Abstract
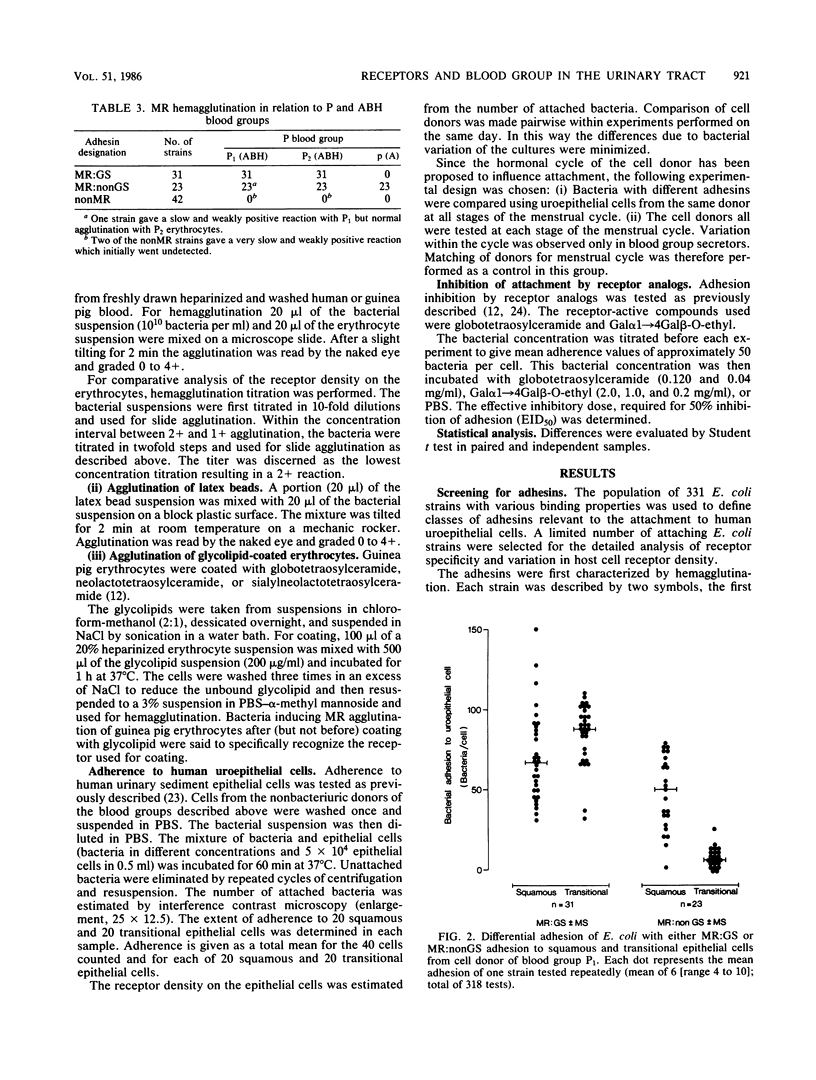
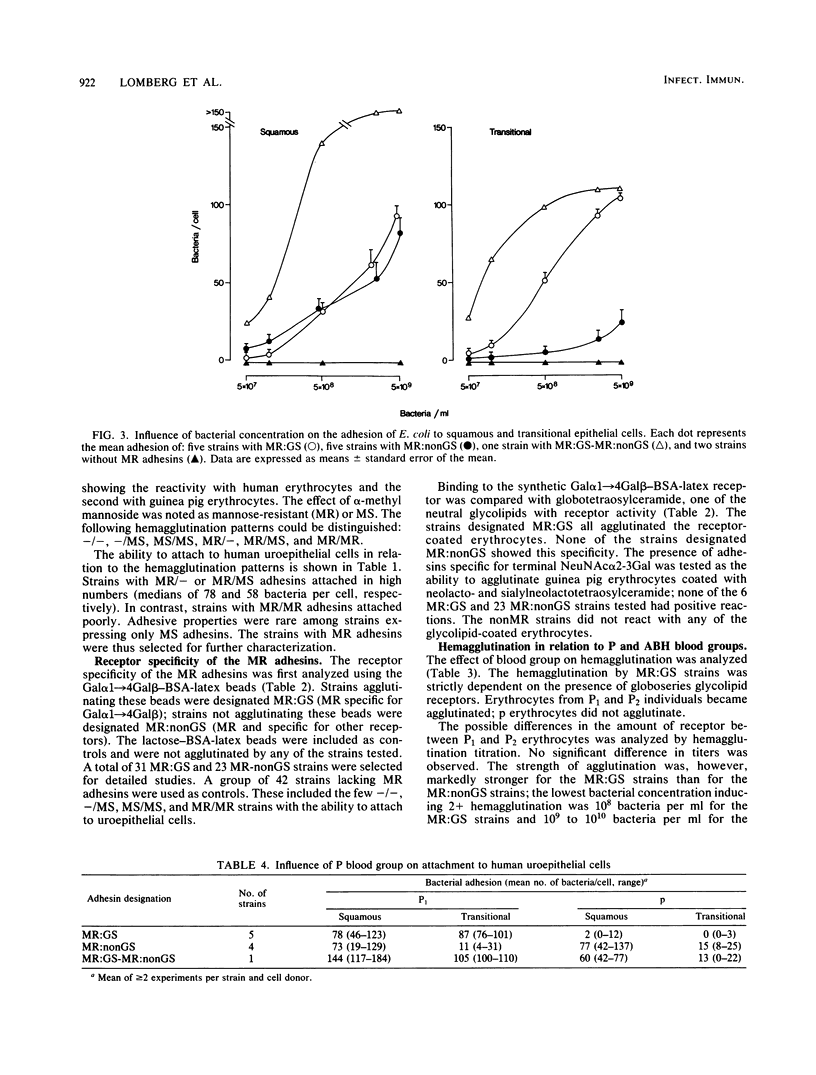
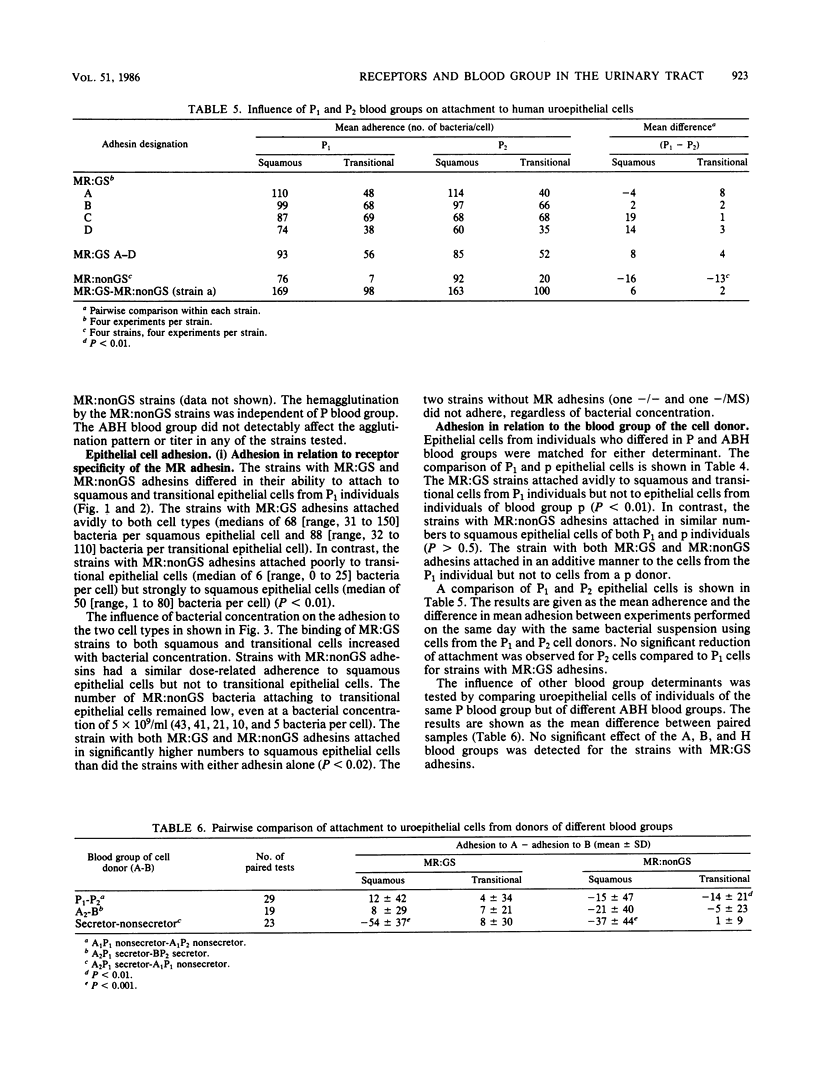
Escherichia coli strains with defined receptor specificity were used as probes to analyze the individual variation in host cell receptors with respect to blood groups. The adhesins were initially characterized as mannose sensitive (MS), mannose resistant (MR), or nonagglutinating (-). The receptor specificity of the strains with MR adhesins was defined by agglutination of synthetic Gal alpha 1----4Gal beta covalently linked via a spacer arm, (CH2)2S(CH2)2CO approximately H-bovine serum albumin (BSA) to BSA-latex beads as specific for the globoseries glycolipid receptors (MR:GS). Strains with MR adhesins not reacting with Gal alpha 1----4Gal beta-BSA-latex were designated MR:nonGS. The attachment and hemagglutination of the MR:GS strains was strictly dependent on Gal alpha 1----4Gal beta-containing receptors, as shown by the absence of binding to cells from individuals of blood group P lacking these structures. Previous reports showed differences in the composition of globoseries glycolipids between erythrocytes from individuals of P1 and P2. No significant difference was found, However, in the mean adhesion to P1 and P2 epithelial cells or in the agglutination titer for P1 and P2 erythrocytes. The MR:GS receptors were equally distributed on squamous and transitional epithelial cells. In contrast, the distribution of MR:nonGS receptors was skewed. Attachment occurred mostly to squamous epithelial