Abstract
Purpose
Endothelial cell (EC) migration is a key event in angiogenesis, and is likely to play an important role in choroidal neovascularization in age-related macular degeneration (AMD). Altered elastin metabolism has been described in AMD, and the present study sought to determine the effects of elastin-derived peptides (EDPs) on choroidal EC migration and proliferation.
Methods
Migration of the chorioretinal EC line Rf/6a and a primary culture of human choroidal ECs through polycarbonate membrane inserts was quantified in the presence of elastin bioactive hexapeptides (BPs), EDPs, bovine serum albumin (BSA), or balanced salt solution. Proliferation assays and in vitro wound closure experiments were also performed in the presence of elastin fragments or balanced salt solution (control). Elastin overlay experiments were performed on sections of human eyes.
Results
For both Rf/6a and human primary choroidal ECs exposed to EDPs or BPs, the number of ECs that migrated through the polycarbonate membrane was significantly higher than ECs exposed to balanced salt solution alone or to BSA (P < 0.05) in all experiments. In contrast, the rate of EC proliferation did not significantly change in comparison to controls. Elastin binding sites were identified on choroidal ECs in human eyes.
Conclusions
Elastin fragments increase choroidal EC migration, whereas they do not appear to increase or decrease EC proliferation. Local or systemic abnormalities in elastin physiology may participate in pathologic neovascular membrane formation in AMD.
Age-related macular degeneration (AMD) is a major cause of blindness. The neovascular (wet) form of AMD is characterized by the abnormal growth of choroidal blood vessels into the sub-retinal space of the macula. This multistep process is likely to be initiated by the breakdown of Bruch's membrane which, when intact, prevents pathologic angiogenesis. In this process, choroidal ECs may migrate from the choroid into the sub-RPE and/or sub-retinal space. These ECs proliferate and form tubes (tubulogenesis), ultimately reorganizing their junctions to increase permeability across the newly formed vascular wall. The neovascular process in AMD can result in serous detachment of the retinal pigmented epithelium (RPE) and/or neurosensory retinal detachment, as well as fibrous disciform scarring beneath the retina, causing a catastrophic decrease in visual acuity.1-5 Current treatments for neovascular AMD are focused primarily on vascular endothelial growth factor (VEGF)-mediated processes.6 While VEGF is a potent inducer of angiogenesis, understanding the role of additional angiogenic stimuli would be invaluable for the development of improved treatments.7
Elastin is a glycoprotein consisting of cross-linked 72 kDa tropoelastin subunits and is an abundant component of the extracellular matrix (ECM) of arteries, lung, and skin.8 Breakdown of elastin results in the formation of elastin-derived peptides (EDPs), cross-linked fragments of tropoelastin of varying sizes.9 These subunits have been shown to initiate EC migration and tubulogenesis in chicken chorio-allantoic membranes, human microvascular ECs (cell line HMEC-1), and human umbilical vein ECs.10 Currently, it is unknown how these peptides are able to activate ECs and whether they are capable of activating ECs from other tissues, such as the choroid. It is plausible that EDPs bind to elastin binding proteins on the cell surface, inducing angiogenic behaviors such as cell migration and/or proliferation.
Several lines of evidence suggest abnormal elastin metabolism occurs in Bruch's membrane in AMD. First, early onset choroidal neovascularization has been shown in patients with pseudoxanthoma elasticum. These patients may be at risk for developing choroidal neovascular membranes because of abnormalities in the elastic layer of Bruch's membrane, including breaks, clinically defined as angioid streaks.11,12 Second, fibulin-5 missense mutations have been identified in association with AMD.13 This mutation may contribute to AMD development by affecting the elastic layer of Bruch's membrane, since fibulin-5 participates in elastogenesis14,15 and is localized to Bruch's membrane in human eyes.16 Third, correlations between the integrity of the elastic layer of Bruch's membrane and AMD have also been made. Light and electron microscopic studies of human eyes have found that Bruch's membrane becomes calcified and increasingly fragmented with increasing severity of AMD.17,18 It is likely that fragmentation of Bruch's membrane in the macula renders it more susceptible to the formation of neovascular membranes.
There is also evidence for abnormal systemic elastin metabolism in AMD. Blumenkranz et al.11 found a correlation between choroidal neovascularization (CNV) and elastotic degeneration. Patients with exudative AMD demonstrated a greater than twofold increase in their susceptibility to elastotic degeneration of relatively sun-protected areas of the skin in dermal biopsies, suggesting that AMD is associated with systemic elastin abnormalities.11 Serum levels of EDPs in patients with exudative AMD have also been found to be significantly higher than levels in non-exudative AMD patients and control patients,19 which may possibly be due to elevated levels of MMP-9.20 Thus, numerous lines of evidence suggest a relationship between altered elastin metabolism and AMD progression. It is feasible that EDPs, derived from serum and/or fragmentation of Bruch's membrane, could promote the pathogenesis observed in AMD. One interpretation of these findings is that systemic and local defects in elastin physiology lead to a compromised barrier for CNV in Bruch's membrane. A second, non-exclusive, possibility is that fragments of elastin itself, generated by systemic or local elastolysis, promote angiogenesis in choroidal ECs.
To clarify the functional impact of elastin on the severity of exudative AMD, we sought to determine whether EDPs promote an angiogenic phenotype in choroidal ECs. During our investigation, we found that EDPs are capable of promoting choroidal EC migration. These results support the idea that abnormal elastin metabolism in AMD patients promotes disease progression toward neovascular membrane formation.
Materials and Methods
EC Cultures
Two EC types were used for these studies, the monkey chorioretinal EC line Rf/6a (ATCC, Manassas, VA) and human choroidal ECs.
