Abstract
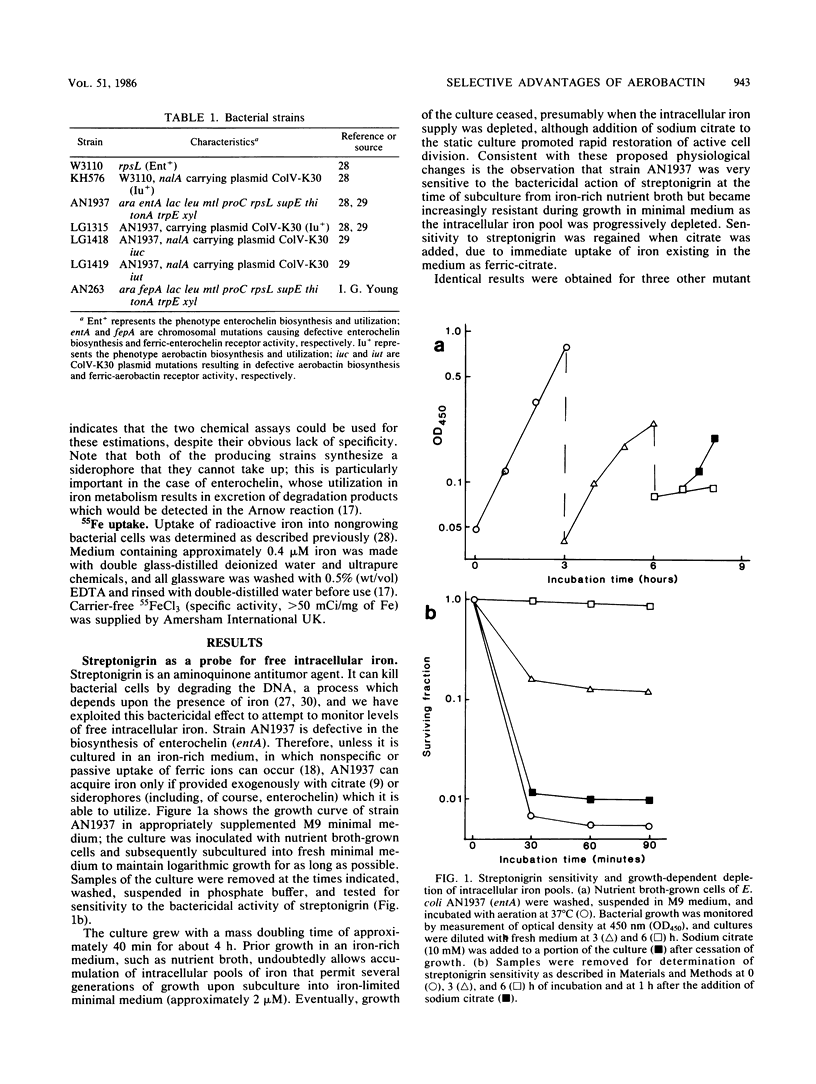
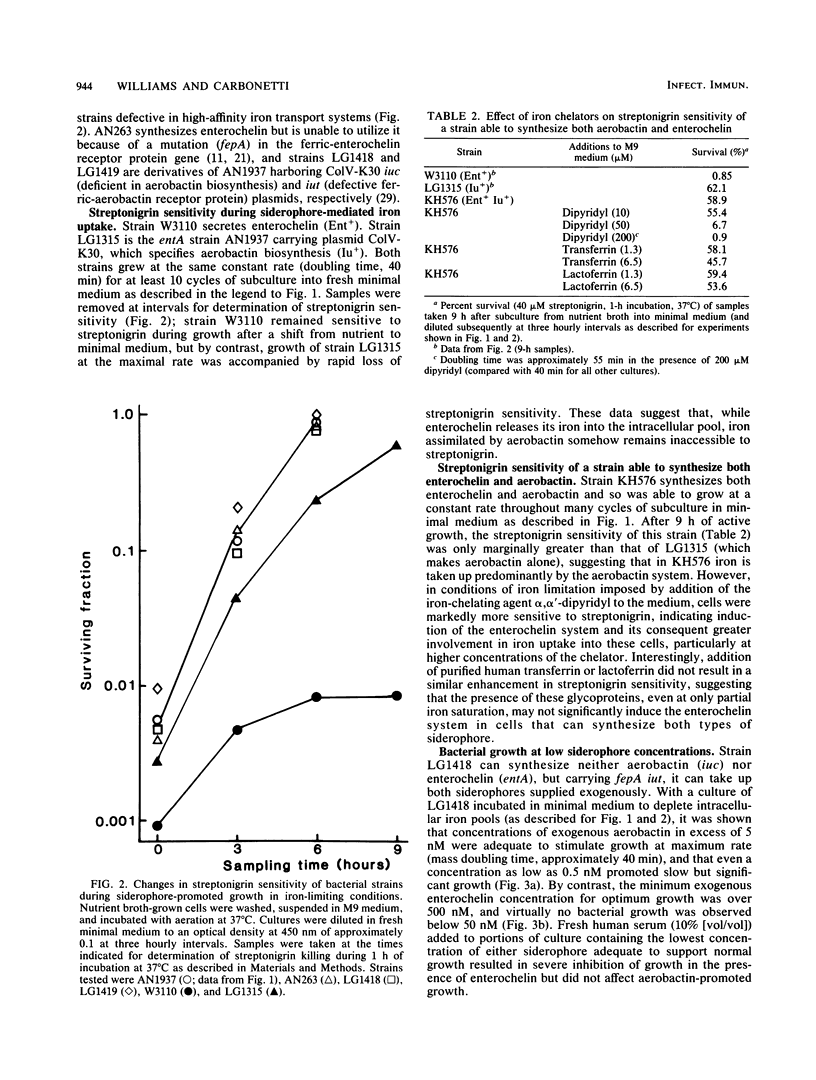
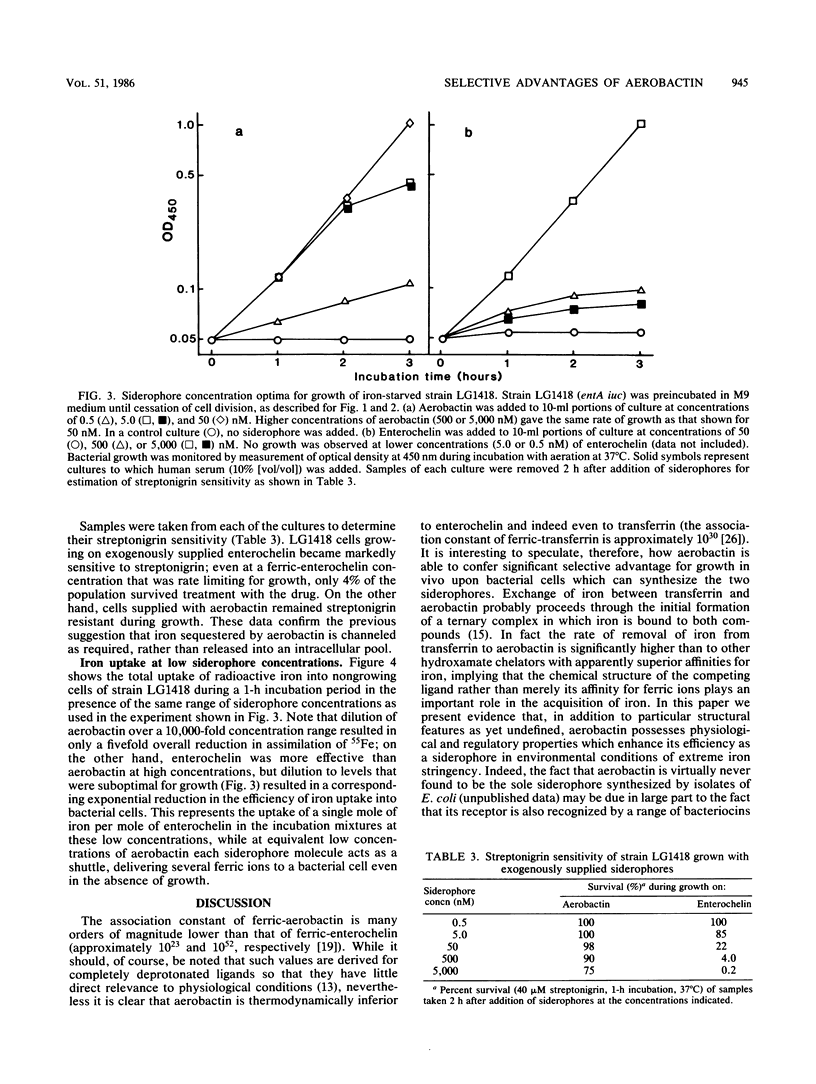
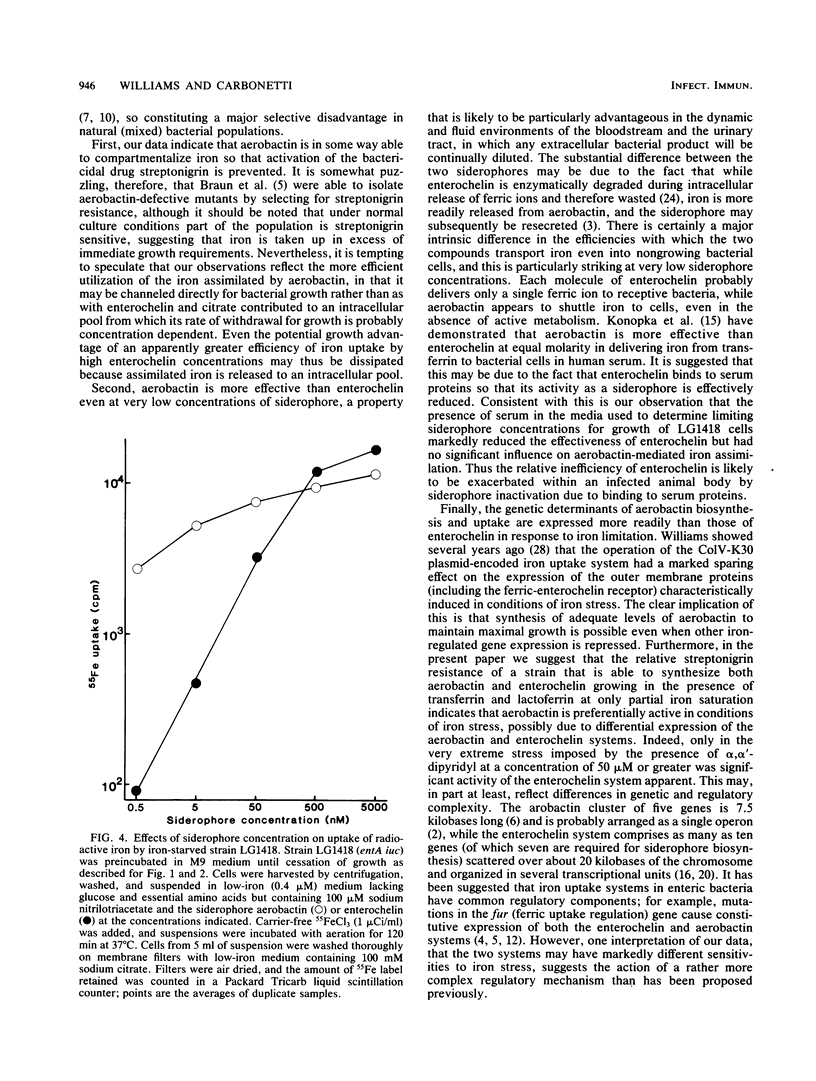
Many strains of Escherichia coli isolated from extraintestinal infections of humans and domestic animals are able to synthesize two siderophores, aerobactin and enterochelin. Although aerobactin has a dramatically lower affinity for iron than enterochelin, it has been shown to provide a significant selective advantage for bacterial growth in conditions of iron limitation, such as in the body fluids and tissues of an infected animal. We have used streptonigrin, which is bactericidal in the presence of iron, as a probe to determine levels of free intracellular iron during bacterial growth promoted by the two siderophores. A strain with only enterochelin remained sensitive to the bactericidal action of streptonigrin, suggesting that assimilated iron was contributed to an intracellular pool from which the rate of its withdrawal for growth is probably concentration dependent. On the other hand, a strain that synthesized aerobactin alone became resistant to streptonigrin, indicating that iron complexed with aerobactin was not made accessible to streptonigrin and suggesting that it may be channeled directly to where it is required for growth. Aerobactin, probably because it is repeatedly reusable, efficiently stimulated bacterial growth at external concentrations some 500-fold lower than those of enterochelin. Moreover, the effective concentration, and thus the siderophore activity, of enterochelin but not of aerobactin was significantly reduced by the presence of human serum in the