Abstract
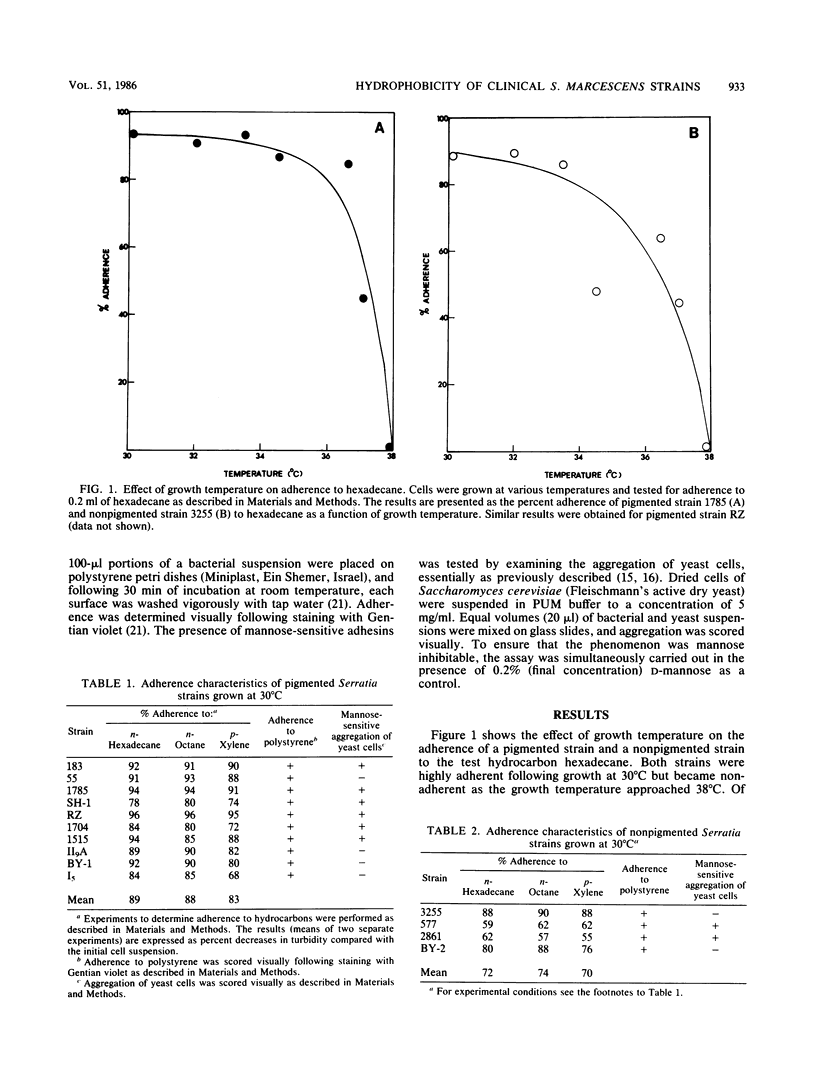
The cell surface hydrophobicity of 10 pigmented and 4 nonpigmented clinical Serratia marcescens strains was studied, based on the ability of the strains to adhere to hydrocarbons and to polystyrene. The cell surface hydrophobicity depended greatly on growth temperature; all of the strains tested were adherent following growth at 30 degrees C, whereas none was adherent following growth at 38 degrees C. In previous studies, the pigment prodigiosin has been cited as responsible for cell surface hydrophobicity in various Serratia strains. However, the observed ability of the nonpigmented strains to adhere to the test hydrocarbons and to polystyrene indicates that Serratia strains can possess hydrophobic surface properties in the absence of this pigment. Moreover, strain 1785 cells were adherent whether they were grown at 30 or 36.5 degrees C, even though pigment was not synthesized at the higher temperature. In Escherichia coli correlations have been noted between increased cell surface hydrophobicity and the presence of mannose-specific adhesins; no such relationship was found in the S. marcescens strains tested. The expression of cell surface hydrophobicity in clinical S. marcescens strains at 30 degrees C and the loss of hydrophobicity at host temperatures raise the possibility that infective cells from the environment are initially hydrophobic, but lose this property upon subsequent proliferation within a host.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adegbola R. A., Old D. C. New fimbrial hemagglutinin in Serratia species. Infect Immun. 1982 Oct;38(1):306–315. doi: 10.1128/iai.38.1.306-315.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard D. C., Syzdek L. D. Water-to-Air Transfer and Enrichment of Bacteria in Drops from Bursting Bubbles. Appl Environ Microbiol. 1982 May;43(5):1001–1005. doi: 10.1128/aem.43.5.1001-1005.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M. J., Williams R. P. Biosynthesis of prodigiosin by white strains of Serratia marcescens isolated from patients. J Clin Microbiol. 1983 Mar;17(3):476–480. doi: 10.1128/jcm.17.3.476-480.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jann K., Schmidt G., Blumenstock E., Vosbeck K. Escherichia coli adhesion to Saccharomyces cerevisiae and mammalian cells: role of piliation and surface hydrophobicity. Infect Immun. 1981 May;32(2):484–489. doi: 10.1128/iai.32.2.484-489.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman D., Altmann G., Eshdat Y. Screening of bacterial isolates for mannose-specific lectin activity by agglutination of yeasts. J Clin Microbiol. 1980 Apr;11(4):328–331. doi: 10.1128/jcm.11.4.328-331.