To create primary human choroidal EC cultures, human donor eyes were obtained from the Iowa Lions Eye Bank (Iowa City, IA) after obtaining informed consent in accordance with the tenets of the Declaration of Helsinki. Anterior segments were removed, leaving the posterior eyecup. The retina was removed and the RPE was scraped away from the choroid. The choroid was minced in F12 medium with 5% fetal bovine serum (FBS) and 1% penicillin/streptomycin and digested in medium with 0.2% protease (Collagenase II; Invitrogen, Grand Island, NY) in a shaker at 37°C for 1 hour. After digestion, the suspension was spun down at 1000g for 8 minutes. Pellets were resuspended in 1 mL Hanks' balanced salt solution (HBSS) with 0.1% bovine serum albumin (BSA). The cell suspension was then passed through a disposable 70-μm cell strainer (Falcon) and the flow-through portion was used for EC isolation. This isolation was completed using anti-CD31 magnetic beads (Dynabeads; Dynal Biotech ASA, Oslo, Norway) according to the manufacturer's instructions. Human choroidal ECs attached to magnetic beads were removed from the suspension using a magnet (Dynal MPC-S; Dynal Biotech ASA).
Choroidal EC purity was assessed by immunocytochemistry using either monoclonal anti-PECAM-1 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) or Ulex europaeus agglutinin I (UEA-1 lectin; Vector Laboratories Inc., Burlingame, CA). Cells were fixed with 4% paraformaldehyde for 10 minutes. For anti-PECAM-1 labeling, cells were blocked with 0.1% BSA for 15 minutes, followed by 1 rinse in phosphate-buffered saline (PBS). Cells were then incubated with 5.5 μg/mL of anti-PECAM-1 for 1 hour, followed by 3 × 5 minute rinses in PBS. Next, cells were incubated with 20 μg/mL donkey anti-mouse Alexa-Fluor 488 (Invitrogen) diluted in PBS for 30 minutes, followed by 3 × 5 minutes in PBS. UEA-1 cytochemistry was performed as described previously.21 Briefly, human choroidal ECs were blocked with 0.1% BSA for 15 minutes, followed by 1 rinse in PBS. Cells were then incubated with 40 μg/mL of UEA-1 for 30 minutes, followed by 3 × 5 minute rinses in PBS. Next, cells were incubated with 25 μg/mL Avidin-Texas Red (Vector Laboratories Inc.) diluted in PBS for 30 minutes, followed by 3 × 5 minutes in PBS. For UEA-1 cytochemistry, the divalent cations CaCl2 and MgCl2 were added to all solutions at 1 mM each. Negative controls included omission of the primary antibody or lectin. Observations were made using a microscope with fluorescence attachments (Olympus BX-41; Olympus, Center Valley, PA) equipped with a digital camera (SPOT-RT; Diagnostic Instruments, Inc., Sterling Heights, MI). Cell cultures used for experiments were chosen based on positive staining with CD31 and UEA-1, as shown in Figure 1.
Figure 1.
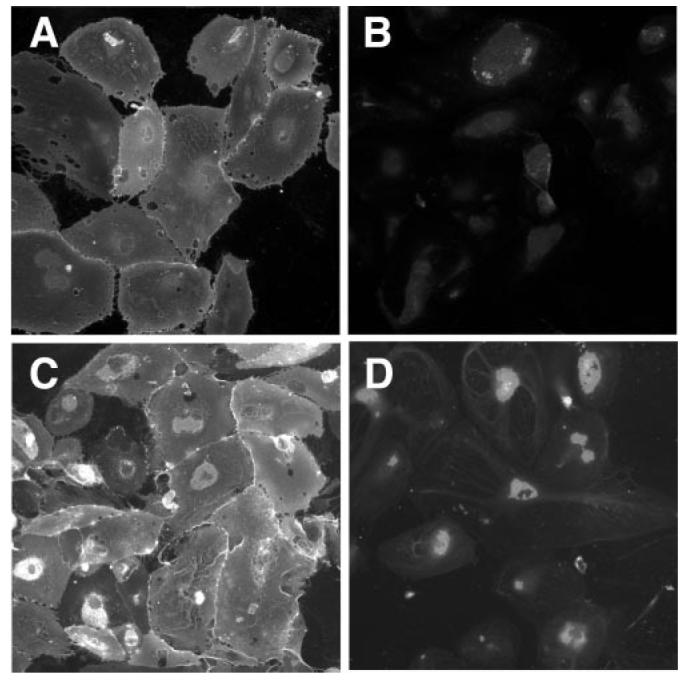
Staining of human choroidal EC primary cultures with anti-CD31 antibodies (A) or with UEA-I lectin (C). Cells that stained positively for these markers were used in migration assays. Controls are shown for anti-CD31 (B) and UEA-1 (D).
Cell purity was also assessed by an acetylated low density lipoprotein (Ac-LDL) uptake assay.22 Confluent cells were incubated in culture medium containing 10 μg/mL Alexa Fluor 488 conjugated Ac-LDL (Invitrogen) for 4 hours, followed by an incubation in medium only for 16 hours. Cells were fixed with 4% paraformaldehyde for 10 minutes and counterstained with 100 μg/mL 4′,6-diamidino-2-phenylindole (DAPI). Cells used in experiments had a punctate pattern of fluorescence around the nucleus, whereas fibroblasts used as a control cell type did not fluoresce.
Elastin-Mediated EC Wound Response Assay
Rf/6a chorioretinal ECs were grown in wells of a 96-well plate until they reached confluency. A 10-μL micropipette tip (Matrix Technologies, Hudson, NH) was used to scrape a line through the cell mono-layer. All scrapes were made by the same individual in the same orientation. The cell monolayer was rinsed 2× with un-supplemented F12 medium and then replaced with F12 medium (with 1% penicillin/streptomycin) containing 1% HBSS buffer, 100 μg/mL EDPs (human aortic; Elastin Products Company, Owensville, MO), or 20% serum. HBSS was used in medium as a negative control since HBSS is the diluent for the peptides, and 20% FBS in medium was used as a positive control as FBS contains VEGF and other factors that support angiogenesis of ECs.23-26 All conditions were tested in triplicate. Cell fields were photographed immediately after creating the gap, and then every 24 hours until the injury gap of one condition had mostly closed using a camera (PupilCAM; Ken-a-vision, Kansas City, MO). At this point, the micrographs were analyzed using ImageJ software (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) calibrated to a stage micrometer. The average width of the injury gap in each picture was measured and the rate of injury closure was determined.