medium. Differential regulation of the genetic determinants of the two siderophores resulted in preferential induction of the aerobactin system in the presence of unsaturated levels of transferrin and lactoferrin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bindereif A., Neilands J. B. Promoter mapping and transcriptional regulation of the iron assimilation system of plasmid ColV-K30 in Escherichia coli K-12. J Bacteriol. 1985 Jun;162(3):1039–1046. doi: 10.1128/jb.162.3.1039-1046.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Burkhardt R. Regulation of the ColV plasmid-determined iron (III)-aerobactin transport system in Escherichia coli. J Bacteriol. 1982 Oct;152(1):223–231. doi: 10.1128/jb.152.1.223-231.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Gross R., Köster W., Zimmermann L. Plasmid and chromosomal mutants in the iron(III)-aerobactin transport system of Escherichia coli. Use of streptonigrin for selection. Mol Gen Genet. 1983;192(1-2):131–139. doi: 10.1007/BF00327658. [DOI] [PubMed] [Google Scholar]
- Carbonetti N. H., Williams P. H. A cluster of five genes specifying the aerobactin iron uptake system of plasmid ColV-K30. Infect Immun. 1984 Oct;46(1):7–12. doi: 10.1128/iai.46.1.7-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P. C., James R. Three immunity types of klebicins which use the cloacin DF13 receptor of Klebsiella pneumoniae. J Gen Microbiol. 1985 Sep;131(9):2313–2318. doi: 10.1099/00221287-131-9-2313. [DOI] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. The inducible citrate-dependent iron transport system in Escherichia coli K12. Biochim Biophys Acta. 1973 Nov 30;330(1):90–101. doi: 10.1016/0005-2736(73)90287-3. [DOI] [PubMed] [Google Scholar]