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofek I., Whitnack E., Beachey E. H. Hydrophobic interactions of group A streptococci with hexadecane droplets. J Bacteriol. 1983 Apr;154(1):139–145. doi: 10.1128/jb.154.1.139-145.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old D. C., Adegbola R., Scott S. S. Multiple fimbrial haemagglutinins in Serratia species. Med Microbiol Immunol. 1983;172(2):107–115. doi: 10.1007/BF02124511. [DOI] [PubMed] [Google Scholar]
- PURKAYASTHA M., WILLIAMS R. P. Association of pigment with the cell envelope of Serratia marcescens (Chromobacterium prodigiosum). Nature. 1960 Jul 23;187:349–350. doi: 10.1038/187349a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg E., Gottlieb A., Rosenberg M. Inhibition of bacterial adherence to hydrocarbons and epithelial cells by emulsan. Infect Immun. 1983 Mar;39(3):1024–1028. doi: 10.1128/iai.39.3.1024-1028.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M. Bacterial adherence to polystyrene: a replica method of screening for bacterial hydrophobicity. Appl Environ Microbiol. 1981 Aug;42(2):375–377. doi: 10.1128/aem.42.2.375-377.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Bayer E. A., Delarea J., Rosenberg E. Role of Thin Fimbriae in Adherence and Growth of Acinetobacter calcoaceticus RAG-1 on Hexadecane. Appl Environ Microbiol. 1982 Oct;44(4):929–937. doi: 10.1128/aem.44.4.929-937.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M. Isolation of pigmented and nonpigmented mutants of Serratia marcescens with reduced cell surface hydrophobicity. J Bacteriol. 1984 Oct;160(1):480–482. doi: 10.1128/jb.160.1.480-482.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh J. D. Antibiotic resistance in Serratia marcescens. Br Med J (Clin Res Ed) 1983 Dec 3;287(6406):1651–1653. doi: 10.1136/bmj.287.6406.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth C. J., Jonsson P., Olsson E., Soderlind O., Rosengren J., Hjertén S., Wadström T. Differences in hydrophobic surface characteristics of porcine enteropathogenic Escherichia coli with or without K88 antigen as revealed by hydrophobic interaction chromatography. Infect Immun. 1978 Nov;22(2):462–472. doi: 10.1128/iai.22.2.462-472.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. P., Gott C. L., Qadri S. M., Scott R. H. Influence of temperature of incubation and type of growth medium on pigmentation in Serratia marcescens. J Bacteriol. 1971 May;106(2):438–443. doi: 10.1128/jb.106.2.438-443.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oss C. J. Phagocytosis as a surface phenomenon. Annu Rev Microbiol. 1978;32:19–39. doi: 10.1146/annurev.mi.32.100178.000315. [DOI] [PubMed] [Google Scholar]