Elastin-Mediated EC Migration
Migration assays were performed using a sterilized culture plate insert with 8-μm diameter pores (Millicell, Cat. # PI8P01250; Millipore, Billerica, MA).
For Rf/6a cells, 200 μL of EC suspension were added to the upper well of each culture insert placed in a 12-well culture plate (Costar, Corning, NY). F12 medium (with 1% penicillin/streptomycin) containing one of the biochemical stimuli (0.5, 1.0, and 100 μg/mL EDPs, 1.0 μg/mL bioactive hexapeptides VGVAPG [BPs], Elastin Products Company) was added to the well of the 12-well culture plate (i.e., the bottom surface of the insert). HBSS (1%) was used in medium as a negative control, and 20% FBS in medium was used again as a positive control. For some experiments, inclusion of an irrelevant protein (BSA, 1.25 μg/mL) was performed to determine the specificity of elastin's effects. The lower concentrations of the EDPs and the BPs were chosen to be similar to concentrations used previously by Robinet et al.,10 and the concentration of EDPs found in the serum of exudative AMD patients by Sivaprasad et al.19 The 100-fold EDP concentration range was chosen to determine whether the degree of cellular response was dependent on the peptide concentration. All conditions were performed in triplicate wells. Cells were incubated for 8 hours at 37°C, 95% humidity, 5% carbon dioxide. After incubation, membranes containing cells were fixed in one-half strength Karnovsky's fix (½ K) for at least 1 hour.27 The membranes were rinsed 2 × 4 minutes in 100 mM cacodylate buffer (pH 7.4) followed by a 30 minute treatment with 1% osmium tetroxide diluted in cacodylate buffer (100 mM). The membranes were then rinsed in double distilled water and then dehydrated. Final dehydration step was achieved with 2 × 10 minute incubations in hexamethyldisilizane (HMDS; Electron Microscopy Sciences, Hatfield, PA). Polycarbonate membranes were mounted on metal stubs with silver mounting medium (Ted Pella, Redding, CA), and then sputter coated with gold ions (K550; Emitech, Ltd., Kent, UK). Membranes were observed using a scanning electron microscope (SEM; S-3400N; Hitachi, Tokyo, Japan) at the University of Iowa Central Microscopy Facility. Ten digital images of the membrane's lower surface were collected for each experimental sample at 150× magnification in a masked fashion, resulting in 30 images per condition due to triplicate wells. Cells were counted for each image using ImageJ software, taking care to count only cells that have established contact onto the membrane beyond the pore opening. In other words, cells that occupied pores but had not attached a portion of their surface membrane to the polymer surface were not included in the counts. Pairwise comparisons were performed using the Student's t-test.
Migration assays were repeated using fourth passage purified human choroidal ECs instead of Rf/6a cells. Elastin fragments were used only at 100 μg/mL for this experiment.
Elastin-Mediated EC Proliferation
Equal numbers of chorioretinal or human choroidal ECs were seeded and grown in wells of a 6-well plate for 48 hours in F12 medium (with 1% penicillin/streptomycin) containing biochemical stimuli. The following concentrations of biochemical stimuli were used: 0.5, 1.0, and 100 μg/mL EDPs, and 1.0 μg/mL BPs. Positive and negative controls included FBS and HBSS, as above. All experimental conditions were performed in triplicate. Time 0 hour seeding populations were fixed with 4% paraformaldehyde and stored suspended in PBS at 4°C until the end of the experiment. After 48 hours, treated cells were trypsinized and centrifuged at 1000g for 5 minutes. Cells were resuspended in 4% paraformaldehyde for 10 minutes and then centrifuged at 1000g for 5 minutes. Cells were resuspended in 0.5 mL PBS with 10 μg/mL propidium iodide (Invitrogen) as well as 100 μL 3.0-μm diameter beads (Fluoresbrite YG Microspheres; Polysciences, Inc., Warrington, PA) diluted in PBS, yielding a final concentration of approximately 1.7 × 109 beads/mL. Cell and bead counts were completed using a flow cytometer (BD LSR; Becton Dickinson) at the University of Iowa Flow Cytometry Facility. Cell to bead ratios were used to standardize the samples and percent growth was calculated for each sample in comparison to the 0-hour cell populations.
Elastin Peptide Overlay Immunohistochemistry
Posterior poles of human donor eye tissue was fixed in 4% paraformaldehyde for 2 hours. Wedges spanning from the macula to the ora serrata were cryoprotected in sucrose and embedded as described previously.28 Sections of tissue were collected at a thickness of 7 μm.
Tissue sections were incubated with 100 ng/mL elastin (Etna Elastin; Elastin Products Company, Inc.) diluted in PBS or in PBS alone overnight at 4°C. Sections were then blocked with 1% horse serum in PBS for 15 minutes, followed by 1 rinse in PBS. Next, the tissue was incubated with 5 μg/mL of a monoclonal anti-elastin antibody (clone 10B8; Millipore) diluted in the blocking solution for 1 hour, followed by 3 × 5 minute rinses in PBS. The primary antibody was visualized using the avidin-biotin-horseradish peroxidase method with a commercial kit according to the manufacturer's instructions (Vectastain Elite kit; Vector Laboratories, Burlingame, CA), and then rinsed 3 × 5 minutes in PBS. Binding was visualized using a colorimetric substrate for horseradish peroxidase (Vector VIP kit; Vector Laboratories). Tissue was dehydrated and permanently mounted.
