GABAA superagonist THIP (20 µM) produces a significantly larger shift in baseline of inhibitory LTS cells than excitatory cells in layer 4 of the mouse somatosensory barrel cortex.
In current clamp, THIP increases conductance in LTS and excitatory cells, with a significantly larger percentage increase in conductance in LTS cells than excitatory cells.
The THIP-induced increase in conductance in LTS cells causes a trend towards an increase in the current required to reach threshold for firing an action potential.
THIP application produces a paradoxical increase in network excitability in layer 4 of the mouse barrel cortex.
DISCUSSION
Tonic inhibition and homeostasis
Our data provide evidence for a selective enhancement of tonic inhibition in layer 4 inhibitory circuits. THIP is a GABA analog but has an increased affinity for δ-containing subunits of the GABAA receptor (Sundstrom-Poromaa et al., 2002). At low concentrations (<30 µM) THIP activates these extrasynaptic receptors (tonic inhibition) without affecting the synaptic GABAA receptors that are associated with phasic inhibition (Drasbek and Jensen, 2006). In the present study, we show that LTS cells are modulated by tonic inhibition; we show an 8 nS change in conductance caused by activation of tonic currents in LTS cells by THIP application. Furthermore, in the tangential slice, which favors horizontal, intralaminar connections, we observe that activation of tonic GABAA receptors in inhibitory neurons favors excitation.
Tonically active GABAA receptors modulate conductance and the membrane time constant, providing a way for ambient GABA to modulate neuronal excitability and gain (Mitchell and Silver, 2003). This modulation provides a powerful mechanism for maintaining the responsiveness (output) of a neuron to diverse levels of incoming synaptic input (Mitchell and Silver, 2003; Semyanov et al., 2004). Tonic inhibition, thus, provides a mechanism of ‘intrinsic homeostatic plasticity’ (Mody, 2005), and cells that possess tonically active GABA receptors have an additional control of intrinsic properties through ambient levels of GABA in the extrasynaptic space. Therefore, tonic inhibition provides a means for dynamic homeostatic plasticity, ‘stabilizing a neurone’s output in the face of a change in its input’ (Mody, 2005). This allows, for example, the dynamic or immediate control of cell excitability in periods of high activity. Although we use the example of one type of inhibitory neuron in a complex circuit, it is important to consider the diverse actions of tonic inhibition might have on the network because of the type of cell that it controls.
Tonic inhibition: extrinsic or intrinsic modulator?
LTS cells are regulated by other mechanisms that operate on a slower time scale. These include both extrinsic and intrinsic modulators (Bacci et al., 2005). Extrinsic modulation is, to some degree, provided by ascending systems (e.g. acetylcholine and serotonin), which are often not associated with a specialized postsynaptic structure (similar to the extrasynaptic nature of the GABAA-mediated tonic inhibition reported here). In LTS cells in particular, a depolarizing (excitatory) current is induced by acetylcholine through nicotinic receptors (Xiang et al., 1998). Slow intrinsic modulation in LTS cells acts through the cannabinoid receptor system and is activated by high levels of LTS cell activity (Bacci et al., 2004). It has been proposed that endocannabinoids do not need to leave the membrane of the LTS cell in which it was produced to inhibit the cell, making this form of inhibition a truly intrinsic means of modulation (Bacci et al., 2005).
Here we report the potential for substantial modulation of the excitability of LTS cells by another mechanism: tonic, extrasynaptic, GABAA-mediated inhibition. The question then becomes, is this a form of intrinsic inhibition or a form of extrinsic inhibition. At first glance, tonic inhibition appears to be a form of intrinsic modulation because LTS cells are GABAergic and, therefore, contribute to the ambient concentration of GABA. However, tonic inhibition might be more of an extrinsic inhibition, acting as the inhibitory correlate of the extrinsic excitatory drive that is provided by acetylcholine.
From Steriade to the future
Steriade noted that ‘synchronous activity among inhibitory interneurons in the neocortex may also be implicated in oscillatory activities, particularly in the generation of fast oscillations (Steriade, 2001). LTS cells are coupled electrically through gap junctions (Gibson et al., 1999; Beierlein et al., 2000). This coupling produces a connected network of LTS cells that produces both irregular and synchronized inhibition (Beierlein et al., 2000). Beierlein et al. (2000) note the striking parallels between this and the irregular, synchronized, synaptic activity seen in wakeful states.