cells. The attachment of strains with MR:nonGS adhesins was independent of the P blood group of the cell donor. The binding ability of MR:GS and MR:nonGS adhesins appeared independent and additive. The attachment was not influenced by the ABH blood group. However, increased binding to epithelial cells from nonsecretors occurred regardless of the P blood group, suggesting a shielding of receptors by products controlled by the secretor genes. These results illustrate how individual variation in cell surface components with and without receptor activity determine the interaction of a ligand with a known receptor.
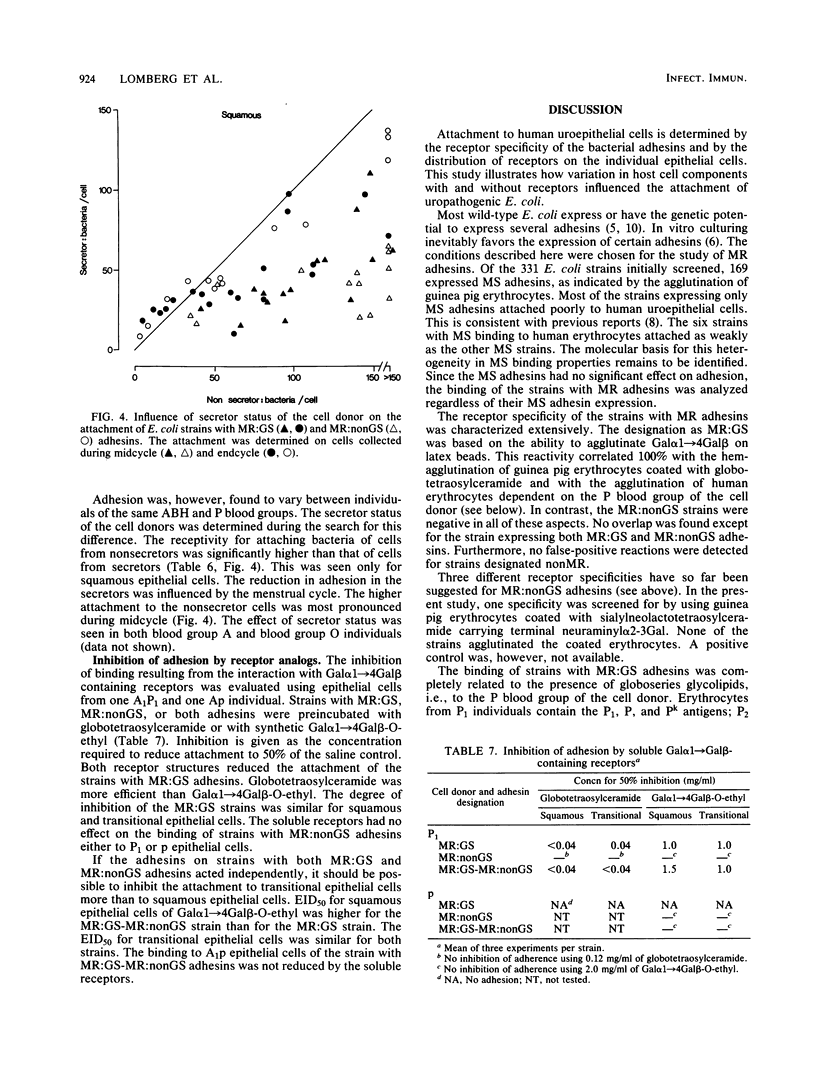
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bock K., Breimer M. E., Brignole A., Hansson G. C., Karlsson K. A., Larson G., Leffler H., Samuelsson B. E., Strömberg N., Edén C. S. Specificity of binding of a strain of uropathogenic Escherichia coli to Gal alpha 1----4Gal-containing glycosphingolipids. J Biol Chem. 1985 Jul 15;260(14):8545–8551. [PubMed] [Google Scholar]
- Breimer M. E., Hansson G. C., Karlsson K. A., Leffler H., Pimlott W., Samuelsson B. E. Selected ion monitoring of glycospingolipid mixtures. Identification of several blood group type glycolipids in the small intestine of an individual rabbit. Biomed Mass Spectrom. 1979 Jun;6(6):231–241. doi: 10.1002/bms.1200060603. [DOI] [PubMed] [Google Scholar]
- Breimer M. E., Jovall P. A. Structural characterization of a blood group A heptaglycosylceramide with globo-series structure. The major glycolipid based blood group A antigen of human kidney. FEBS Lett. 1985 Jan 1;179(1):165–172. doi: 10.1016/0014-5793(85)80213-1. [DOI] [PubMed] [Google Scholar]