Statistical Analysis
Pairwise comparisons were made between one experimental group and the control in each experimental set using Student's t-test. All P values are representative of protein conditions compared to HBSS.
Results
Elastin-Mediated EC Wound Response Assay
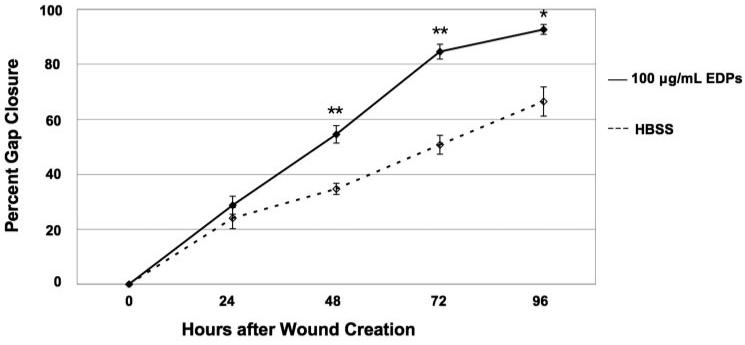
To determine whether EDPs elicit increased wound closure in ECs, a wound response assay was performed. A greater rate of Rf/6a EC wound response was observed, including migration and/or proliferation, in the presence of elastin fragments. In the presence of 100 μg/mL EDPs, 91% of the gap was closed at 96 hours in comparison to only 65% closed by cells in buffer alone (Fig. 2). Rf/6a ECs exposed to 0.5 μg/mL EDPs or 100 μg/mL BPs had responses similar to those exposed to 100 μg/mL EDPs; however, the levels were not of statistical significance (data not shown). The trends implicated a dependence of EC migration and/or proliferation on the presence of elastin fragments, especially at a concentration of 100 μg/mL.
Figure 2.
Elastin-mediated wound healing response. The chorioretinal EC line Rf/6a responded to 100 μg/mL EDPs more than to HBSS by closing the gap to a greater extent after 96 hours. Comparison was statistically significant with a P value of 0.01. Cells exposed to other concentrations and types of elastin fragments demonstrated similar trends in comparison to HBSS exposed cells; however, the levels did not reach statistical significance. *P < 0.05; **P < 0.01.
Elastin-Mediated EC Migration
To determine whether elastin fragments elicit increased migration of ECs, a migration study using cell culture plate inserts was performed. The diffusion of FBS proteins across the culture insert membrane from lower chamber to upper chamber was analyzed at 0, 2, 4, and 8 hours in the absence of cells using SDS-PAGE to assess whether a chemotactic gradient is maintained across the membrane. After 8 hours, 70% of the original protein remained in the lower chamber, indicating that a gradient was still present (data not shown).
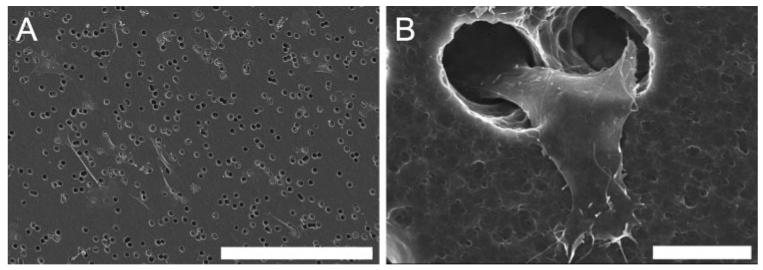
Cell migration in response to EDPs was quantified in a masked fashion. Cells included in the migrated count had established a portion of their membrane onto the polymer membrane. Examples of cells counted are pictured in Figure 3. In initial experiments, Rf/6a cells migrated to a greater extent in the presence of either 100 μg/mL EDPs (40% greater) or 1 μg/mL BPs (19% greater) than in the presence of HBSS. Cells exposed to BSA showed a migratory response similar (only 4% greater) to those exposed to HBSS (Fig. 4). Statistical analyses of the number of migrated cells were determined for each experimental stimulus in comparison to HBSS. Both EDPs and FBS had P values less than 0.001 and BPs had a P value equal to 0.01.
Figure 3.
SEM images of ECs which have migrated to the bottom surface of an 8-μm diameter pore polycarbonate membrane. (A) An example image of Rf/6a chorioretinal ECs used for quantitative analysis. (B) Cells were counted only if a portion of the cell had begun to emerge from the pore, as shown in detail in this micrograph of a human choroidal EC. Scale bar: (A) 300 μm; (B) 10 μm.
Figure 4.
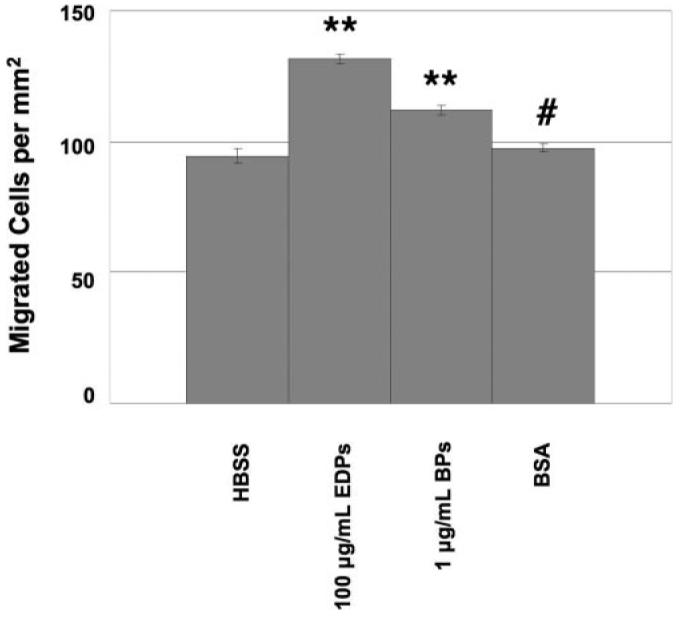
Preliminary study of chorioretinal EC migration through a polycarbonate membrane due to a chemotactic gradient. BSA was added to determine whether an irrelevant protein would promote an increase in EC migration. **P < 0.01; #P > 0.50.
