Abstract
Progressive decline of memory functions has been observed in patients with chronic medication-resistant epilepsy. The progression likely relates to the effects of epileptiform discharges, seizures, and medications on the processes of encoding and retrieval. The goal of this study was to use functional MRI (fMRI) to examine the effects of chronic epilepsy on verbal recognition memory. We enrolled 12 patients with medication-resistant epilepsy (5 with right and 7 in left-hemispheric seizure onset) and 18 healthy controls matched for age, gender, and handedness. Subjects underwent fMRI at 3T using a word recognition task during which they had to recall if words presented during scanning were words they had learned prior to scanning. Although we noted many similarities in the fMRI activation patterns between the epilepsy and healthy subjects in areas typically involved in memory processing, testing of the interaction effects for the target-foil differences between groups revealed several differences in activation including the right insula, the left cuneus and bilateral subgenual anterior cingulate cortex (ACC). In patients with epilepsy these regions exhibited greater activation for targets than foils but in healthy subjects the difference is reversed (right insula), absent (left cuneus), or includes deactivation to target words (pregenual ACC). These differences were seen despite similar performance during the memory task, suggesting that activations seen in these additional regions may represent compensatory processes for verbal recognition memory that are induced by chronic brain injury related to recurrent seizures.
Keywords: epilepsy, fMRI, memory, recognition memory, compensatory strategies
INTRODUCTION
From recent neuroimaging work we have learned that multiple brain areas are involved in encoding and retrieval. These regions include bilateral lingual and fusiform gyri, bilateral hippocampi, predominantly left lateral parietal cortex and medial parietal cortex. Other areas are also involved in this process including left anterior prefrontal cortex near Brodmann area 10, anterior insula, thalamus, anterior cingulate cortex (ACC), inferior frontal gyrus, premotor cortex, and presupplementary motor area.(1–5) More specific memory processes, namely encoding or retrieval, appear to be lateralized depending on the type of memory encounter; left-hemispheric regions appear to participate in the verbal and right-hemispheric regions in the non-verbal encoding and retrieval of memories.(6)
One specific form of memory, verbal recognition memory, entails the ability to discriminate a list of previously memorized words from words not on the list.(4) The decision as to whether a word has been previously studied or not depends upon two mental processes, recollecting the actual episode of studying a word (remembering) or feeling a sense of familiarity that the word was studied (knowing). Remembering (or recollecting) is frequently defined as “mental reinstatement of previous events” while familiarity (or knowing) is defined as “mental awareness that an event has been experienced.”(4) Specific brain regions make distinct contributions to recollection or familiarity judgments. Regions including left prefrontal and parietal cortices support recollection of words and regions including right lateral and medial prefrontal cortex support word familiarity judgments.(7) Overall, neuroimaging studies in healthy subjects have found that the specific brain areas that participate in familiarity judgment include dorsal and ventral lateral prefrontal cortex (PFC), medial prefrontal cortex, thalamus, insula, medial and lateral parietal cortex, and occipital and fusiform regions.(3, 7)
There is neuropsychological evidence from amnesic patients with temporal lobe damage that recollection may be affected by injury to this region more than other memory processes.(8, 9) Similar effects on memory processes may be observed in aging (10) or in patients with brain damage that diminishes memory capacity including mild cognitive impairment and Alzheimer’s disease, traumatic brain injury, and epilepsy.(4)
Medically intractable epilepsy may negatively impact memory by disrupting memory processes through the presence of chronic and persistent seizures and concomitant progressive brain injury. A younger age of onset and longer disease duration correlate with reduced memory capacity in patients.(11, 12) Further, left and right medial temporal seizure onset are usually associated with verbal and non-verbal (or visuo-spatial) memory deficits, respectively.(13, 14) Using an encoding functional MRI (fMRI) task, we have recently shown that chronic focal epilepsy may influence the functional neuroanatomy of memory with different memory lateralization patterns in patients with left or right hemispheric epilepsy.(15) In that study, patients with left hemispheric epilepsy showed right-lateralized activation that differed significantly from controls and patients with right hemispheric epilepsy. In contrast, patients with right hemispheric seizure onset showed a non-significant increase in the degree of left lateralization. Further, neuropsychological measures of memory (WMS-III Story Recall) across epilepsy patients predicted memory lateralization with fMRI. We did not find age of onset or duration of epilepsy to be significantly related to fMRI memory lateralization.(15) Another fMRI study that utilized a different memory retrieval task confirmed the lack of relationship between memory lateralization and age of epilepsy onset, epilepsy duration and seizure frequency but found that the distribution pattern of epileptiform discharges may affect memory lateralization.(16)
One of the first studies of recognition memory in patients with heterogeneous epilepsy suggested, after adjusting for lower IQ in epilepsy patients, that there is no difference in recognition of verbal material compared with healthy controls.(17) This suggests that epilepsy patients may develop compensatory strategies to maintain memory capabilities that are adversely affected by their disease. Unfortunately, little is known about these compensatory strategies or what brain networks might be recruited for compensation.