- Dahmén J., Frejd T., Magnusson G., Noori G., Carlström A. S. Synthesis of spacer-arm, lipid, and ethyl glycosides of the trisaccharide portion [alpha-D-Gal-(1----4)-beta-D-Gal-(1----4)-beta-D-Glc] of the blood-group Pk antigen: preparation of neoglycoproteins. Carbohydr Res. 1984 Apr 2;127(1):15–25. doi: 10.1016/0008-6215(84)85102-2. [DOI] [PubMed] [Google Scholar]
- Duguid J. P., Clegg S., Wilson M. I. The fimbrial and non-fimbrial haemagglutinins of Escherichia coli. J Med Microbiol. 1979 May;12(2):213–227. doi: 10.1099/00222615-12-2-213. [DOI] [PubMed] [Google Scholar]
- Eden C. S., Eriksson B., Hanson L. A. Adhesion of Escherichia coli to human uroepithelial cells in vitro. Infect Immun. 1977 Dec;18(3):767–774. doi: 10.1128/iai.18.3.767-774.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Freter R., Hagberg L., Hull R., Hull S., Leffler H., Schoolnik G. Inhibition of experimental ascending urinary tract infection by an epithelial cell-surface receptor analogue. Nature. 1982 Aug 5;298(5874):560–562. doi: 10.1038/298560a0. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Hanson L. A., Jodal U., Lindberg U., Akerlund A. S. Variable adherence to normal human urinary-tract epithelial cells of Escherichia coli strains associated with various forms of urinary-tract infection. Lancet. 1976 Sep 4;1(7984):490–492. [PubMed] [Google Scholar]