After further testing, it was verified that for both EDPs and BPs, the highest concentration (100 μg/mL) did not show significantly more migration than the lowest concentration (0.5 μg/mL), P = 0.3; however, all concentrations yielded significant increases in migration in comparison to HBSS with P values all less than 0.05 (Fig. 5).
Figure 5.
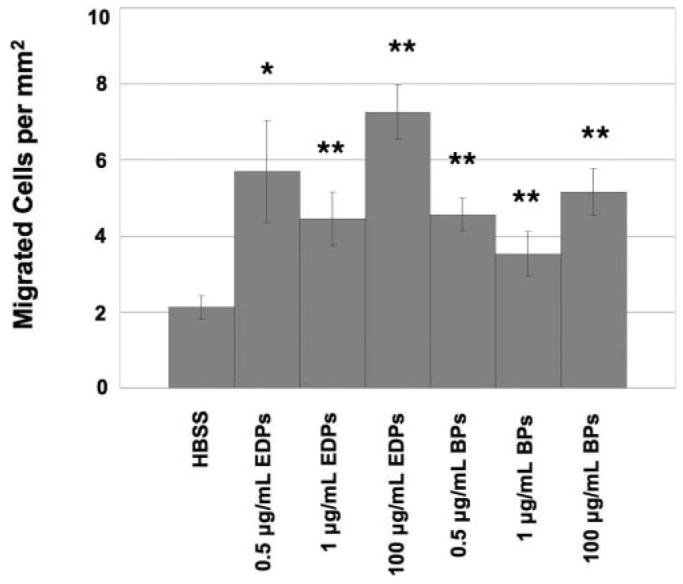
Chorioretinal EC migration through a polycarbonate membrane due to a chemotactic gradient. Different types of elastin-derived peptides as well as different concentrations of the peptides increased the amount of EC migration. *P < 0.05; **P < 0.01.
To confirm that results in the Rf/6a cell line were applicable to human ECs, migration experiments were also performed on a culture of human choroidal ECs. As noted for Rf/6a cells, human choroidal ECs increased their migration in response to EDPs (Fig. 6).
Figure 6.
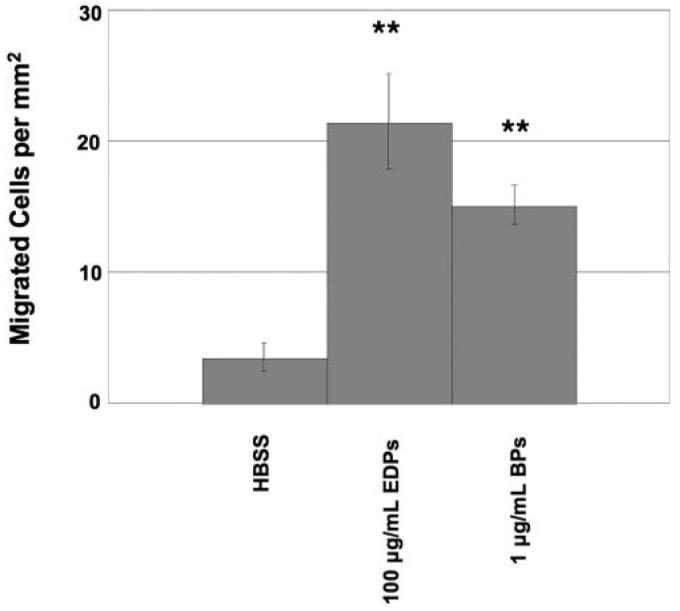
Human choroidal EC migration through a polycarbonate membrane. For all experiments, cells cultured in the presence of EDPs or BPs had a greater degree of migration than those cultured in the presence of HBSS alone. **P < 0.01.
Cells exposed to the positive control, FBS, showed the greatest migratory response in all experiments. Statistical analyses of cell migration rates were determined for each experimental stimulus in comparison to HBSS. Both BPs and FBS had P values less than 0.01 and EDPs had a P value less than 0.05.
Elastin-Mediated EC Proliferation
To determine whether EDPs and/or BPs increase EC proliferation, growth changes were quantified using flow cytometry. Cells exposed to elastin fragments did not show an altered degree of proliferation in comparison to cells exposed to HBSS after 48 hours (Figs. 7 and 8).
Figure 7.
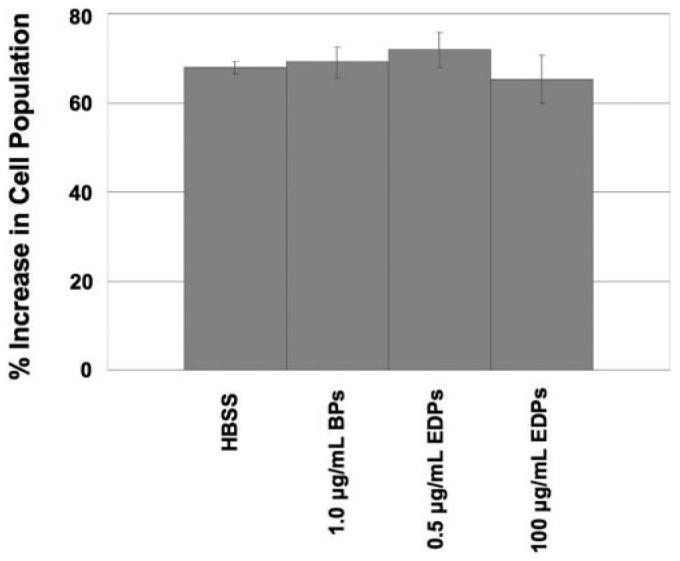
Chorioretinal EC proliferation in response to elastin fragments. None of the experimental conditions caused the cells to proliferate more than the HBSS control. All error bars are SEM.