In view of the above findings we conducted a pilot study employing a word recognition task that engages memory circuits. The goal of our study was to test whether chronic, medication resistant epilepsy affects memory retrieval and to understand what brain circuits are involved in compensatory strategies. We hypothesized that epilepsy patients would exhibit additional extratemporal areas of increased blood oxygenation-level dependent (BOLD) contrast during memory retrieval compared to a group of age, gender and handedness matched healthy controls.
MATERIAL AND METHODS
Subjects
Twelve patients with medication-refractory epilepsy were recruited from the Epilepsy Monitoring Unit at the University Hospital, Cincinnati, OH (Table 1). These subjects were recruited from a larger cohort of epilepsy patients participating in a study of language lateralization using fMRI and intracarotid amobarbital procedure.(5, 15, 18) Antiepileptic drugs (AEDs) were not adjusted or changed for the purpose of the study and all patients were seizure-free for at least 24 hours prior to scanning in order to avoid possible effects of seizures on fMRI BOLD signal responses.(19) The diagnosis of epilepsy was confirmed by prolonged video EEG monitoring (PVEM) in conjunction with neuropsychological testing, PET, and MRI. Eighteen healthy subjects were recruited by word of mouth and group-matched to the epilepsy subjects by age, gender, and handedness. All subjects provided written informed consent on separate forms approved by the Institutional Review Boards of the University of Cincinnati and the Cincinnati Children’s Hospital Medical Center (CCHMC). Each participant completed the Edinburgh Handedness Inventory prior to the scanning procedure.(20) Demographic data for both subject groups are reported in Table 2.
Table 1.
Clinical and Demographic Information for Epilepsy subjects.
| Right hemispheric seizure onset patients | |||||||
|---|---|---|---|---|---|---|---|
| Patient | Age | Age of onset | Sex | EHI* | Seizure onset | Structural MRI | Etiology/Risk Factor |
| 1 | 17 | 16 | F | 58 | R medial temporal | R medial temporal tip lesion | Cavernoma |
| 2 | 24 | 14 | F | 88 | R medial temporal | R MTS | Febrile seizures |
| 3 | 30 | 11 | M | 100 | R lateral temporal | R post. Temporal DNET | DNET |
| 4 | 30 | 9 | F | 100 | R medial temporal | R MTS | Febrile seizures |
| 5 | 38 | 4 | F | 100 | R medial temporal | Bilateral hippocampal volume loss R>L | Febrile seizures |
| Left hemispheric seizure onset patients | |||||||
| 6 | 33 | 19 | F | 67 | L medial temporal | Normal | none |
| 7 | 36 | 20 | F | 100 | L frontal | L lateral grade II oligodendroglioma | Tumor |
| 8 | 39 | 20 | F | −50 | L medial temporal | Normal | none |
| 9 | 51 | <1 | M | 100 | L medial temporal | L MTS | Perinatal insult |
| 10 | 51 | 5 | M | 33 | L medial temporal | L MTS | Febrile seizures |
| 11 | 52 | 36 | M | 85 | L lateral temporal | L MTS | Meningitis |
| 12 | 53 | 47 | M | 100 | L medial temporal | Normal | none |
Personal handedness measured using Edinburgh Handedness Inventory (EHI) (Oldfield, 1971)
Table 2.