- Edén C. S., Larsson P., Lomberg H. Attachment of Proteus mirabilis to human urinary sediment epithelial cells in vitro is different from that of Escherichia coli. Infect Immun. 1980 Mar;27(3):804–807. doi: 10.1128/iai.27.3.804-807.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K. E., Karlsson K. A., Samuelsson B. E. Proton nuclear magnetic resonance analysis of anomeric structure of glycosphingolipids. The globo-series (one to five sugars). Arch Biochem Biophys. 1979 Jan;192(1):164–176. doi: 10.1016/0003-9861(79)90082-1. [DOI] [PubMed] [Google Scholar]
- Hagberg L., Jodal U., Korhonen T. K., Lidin-Janson G., Lindberg U., Svanborg Edén C. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect Immun. 1981 Feb;31(2):564–570. doi: 10.1128/iai.31.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P., Orskov I., Orskov F. F7 and type 1-like fimbriae from three Escherichia coli strains isolated from urinary tract infections: protein chemical and immunological aspects. Infect Immun. 1982 May;36(2):462–468. doi: 10.1128/iai.36.2.462-468.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler H., Svanborg-Edén C. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect Immun. 1981 Dec;34(3):920–929. doi: 10.1128/iai.34.3.920-929.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomberg H., Hanson L. A., Jacobsson B., Jodal U., Leffler H., Edén C. S. Correlation of P blood group, vesicoureteral reflux, and bacterial attachment in patients with recurrent pyelonephritis. N Engl J Med. 1983 May 19;308(20):1189–1192. doi: 10.1056/NEJM198305193082003. [DOI] [PubMed] [Google Scholar]