Figure 8.
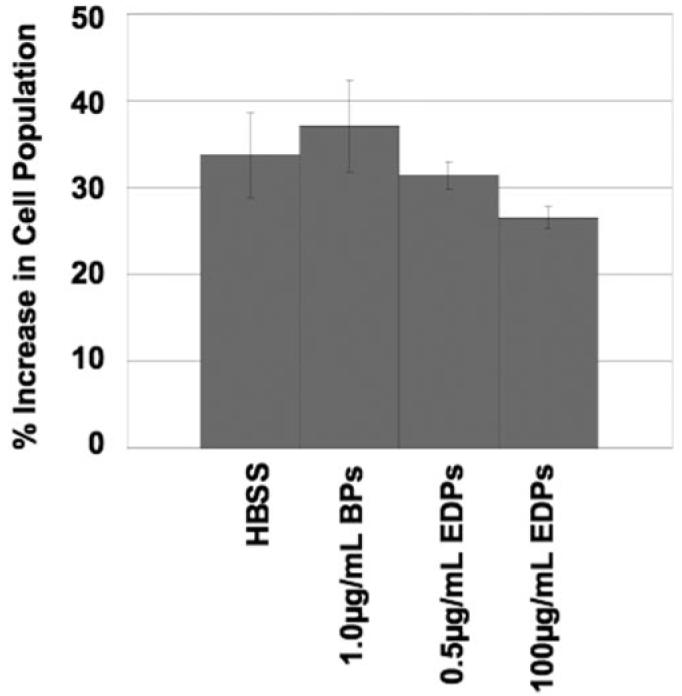
Human choroidal EC proliferation in response to elastin fragments. Similar to the Rf/6a chorioretinal EC proliferation experiment, none of the experimental conditions caused an increase in cell proliferation. All error bars are SEM.
Elastin Peptide Overlay Immunohistochemistry
To examine the distribution of elastin/EDP-binding proteins in human eyes, elastin (Etna Elastin; Elastin Products Company, Inc.) overlay experiments were performed on sections of several different human eyes. Tissue sections incubated with elastin or PBS both exhibited elastin labeling in Bruch's membrane, in the capillary extra-cellular matrices, as well as in vessel walls of choroidal arteries. In contrast, tissue pre-incubated with elastin showed increased labeling of the RPE, choriocapillaris and choroidal endothelium, indicating the presence of elastin/EDP binding sites on human choroidal ECs in vivo. Some heterogeneity between donors and between capillaries within donors was observed; however, the pattern of labeling was similar in 11 out of 13 cases. An example of this labeling is shown in Figure 9.
Figure 9.
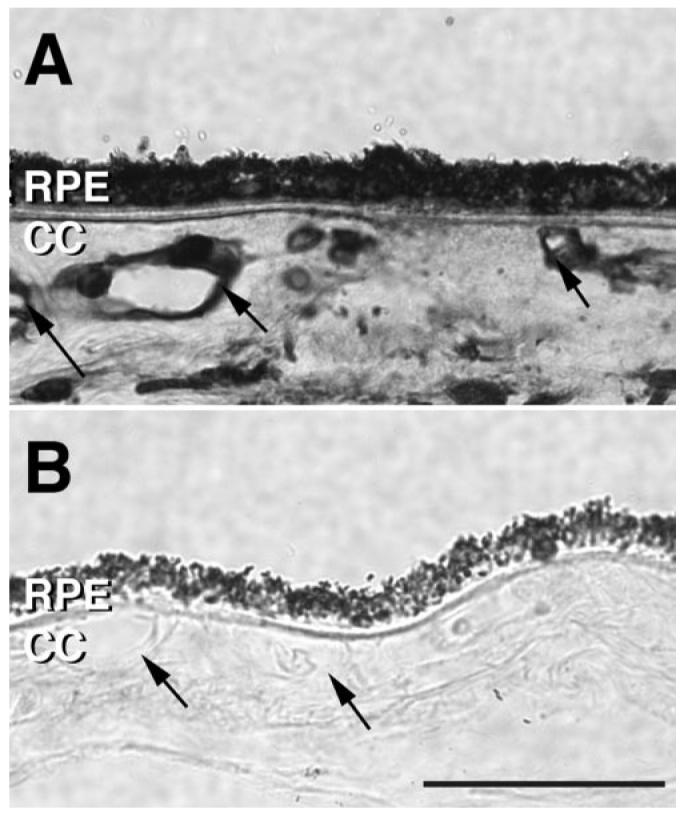
Light micrographs of elastin (Etna Elastin; Elastin Products Company, Inc.) overlay immunohistochemistry. (A) Tissue section of the human choroid incubated with elastin overnight and labeled with an elastin antibody. (B) Adjacent section incubated with PBS overnight and labeled with an elastin antibody. Labeling of choriocapillary and choroidal vessels in the tissue exposed to etna elastin (arrows) suggests the presence of elastin/elastin-derived peptide binding sites on the endothelium. Although intensity of labeling was variable, this pattern of elastin binding sites was similar in 11 out of 13 donor eyes. Scale bar, 50 μm. CC, choriocapillaris.
Discussion
Elastin is a glycoprotein that provides structural support as well as elasticity to tissues such as arteries, lung, and skin. Elastin is also the major component of the central layer of Bruch's membrane, which is located between the RPE and the choriocapillaris. Bruch's membrane likely functions as a physical barrier, allowing for the diffusion of essential nutrients and gases to the retina and removal of waste from the retina, while preventing the choroidal blood vessels from growing into and disrupting the delicate structure of the retina. Disruption of Bruch's membrane by disease or experimental intervention is associated with CNV.12,17,18
As discussed above, elastin within Bruch's membrane is abnormally metabolized in AMD. In this study we sought to determine whether, in addition to disruption of the physical barrier, elastolysis may affect the behavior of choroidal ECs, and found that EDPs increase the migration of these cells.