Demographic comparisons between epilepsy and comparison subjects
| Characteristic | Epilepsy subjects (N=12) | Healthy subjects (N=18) | Significance (p) |
|---|---|---|---|
| Age, years (std. dev.) | 38 (12) | 37 (9) | 0.79 |
| Age range, min-max | 17–53 | 23–52 | |
| % Female | 58 | 72 | 0.45 |
| Education, years (std. dev.) | 15 (4) | 17 (3) | 0.13 |
| Edinburgh Handedness Inventory (EHI)* (std. dev.) | 77 (49) | 93 (9) | 0.30 |
| Familial Handedness Inventory (FHI)** (std. dev.) | 83 (24) | 88 (16) | 0.56 |
Left-handed subject scored −71 on EHI. Without this subject average is 91(15), p>0.7
Left-handed subject scored 60 on FHI. Without this subject average is 86(24), p>0.7
Functional MRI procedures
Upon completion of standard MRI screening procedures, we acquired a high-resolution anatomical scan and fMRI for several tasks including the word recognition paradigm reported here. Imaging was performed on a 3T Bruker Biospec 30/60 (Bruker Medizintechnik, Karlsruhe, Germany) running Bruker’s Paravision™ (ver. 2.2) under the IRIX operating system (ver. 6.5) in the Imaging Research Center at Cincinnati Children’s Hospital Medical Center. A Bruker quadrature radio frequency (RF) head coil was used to both transmit excitation pulses and receive the NMR signal on the scanner. The scanner is equipped with an audiovisual system for presentation of task stimuli (SV 4120; Avotech Systems Inc., Jensen Beach, FL). Foam padding was used to constrain head movement. The subjects were given buttons for responding to the task and a separate emergency response button to alert the MRI technologist to problems.
Subjects were positioned in the scanner and an initial alignment scan was done to identify locations of the axial planes for fMRI. Echo Planar Imaging (EPI) fMRI scans were performed using thirty-two 5 mm thick slices covering the entire brain. One hundred forty five EPI images were obtained using a T2*-weighted gradient-echo EPI pulse sequence (TR/TE 3000/38ms, FOV 25.6×25.6cm, matrix 64×64 pixels, slice thickness 5mm, flip angle 90°). Finally, a high-resolution T1-weighted 3-D anatomical scan was obtained using a modified driven equilibrium Fourier transform (MDEFT) protocol (TR 15 ms, TI 550ms, TE 4.5 ms, FOV 192×192×256, Matrix 128×128×256, flip angle = 20°; spatial resolution of 1.5mm × 1.5mm × 1mm) to provide images for anatomical localization of brain activation maps.(21, 22)
We used a word recognition task that was developed based on a previous study.(23) Thirty to 45 minutes prior to the fMRI procedure a list of 10 unrelated words (alligator, chart, cover, defeat, feature, generosity, gentleman, infraction, pompous, remedy) was presented to the subject one word at a time for three seconds each. The list was repeated until the subjects memorized all words. Subjects proceeded to the scanner only after they correctly recalled all 10 items.
During the fMRI scan, the word recognition task was presented in a blocked design alternating the active (recognition) and control conditions. The paradigm, which lasted 7 min 15 seconds, began with a 15 seconds control block to allow for T1 equilibration and then alternated between 30 seconds of the recognition condition and 30 seconds of the control condition for the next 14 blocks. Seventy words were presented, 22 target words and 48 novel words. During each block of the recognition condition 10 words were presented one every 3 sec for 2.5 sec on a white background followed by a blank screen for 0.5 seconds. Words were presented in pseudo-randomized order. Each target word appeared twice, except for two words that appeared three times. Subjects were instructed to respond to all target words with a dominant hand button press, and respond to all foils with a non-dominant hand button press. During the blocks of the control condition a single asterisk (“*”) was presented, once every three seconds for 2.5 sec, followed by a blank screen for 0.5 sec. Subjects were instructed to press the dominant hand button in response to asterisk presentation. The control task thus accounted for stimulation, attention, and response elements and prevented the performance of unrelated tasks.
The visual paradigm was presented in PsyScope (http://psyscope.psy.cmu.edu/). Response information was acquired during the fMRI runs and consisted of response choice and reaction time (RT) for responses made during the blocks of the recognition condition. Response choice, RT, and number of errors, both response and non-response errors, were used as dependent measures in t-tests contrasting the two groups’ performance (Table 3).
Table 3.