- Lomberg H., Hellström M., Jodal U., Leffler H., Lincoln K., Svanborg Edén C. Virulence-associated traits in Escherichia coli causing first and recurrent episodes of urinary tract infection in children with or without vesicoureteral reflux. J Infect Dis. 1984 Oct;150(4):561–569. doi: 10.1093/infdis/150.4.561. [DOI] [PubMed] [Google Scholar]
- Marcus D. M., Kundu S. K., Suzuki A. The P blood group system: recent progress in immunochemistry and genetics. Semin Hematol. 1981 Jan;18(1):63–71. [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Oriol R., Cartron J. P., Cartron J., Mulet C. Biosynthesis of ABH and Lewis antigens in normal and transplanted kidneys. Transplantation. 1980 Mar;29(3):184–188. doi: 10.1097/00007890-198003000-00003. [DOI] [PubMed] [Google Scholar]
- Parkkinen J., Finne J., Achtman M., Väisänen V., Korhonen T. K. Escherichia coli strains binding neuraminyl alpha 2-3 galactosides. Biochem Biophys Res Commun. 1983 Mar 16;111(2):456–461. doi: 10.1016/0006-291x(83)90328-5. [DOI] [PubMed] [Google Scholar]
- Väisänen-Rhen V., Korhonen T. K., Finne J. Novel cell-binding activity specific for N-acetyl-D-glucosamine in an Escherichia coli strain. FEBS Lett. 1983 Aug 8;159(1-2):233–236. doi: 10.1016/0014-5793(83)80453-0. [DOI] [PubMed] [Google Scholar]
- Väisänen V., Korhonen T. K., Jokinen M., Gahmberg C. G., Ehnholm C. Blood group M specific haemagglutinin in pyelonephritogenic Escherichia coli. Lancet. 1982 May 22;1(8282):1192–1192. doi: 10.1016/s0140-6736(82)92264-4. [DOI] [PubMed] [Google Scholar]