FS cells are another class of inhibitory neuron found in the cortex. FS cells differ from LTS cells in three ways that are relevant to this discussion. First, FS cells are fast-spiking, which, we speculate might mean that they contribute more to the ambient concentration of GABA than LTS cells. Second, FS cells receive direct thalamocortical input (Gibson et al., 1999; Beierlein et al., 2000; Porter et al., 2001; Swadlow, 2003), which puts them in a more direct position to be regulated by thalamocortical bursting than LTS cells. Finally, FS cells are inhibited, whereas LTS cells are excited, by acetylcholine (Xiang et al., 1998). Acetylcholine input from the basal forebrain increases during alert wakefulness and REM sleep (Steriade, 1997; Beierlein et al., 2000). Together, these observations lead us to hypothesize that FS cells are more active during slow-wave activity states, whereas LTS cells are inhibited during these times through tonic inhibition, possibly from the GABA released from FS cells. LTS cells, in turn, would be more active during times of alertness, through excitation from the acetylcholinergic system. In this way, tonic inhibition on LTS cells would be an extrinsic, rather than intrinsic, regulator. This role of tonic inhibition in the cortex favoring slow wave activity associated with sleep fits with tonic inhibition in the thalamus promoting bursting and slow-wave activity (Belelli et al., 2005; Cope et al., 2005). With regard to this hypothesis, it is of interest whether FS cells have the same degree of tonic GABAA-mediated inhibition as LTS cells. Ongoing studies in our laboratory are examining the cell-type specificity of tonic inhibition in cells other than excitatory and LTS cells.
REFERENCES
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–316. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Modulation of neocortical interneurons: extrinsic influences and exercises in self-control. Trends in Neurosciences. 2005;28:602–610. doi: 10.1016/j.tins.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Bacci A, Rudolph U, Huguenard JR, Prince DA. Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. Journal of Neuroscience. 2003;23:9664–9674. doi: 10.1523/JNEUROSCI.23-29-09664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nature Neuroscience. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. Journal of Neurophysiology. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. Journal of Neuroscience. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. Journal of Physiology. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. British Journal of Pharmacology. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. Journal of Neuroscience. 2005;25(50):11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cerebral Cortex. 2006;16:1134–1141. doi: 10.1093/cercor/bhj055. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nature Reviews Neuroscience. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fleidervish IA, Binshtok AM, Gutnick MJ. Functionally distinct NMDA receptors mediate horizontal connectivity within layer 4 of mouse barrel cortex. Neuron. 1998;21:1055–1065. doi: 10.1016/s0896-6273(00)80623-6. [DOI] [PubMed] [Google Scholar]
- Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. Shunting inhibition modulates neuronal gain during synaptic excitation. Neuron. 2003;38:433–445. doi: 10.1016/s0896-6273(03)00200-9. [DOI] [PubMed] [Google Scholar]
- Mody I. Aspects of the homeostaic plasticity of GABAA receptor-mediated inhibition. Journal of Physiology. 2005;562:37–46. doi: 10.1113/jphysiol.2004.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, Lambert JD, Heinemann U. Low extracellular magnesium induces epileptiform activity and spreading depression in rat hippocampal slices. Journal of Neurophysiology. 1987;57:869–888. doi: 10.1152/jn.1987.57.3.869. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. Journal of Neuroscience. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski PI, Turner JR, Barberis A, Motamedi G, Yasuda RP, Wolfe BB, et al. Deletion of the GABA(A) receptor alpha1 subunit increases tonic GABA(A) receptor current: a role for GABA uptake transporters. Journal of Neuroscience. 2006;26:9323–9331. doi: 10.1523/JNEUROSCI.2610-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JT, Johnson CK, Agmon A. Diverse types of inter-neurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. Journal of Neuroscience. 2001;21:2699–2710. doi: 10.1523/JNEUROSCI.21-08-02699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzkroin PA, Prince DA. Cellular and field potential properties of epileptogenic hippocampal slices. Brain Research. 1978;147:117–130. doi: 10.1016/0006-8993(78)90776-x. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nature Neuroscience. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA A receptors: modulating gain and maintaining the tone. Trends in Neurosciences. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Steriade M. Synchronized activities of coupled oscillators in the cerebral cortex and thalamus at different levels of vigilance. Cerebral Cortex. 1997;7:583–604. doi: 10.1093/cercor/7.6.583. [DOI] [PubMed] [Google Scholar]
- Steriade M. The Intact and Sliced Brain. MIT Press; 2001. [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, et al. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nature Neuroscience. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA. Fast-spike interneurons and feedforward inhibition in awake sensory neocortex. Cerebral Cortex. 2003;13:25–32. doi: 10.1093/cercor/13.1.25. [DOI] [PubMed] [Google Scholar]
- White EL, Keller A. Intrinsic circuitry involving the local axon collaterals of corticothalamic projection cells in mouse SmI cortex. Journal of Comparative Neurology. 1987;262:13–26. doi: 10.1002/cne.902620103. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. The description of a cortical field composed of discrete cytoarchitec-tonic units. Brain Research. 1970;17:205–242. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]