Behavioral performance of epilepsy and healthy comparison subjects on memory task
| Measure | Epilepsy Subjects | Healthy Subjects | Significance (p) |
|---|---|---|---|
| Total RT, mean(std. dev.) | 1136 (38) | 1168 (32) | 0.51 |
| Correct RT | 1128 (35) | 1160 (34) | 0.51 |
| Total Errors* | 1.75 (0.81) | 1.00 (0.33) | 0.32 |
| Response Errors, false alarms | 0.42 (0.27) | 0.56 (0.22) | 0.51 |
| Non-response errors, misses | 1.33 (0.67) | 0.44 (0.19) | 0.13 |
| Number of subjects making errors | 6 | 9 |
The average number of errors per subject
Image Processing
Image analysis was performed using a combination of CCHIPS© (Cincinnati Children’s Hospital Image Processing System; http://irc.cchmc.org/software/cchips.php) developed by the Imaging Research Center in IDL (Research Systems Inc., Boulder, CO) and AFNI (Analysis of Functional NeuroImages; http://afni.nimh.nih.gov). Image processing proceeded in several stages beginning with the following steps in CCHIPS. Geometric distortion due to B0 field inhomogeneity was corrected during image reconstruction using a multi-echo reference scan.(24) FMRI EPI data were co-registered to the anatomical scans using scanner coordinates, motion corrected using a pyramid iterative algorithm,(25) and normalized to Talairach space by the identification of brain landmarks on the MDEFT including the anterior and posterior commissures.(26) The remaining steps were performed in AFNI: Gaussian blurring of normalized EPI time series with 8mm full-width at half-maximum kernel, estimation of individual brain activations based on behavioral paradigm using a deconvolution algorithm, and calculation of group activation maps using t-tests or multiple linear regression.
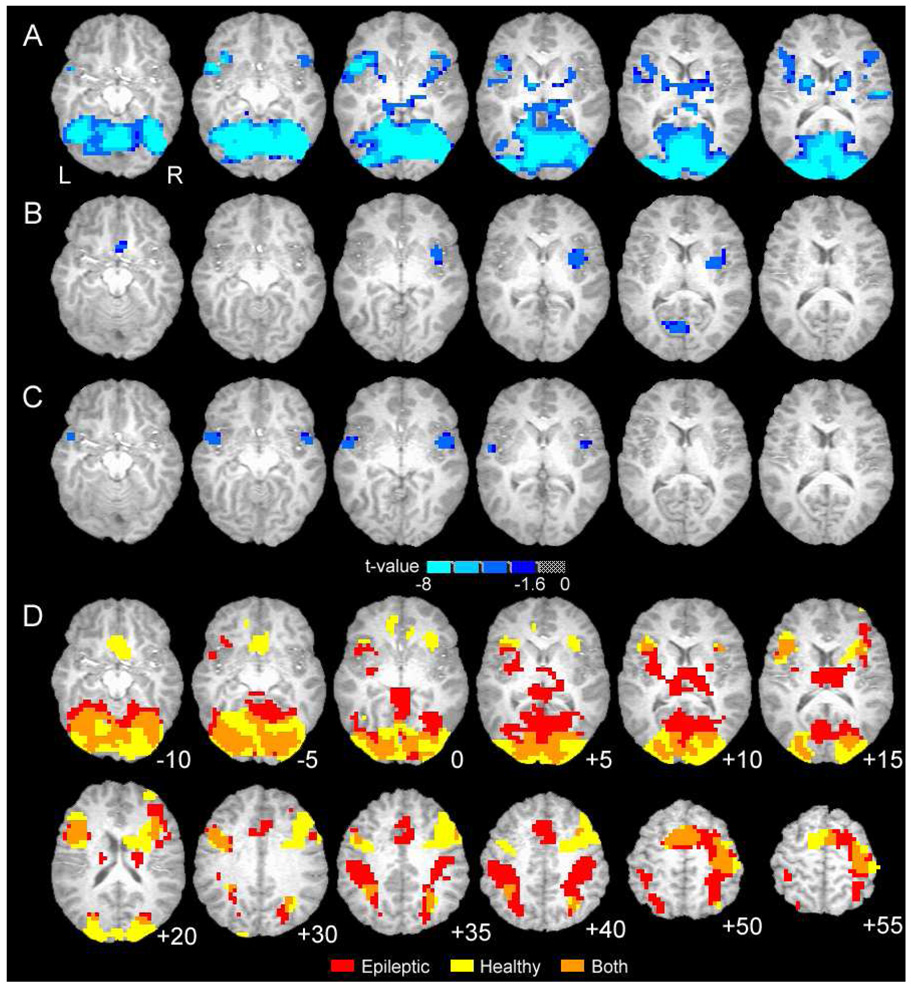
Using an event-related approach we estimated brain activation magnitudes (fit-coefficients) for target words and foil words relative to the control task baseline. These two fit coefficients were then used as dependent variables in t-tests or linear regression. One-way t-tests comparing fit coefficients for target words or foils to zero were conducted for healthy and epileptic subjects separately (Figures 1A and B). These images indicate which brain regions are activated by target word recognition or foil words. A two-way t-test compared target word activation between healthy and epilepsy subjects (Figure 1C), i.e., quantitatively compared data underlying Figure 1A to 1B. The linear regression analysis included both foil and target word fit coefficients as dependent measures. Group (healthy or epilepsy) and condition (target or foil) served as predictor variables, and the model included the main effects of group and condition and the two-way interaction between group and condition. These group activation maps were thresholded with a voxel-level p-value ≤ 0.005 and a cluster of 1120 or more microliters (14 contiguous voxels), as determined by Monte Carlo simulation, reflecting a corrected p-value ≤ 0.05.