- Grewal K. K., Warner P. J., Williams P. H. An inducible outer membrane protein involved in aerobactin-mediated iron transport by co1V strains of Escherichia coli. FEBS Lett. 1982 Apr 5;140(1):27–30. doi: 10.1016/0014-5793(82)80513-9. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Hantke K., Braun V. Iron transport of Escherichia coli K-12: involvement of the colicin B receptor and of a citrate-inducible protein. J Bacteriol. 1976 Sep;127(3):1370–1375. doi: 10.1128/jb.127.3.1370-1375.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182(2):288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- Johansson B. G. Isolation of crystalline lactoferrin from human milk. Acta Chem Scand. 1969;23(2):683–684. doi: 10.3891/acta.chem.scand.23-0683. [DOI] [PubMed] [Google Scholar]
- Konopka K., Bindereif A., Neilands J. B. Aerobactin-mediated utilization of transferrin iron. Biochemistry. 1982 Dec 7;21(25):6503–6508. doi: 10.1021/bi00268a028. [DOI] [PubMed] [Google Scholar]
- Laird A. J., Young I. G. Tn5 mutagenesis of the enterochelin gene cluster of Escherichia coli. Gene. 1980 Nov;11(3-4):359–366. doi: 10.1016/0378-1119(80)90075-x. [DOI] [PubMed] [Google Scholar]
- Langman L., Young I. G., Frost G. E., Rosenberg H., Gibson F. Enterochelin system of iron transport in Escherichia coli: mutations affecting ferric-enterochelin esterase. J Bacteriol. 1972 Dec;112(3):1142–1149. doi: 10.1128/jb.112.3.1142-1149.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Pierce J. R., Pickett C. L., Earhart C. F. Two fep genes are required for ferrienterochelin uptake in Escherichia coli K-12. J Bacteriol. 