Choroidal neovascularization is a multistep process including the breakdown of Bruch's membrane, choroidal EC migration from the choroid into the sub-RPE and/or sub-retinal space, as well as EC proliferation and tubulogenesis. In this study we have demonstrated that EDPs may play a role in choroidal angiogenesis. First, we observed that on disrupting a monolayer of chorioretinal ECs by scraping a gap into them, cells in the presence of EDPs were able to close the gap more efficiently. Increased gap closure may result from cell proliferation, cell migration, or both. Therefore it was necessary to test these events separately. When testing migration responses, EDPs played a significant role as a chemoattractant for both human and monkey choroidal ECs, as these cells migrated to a greater extent in the presence of distinct forms of elastin. The magnitude of the migratory response was dependent on the concentration of EDPs used. The greatest response was elicited when the concentration was 100 μg/mL compared to 0.5 μg/mL, which also yielded a cell migratory response significantly greater than buffer alone. In contrast, proliferation assays indicated that choroidal ECs replicate at similar rates in the presence of EDPs as in the presence of buffer alone, suggesting that some events in angiogenesis are not driven by EDPs.
While these in vitro studies provide important information, these studies have their limitations due to the fact that the in vivo microenvironment is far more complex and dynamic. Current investigations include an in vivo mouse model, which may provide new information about EDP mediated effects on EC behavior. Our observation that binding sites for elastin fragments are present on the choroidal vascular endothelium supports the possibility that choroidal ECs may be capable of responding to EDPs in the aging eye in vivo.
With respect to the events occurring in neovascular AMD, it is plausible that the ability of choroidal ECs to demonstrate angiogenic behaviors in response to elevated EDPs plays a role in the progression of the disease pathogenesis. Morphologically, it has been demonstrated that the elastic layer of Bruch's membrane is thinner and more fragmented in patients with AMD.18 Loss of the barrier function of Bruch's membrane likely permits choroidal ECs to migrate through into the sub-retinal space. The breakdown of this membrane provides a local increase in soluble elastin fragments that are capable of binding to and activating ECs to migrate. Recent investigations of mice deficient in lysyl oxidase-like 1 (LOXL1; the enzyme that facilitates elastin polymerization) showed that abnormal elastin polymerization leads to increased soluble elastin-derived peptides, fragmentation of the elastic lamina in Bruch's membrane, as well as an increased severity of laser-induced choroidal neovascular membrane formation.29 Clinically, EDPs are at higher levels in the serum of AMD patients, especially those with neovascular AMD, in comparison to patients without AMD, indicating that there may be a systemic source of soluble elastin fragments in AMD patients as well. Therefore, choroidal ECs in a patient with AMD may potentially be activated by an increased level of EDPs, both from the serum and from elastolysis of Bruch's membrane, to become angiogenic. Coupled with the decreased integrity of Bruch's membrane in AMD patients, these cells may migrate into the sub-retinal space where they proliferate and form new vessels, beginning the cascade of events involved in neovascular membrane formation.
In summary, elastin degradation plays a role in both the weakening of the barrier to EC migration into the sub-retinal space as well as potentially activating ECs to migrate. We suggest that elastin degradation may play an important role in choroidal neovascularization during late stage AMD. Halting this breakdown of elastin or interfering with EDP signaling may provide a target for slowing the progression of AMD toward neovascularization.
Acknowledgments
The authors thank Iowa Lions Eye Bank for their dedicated work in obtaining donated human eyes, Markus Kuehn and John Fingert for helpful discussions, as well as Elizabeth Faidley and Justin Fishbaugh for technical assistance. The monoclonal antibody to PECAM-1, developed by Elizabeth Wayner and Greg Vercellotti, was obtained from the Developmental Studies Hybridoma Bank under the auspices of the NICHD and maintained by the Department of Biological Sciences, University of Iowa, Iowa City, IA.
Supported in part by NEI Grant EY017451 (RFM), Macula Vision Research Foundation (RFM), a center grant from The Foundation Fighting Blindness, and Alcon Research, Ltd. (RFM).
Footnotes
Disclosure: J.M. Skeie, Alcon Research Ltd. (F); R.F. Mullins, Alcon Research Ltd. (F)
Presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Fort Lauderdale, Florida, May 2007.