Figure 1.
Group brain activation maps showing statistical results (A–C) and word recognition activation similarity (D). A. Contrast between healthy and epileptic subjects for word recognition task, includes target and foil words (main effect of Group from regression). B. Difference in brain activation between healthy and epileptic subjects for target-foil word difference (Group [healthy/epileptic] by Condition [target/foil] interaction from regression). C. Difference in brain activation between healthy and epileptic subjects for target words (two-way t-test comparing patterns in D). For A–C blue-cyan indicates negative t-value and greater activation for epileptic subjects. D. Overlap of activation patterns for healthy and epileptic subjects during recognition of memorized (target) words only (overlap of each group’s one-way t-test). Additional superior brain regions are shown. For D, red is epilepsy patients only, yellow is healthy subjects only, orange is overlap of both groups.
RESULTS
Statistical tests of the demographic characteristics and behavioral measures indicated no significant differences between the two groups (Table 2 and Table 3). Overall, epilepsy subjects had higher number of errors on recall during the scanning and higher number of non-responses but these differences between the groups were not significant.
The initial analysis of the fMRI data involved a comparison of the distribution of the BOLD signal changes between the patients with right or left hemispheric epilepsy onset. Minimal differences between groups were noted predominantly in the left motor and premotor cortices for target events only with more activation for right hemisphere patients than left. There were no significant differences for foils or the combination of targets and foils. This might reflect the varied use of dominant hand responses for targets and non-dominant hands for foil responses. However, the activation did not correspond to typical memory processing areas and was positioned at the edge of the imaging slab (data not shown). Therefore, the right and left hemispheric epilepsy onset patients were combined in one larger group for further comparisons with healthy controls.
The fMRI data indicate similarity in the regions of brain activation for the two groups (epilepsy patients, healthy controls), but also identify several regions where the groups differ in activation magnitude or in the pattern of activation for target and foil words. The main effect of the regression analysis indicates extensive regions with greater activation for epilepsy patients than comparison subjects during memory task performance (Fig. 1A, Table 4). The main effect of the regression combines both target and foil word events when contrasting the two groups and represents, in effect, a block design analysis. Regions of activation include widespread areas of the occipital lobes, medial, dorsolateral, and ventrolateral frontal regions, striatum, thalamus and smaller temporal and parietal lobe sites.
Table 4.
Activation differences between Healthy and Epilepsy subjects: main effect of regression.
| Cluster | Volume (µL) | Max t-score | Mean | SEM | X | Y | Z | Region | BA |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 153200 | −22.67 | −6.70 | 0.07 | 34 | −61 | −5 | R FFG | 19 |
| sub-maxima | −16.16 | 27 | −93 | 15 | R MOG | 19 | |||
| −17.02 | −21 | −89 | 20 | L MOG | 18 | ||||
| −15.08 | 11 | −73 | 5 | R Lingual | 18 | ||||
| −11.88 | −41 | −57 | −10 | L FFG | 37 | ||||
| 2 | 94640 | −11.99 | −4.70 | 0.04 | −6 | 3 | 45 | L ACC | 24 |
| sub-maxima | −7.25 | −34 | −1 | 50 | L MFG | 6 | |||
| −9.54 | −46 | 15 | −5 | L IFG | 47 | ||||
| −6.57 | −50 | −21 | 55 | L PostCentrG | 1 | ||||
| −5.79 | 18 | −5 | 20 | R Caudate | |||||
| −6.07 | −18 | −1 | 15 | L Putamen | |||||
| −7.06 | 42 | 7 | 25 | R IFG | 9 | ||||
| −5.12 | 54 | 7 | 35 | R MFG | 9 | ||||
| 3 | 4560 | −10.85 | −5.39 | 0.25 | 22 | −53 | 55 | R SPL | 7 |
| 4 | 4400 | −5.90 | −4.02 | 0.11 | −30 | −49 | 40 | L IPL | 7 |
| 5 | 2240 | −6.76 | −4.67 | 0.20 | 34 | 11 | −30 | R STG | 38 |
| 6 | 1520 | −5.31 | −3.83 | 0.13 | 30 | 27 | 0 | R IFG | 47 |
| 7 | 1280 | −7.08 | −4.58 | 0.32 | 54 | −13 | 15 | R PostCentrG | 43 |
Negative differences indicate greater activation for Epilepsy subjects. µL – microliters, SEM – standard error of the mean, L – left, R – right, FFG – fusiform gyrus, MOG – middle occipital gyrus, ACC – anterior cingulated cortex, MFG – middle frontal gyrus, IFG – inferior frontal gyrus, PostCentrG – post-central gyrus, SPL – superior parietal lobule, IPL – inferior parietal lobule, STG – superior temporal gyrus.