1983 Jul;155(1):330–336. doi: 10.1128/jb.155.1.330-336.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Characterization of group B colicin-resistant mutants of Escherichia coli K-12: colicin resistance and the role of enterochelin. J Bacteriol. 1976 Jul;127(1):218–228. doi: 10.1128/jb.127.1.218-228.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugsley A. P., Reeves P. Increased production of the outer membrane receptors for colicins B, D and M by Escherichia coli under iron starvation. Biochem Biophys Res Commun. 1976 Jun 7;70(3):846–853. doi: 10.1016/0006-291x(76)90669-0. [DOI] [PubMed] [Google Scholar]
- Warner P. J., Williams P. H., Bindereif A., Neilands J. B. ColV plasmid-specific aerobactin synthesis by invasive strains of Escherichia coli. Infect Immun. 1981 Aug;33(2):540–545. doi: 10.1128/iai.33.2.540-545.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. R., Yeowell H. N. Iron enhances the bactericidal action of streptonigrin. Biochem Biophys Res Commun. 1982 May 31;106(2):407–411. doi: 10.1016/0006-291x(82)91125-1. [DOI] [PubMed] [Google Scholar]
- Williams P. H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979 Dec;26(3):925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H., Warner P. J. ColV plasmid-mediated, colicin V-independent iron uptake system of invasive strains of Escherichia coli. Infect Immun. 1980 Aug;29(2):411–416. doi: 10.1128/iai.29.2.411-416.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeowell H. N., White J. R. Iron requirement in the bactericidal mechanism of streptonigrin. Antimicrob Agents Chemother. 1982 Dec;22(6):961–968. doi: 10.1128/aac.22.6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]