References
- 1.Green WR. Histopathology of age-related macular degeneration. Mol Vis. 1999;5:27. [PubMed] [Google Scholar]
- 2.Grossniklaus HE, Green WR. Histopathologic and ultrastructural findings of surgically excised choroidal neovascularization. Sub-macular Surgery Trials Research Group. Arch Ophthalmol. 1998;116(6):745–749. doi: 10.1001/archopht.116.6.745. [DOI] [PubMed] [Google Scholar]
- 3.Spraul CW, Lang GE, Grossniklaus HE, Lang GK. Histologic and morphometric analysis of the choroid, Bruch's membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic examination of surgically excised choroidal neovascular membranes. Surv Ophthalmol. 1999;44(suppl 1):S10–32. doi: 10.1016/s0039-6257(99)00086-7. [DOI] [PubMed] [Google Scholar]
- 4.Grossniklaus HE, Martinez JA, Brown VB, et al. Immunohistochemical and histochemical properties of surgically excised subretinal neovascular membranes in age-related macular degeneration. Am J Ophthalmol. 1992;114(4):464–472. doi: 10.1016/s0002-9394(14)71859-8. [DOI] [PubMed] [Google Scholar]
- 5.Vine AK, Stader J, Branham K, Musch DC, Swaroop A. Biomarkers of cardiovascular disease as risk factors for age-related macular degeneration. Ophthalmology. 2005;112(12):2076–2080. doi: 10.1016/j.ophtha.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Stone EM. A very effective treatment for neovascular macular degeneration. N Engl J Med. 2006;355(14):1493–1495. doi: 10.1056/NEJMe068191. [DOI] [PubMed] [Google Scholar]
- 7.van Wijngaarden P, Coster DJ, Williams KA. Inhibitors of ocular neovascularization: promises and potential problems. JAMA. 2005;293(12):1509–1513. doi: 10.1001/jama.293.12.1509. [DOI] [PubMed] [Google Scholar]
- 8.Hayden MR, Sowers JR, Tyagi SC. The central role of vascular extracellular matrix and basement membrane remodeling in metabolic syndrome and type 2 diabetes: the matrix preloaded. Cardiovasc Diabetol. 2005;4(1):9. doi: 10.1186/1475-2840-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brooke BS, Karnik SK, Li DY. Extracellular matrix in vascular morphogenesis and disease: structure versus signal. Trends Cell Biol. 2003;13(1):51–56. doi: 10.1016/s0962-8924(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 10.Robinet A, Fahem A, Cauchard JH, et al. Elastin-derived peptides enhance angiogenesis by promoting endothelial cell migration and tubulogenesis through upregulation of MT1-MMP. J Cell Sci. 2005;118(Pt 2):343–363. doi: 10.1242/jcs.01613. [DOI] [PubMed] [Google Scholar]
- 11.Blumenkranz MS, Russel SR, Robey MG, Kott-Blumenkranz R, Penneys N. Risk factors in age-related maculopathy complicated by choroidal neovascularization. Ophthalmology. 1986;93(5):552–558. doi: 10.1016/s0161-6420(86)33702-3. [DOI] [PubMed] [Google Scholar]
- 12.Hogan MJ. Bruch's membrane and disease of the macula. Role of elastic tissue and collagen. Trans Ophthalmol Soc UK. 1967;87:113–161. [PubMed] [Google Scholar]
- 13.Stone EM, Braun TA, Russell SR, et al. Missense variations in the fibulin 5 gene and age-related macular degeneration. N Engl J Med. 2004;351(4):346–353. doi: 10.1056/NEJMoa040833. [DOI] [PubMed] [Google Scholar]
- 14.Yanagisawa H, Davis EC, Starcher BC, et al. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415(6868):168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura T, Lozano PR, Ikeda Y, et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415(6868):171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 16.Mullins RF, Olvera MA, Clark AF, Stone EM. Fibulin-5 distribution in human eyes: relevance to age-related macular degeneration. Exp Eye Res. 2007;84(2):378–380. doi: 10.1016/j.exer.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spraul CW, Grossniklaus HE. Characteristics of Drusen and Bruch's membrane in postmortem eyes with age-related macular degeneration. Arch Ophthalmol. 1997;115(2):267–273. doi: 10.1001/archopht.1997.01100150269022. [DOI] [PubMed] [Google Scholar]
- 18.Chong NH, Keonin J, Luthert PJ, et al. Decreased thickness and integrity of the macular elastic layer of Bruch's membrane correspond to the distribution of lesions associated with age-related macular degeneration. Am J Pathol. 2005;166(1):241–251. doi: 10.1016/S0002-9440(10)62248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivaprasad S, Chong NV, Bailey TA. Serum elastin-derived peptides in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2005;46(9):3046–3051. doi: 10.1167/iovs.04-1277. [DOI] [PubMed] [Google Scholar]
- 20.Chau KY, Sivaprasad S, Patel N, Donaldson TA, Luthert PJ, Chong NV. Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye. 2007;21(12):1511–1515. doi: 10.1038/sj.eye.6702722. [DOI] [PubMed] [Google Scholar]
- 21.Mullins RF, Grassi MA, Skeie JM. Glycoconjugates of choroidal neovascular membranes in age-related macular degeneration. Mol Vis. 2005;11:509–517. [PubMed] [Google Scholar]
- 22.DeCarlo AA, Cohen JA, Aguado A, Glenn B. Isolation and characterization of human gingival microvascular endothelial cells. J Periodontal Res. 2008;43(2):246–254. doi: 10.1111/j.1600-0765.2007.01015.x. [DOI] [PubMed] [Google Scholar]
- 23.McLaughlin AP, De Vries GW. Role of PLCgamma and Ca(2+) in VEGF- and FGF-induced choroidal endothelial cell proliferation. Am J Physiol Cell Physiol. 2001;281(5):C1448–1456. doi: 10.1152/ajpcell.2001.281.5.C1448. [DOI] [PubMed] [Google Scholar]
- 24.Pepper MS, Mandriota SJ, Jeltsch M, Kumar V, Alitalo K. Vascular endothelial growth factor (VEGF)-C synergizes with basic fibroblast growth factor and VEGF in the induction of angiogenesis in vitro and alters endothelial cell extracellular proteolytic activity. J Cell Physiol. 1998;177(3):439–452. doi: 10.1002/(SICI)1097-4652(199812)177:3<439::AID-JCP7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 25.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18(1):4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Prog Horm Res. 2000;55:15–35. discussion 35–36. [PubMed] [Google Scholar]
- 27.Schneeberger-Keeley EE, Karnovsky MJ. The ultrastructural basis of alveolar-capillary membrane permeability to peroxidase used as a tracer. J Cell Biol. 1968;37(3):781–793. doi: 10.1083/jcb.37.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barthel LK, Raymond PA. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J Histochem Cytochem. 1990;38(9):1383–1388. doi: 10.1177/38.9.2201738. [DOI] [PubMed] [Google Scholar]
- 29.Yu HG, Liu X, Kiss S, et al. Increased choroidal neovascularization following laser induction in mice lacking lysyl oxidase-like 1. Invest Ophthalmol Vis Sci. 2008;49(6):2599–2605. doi: 10.1167/iovs.07-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]