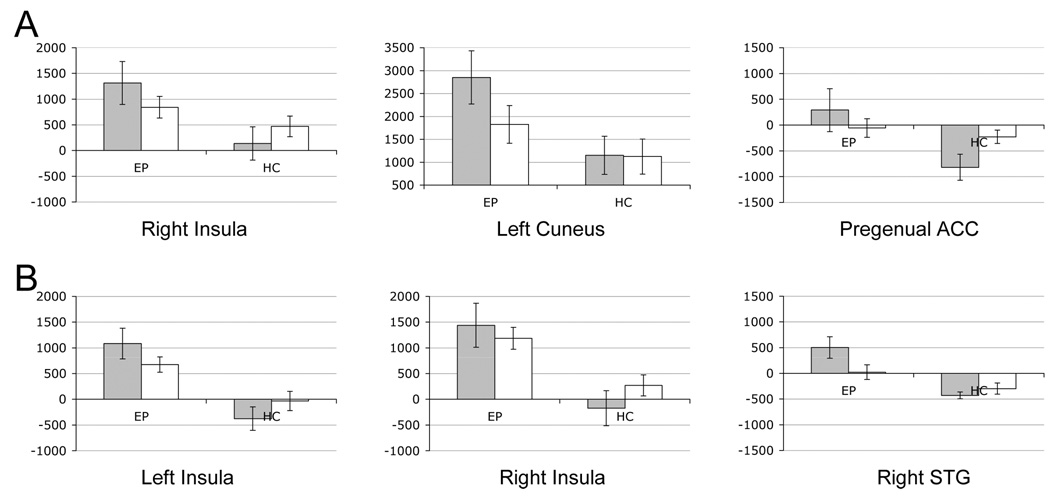
Further regression analysis of the interaction effect indicates three regions with distinct target-foil differences for epilepsy and healthy subjects (Fig. 1B, 2A, Table 5). These regions include the right insula, the left cuneus and bilateral subgenual anterior cingulate cortex. In epileptic patients these regions exhibit greater activation for targets than foils but in healthy subjects the difference is reversed (right insula), absent (left cuneus), or includes deactivation to target words (pregenual ACC). Although not apparent from the color scale, the pregenual ACC and the right superior temporal gyrus (STG; described next, see Figure 2B; fMRI data not shown) were the only two regions to exhibit significant task-related deactivations in either group in our one-way t-tests (see Fig. 1D).
Figure 2.
Target (gray) and distracter (white) activation level in regions identified by regression analysis (A) or t-test results (B). For regression analysis, target word activation is greater than distracter for epilepsy patients (EP) but not for comparison subjects (HC). For t-test results target words yield positive activations for epilepsy patients (EP, gray bars) but deactivations for comparison subjects (HC gray bars). Activation is in raw MR units.
Table 5.
Activation differences between Healthy and Epileptic subjects for target-foil difference: interaction effect of regression.
| Cluster | Volume (µL) | Max t-score | X | Y | Z | Region | BA |
|---|---|---|---|---|---|---|---|
| 1 | 3440 | −4.61 | 35 | 3 | 5 | R Insula | 13 |
| 2 | 1360 | −3.57 | −9 | −69 | 10 | L Cuneus | 30 |
| 3 | 1200 | −3.63 | 3 | 15 | −10 | SubGen ACC | 25 |
SubGen – subgenual; other abbreviations as in table 4.
We contrasted target or foil words between groups in two separate t-tests. The pattern of activation between groups for foils did not differ significantly in any brain region (data not shown). For target words, three regions exhibited significantly greater activation in epileptic patients than in healthy controls (Fig. 1C, Fig. 2B, Table 6). These regions exhibit positive activation for epileptic subjects during recognition of targets and little or no deactivation (on average) for comparison subjects. Figure 2B also illustrates the findings of Figure 1A, where, in general, epileptic patients exhibit greater activation for both word events.
Table 6.
Activation differences between Healthy and Epileptic subjects for target word recognition.
| Cluster | Volume (µL) | Max t-score | X | Y | Z | Region | BA |
|---|---|---|---|---|---|---|---|
| 1 | 2320 | −4.76 | −49 | 7 | −5 | L STG | 22 |
| 2 | 2160 | −4.04 | 47 | 3 | 0 | R STG | 22 |
| 3 | 1120 | −4.04 | 31 | 3 | −30 | R polar STG | 38 |
Polar – temporal pole; other abbreviations as in table 4.
Also, the general pattern of activation for target words is similar between the groups (overlap regions in Fig. 1D). Although qualitative differences in target word activation are apparent in parietal regions, thalamus, striatum and medial frontal cortex (supragenual ACC), these differences did not retain statistical significance in our voxel-by-voxel analysis. Despite the significant differences reported here, the degree of similarity in task activation suggests that the two groups, by and large, use similar neural circuits to identify target words and to filter out distractors. The additional brain regions with distinct activation in patients with epilepsy may indicate compensatory areas engaged in support of recognition in these patients.
DISCUSSION
In this study we focused on short-term word recognition memory in patients with medication-refractory epilepsy. As expected, epilepsy patients exhibit additional areas of increased BOLD signal during memory retrieval compared to a group of age, gender, education, and handedness matched healthy controls. Overall, the areas different between epilepsy patients and controls include right insula, left cuneus and bilateral subgenual anterior cingulate cortex. In epileptic patients, these regions exhibit greater activation for targets than foils but in healthy subjects the difference is either reversed (as in right insula), absent as in left cuneus, or appears as deactivation to target words (pregenual ACC). Given the similarities between groups, including lack of differences in behavioral performance (Table 2 and Table 3) and general activation patterns, we focus our discussion on regions of distinct activations as these differences between healthy controls and epilepsy patients likely represent compensatory strategies (i.e., cortical reorganization as a response to a chronic insult) rather than cortical dysfunction.
Relatively little is known about the functional substrates of compensatory strategies involved in memory retrieval when the brain is adversely affected by disease. Studies have shown that patients with left temporal epilepsy have decreased recognition memory when compared to right temporal lobe epilepsy patients and make significantly more false positive errors.(27) The errors these patients make are more frequently semantic in nature, which is consistent with predominantly verbal memory deficits either preceding or following epilepsy surgery in patients with dominant temporal lobe epilepsy. In our study, this group of patients had a lower rate of false positive errors and a higher rate of false negative errors (misses), and while neither was significant, only the misses approached a statistical trend. Also, although memory deficits are one of the most frequent cognitive complaints of patients with temporal lobe epilepsy, they may be, in part, related to language rather than memory impairment.(28, 29) Although the patients with temporal lobe epilepsy frequently have focal memory deficits, these are likely a result of a combined, focal and global network dysfunction.(30–32) Bilateral anatomical and biochemical substrates may underlie such dysfunction despite the seizures being unilateral in their onset.(33, 34)
Previous neuroimaging studies of healthy subjects have shown various brain areas involved in verbal memory retrieval. These areas include bilateral parahippocampal and fusiform gyri, occipital cortex, left parietal and verntrolateral frontal cortex.(32) Additional areas were noted by others including anterior cingulate, premotor cortex, and thalamus.(3, 7) Patients with epilepsy usually express brain activations related to retrieval in these and other brain areas including lingual and posterior cingulate gyri (30, 31) but usually do not activate the temporal structures including bilateral parahippocampal gyri.(32) Here, we show that patients with epilepsy have additional areas of the brain involved in a short-term recall process when compared to healthy controls. These newly identified additional areas of activations are similar to previous studies. As the performance of the epilepsy patients is similar to the performance of the controls and in view of our previous studies, (12, 15) we assume that these additional areas of activation related to verbal memory retrieval in epilepsy patients reflect functional compensation to permit equivalent performance. Activation of these regions could represent brain plasticity related to chronic insult (seizures and/or epileptiform discharges).(16, 35) This notion is supported by a study that showed normalization of metabolic brain abnormalities in response to successful epilepsy surgery.(33) Therefore, we suspect that the differences in activation pattern between healthy controls and epilepsy patients reflect the effects of epileptiform discharges and seizures on the overall brain function and connectivity. Given that our patients are pre-surgical and epilepsy surgery normalizes metabolic abnormalities, one explanation for the activations seen in Fig. 1B and C is that epilepsy affects the resting or default state of the brain. The pregenual ACC, pre-cuneus (near our cuneus cluster) and insular regions, all appear to be part of a network in the brain associated with either resting state correlation (36, 37) or task-related deactivation.(38) Five of the six activation patterns observed in Fig. 2 for healthy subjects are reversed for epileptic patients. This could mean that the patients’ judgments of recollection and familiarity are occurring against an abnormal background brain activity level requiring compensation in these particular regions to maintain equivalent performance.
Several limitations of this preliminary study should be noted. These include relatively small number of subjects and inclusion of subjects with various types and etiologies of epilepsy (medial temporal, neocortical, and lesional). Therefore, we cannot exclude the possibility that the observed effects may be attributed to these factors. Further, we were not able to account for the possible effects of duration of epilepsy, lifetime number of seizures, and medication effects all of which may affect memory functions.(11, 12, 39, 40) As this is a preliminary study, a larger study addressing the above listed limitations and possibly introducing an intervention should be considered.
ACKNOWLEDGEMENTS
This work was supported in part by a grant from The Neuroscience Institute in Cincinnati (JPS) and in part by NIH RO1-HD38578 (to SKH). Drs. Szaflarski and Eliassen are currently supported by NIH K23 NS052468 and K01-DA020485, respectively. This paper was presented in part at the 57th Annual Meeting of the American Academy of Neurology, Miami, FL, 4/05.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Aggleton JP, Vann SD, Denby C, Dix S, Mayes AR, Roberts N, et al. Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia. 2005;43(12):1810–1823. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Kapur N, Friston KJ, Young A, Frith CD, Frackowiak RS. Activation of human hippocampal formation during memory for faces: a PET study. Cortex. 1995 Mar;31(1):99–108. doi: 10.1016/s0010-9452(13)80108-6. [DOI] [PubMed] [Google Scholar]
- 3.Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. Neuroimage. 2000 Sep;12(3):276–286. doi: 10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- 4.Skinner EI, Fernandes MA. Neural correlates of recollection and familiarity: a review of neuroimaging and patient data. Neuropsychologia. 2007 Jun 11;45(10):2163–2179. doi: 10.1016/j.neuropsychologia.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Szaflarski JP, Holland SK, Schmithorst VJ, Dunn RS, Privitera MD. High-resolution functional MRI at 3T in healthy and epilepsy subjects: hippocampal activation with picture encoding task. Epilepsy Behav. 2004 Apr;5(2):244–252. doi: 10.1016/j.yebeh.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Wagner AD, Poldrack RA, Eldridge LL, Desmond JE, Glover GH, Gabrieli JD. Material-specific lateralization of prefrontal activation during episodic encoding and retrieval. Neuroreport. 1998 Nov 16;9(16):3711–3717. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- 7.Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: an event-related functional magnetic resonance imaging study. J Neurosci. 1999 May 15;19(10):3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holdstock JS, Mayes AR, Gong QY, Roberts N, Kapur N. Item recognition is less impaired than recall and associative recognition in a patient with selective hippocampal damage. Hippocampus. 2005;15(2):203–215. doi: 10.1002/hipo.20046. [DOI] [PubMed] [Google Scholar]
- 9.Kapur N, Brooks DJ. Temporally-specific retrograde amnesia in two cases of discrete bilateral hippocampal pathology. Hippocampus. 1999;9(3):247–254. doi: 10.1002/(SICI)1098-1063(1999)9:3<247::AID-HIPO5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 10.Healy MR, Light LL, Chung C. Dual-process models of associative recognition in young and older adults: evidence from receiver operating characteristics. J Exp Psychol Learn Mem Cogn. 2005 Jul;31(4):768–788. doi: 10.1037/0278-7393.31.4.768. [DOI] [PubMed] [Google Scholar]
- 11.Hermann B, Seidenberg M. Neuropsychology and temporal lobe epilepsy. CNS Spectr. 2002 May;7(5):343–348. doi: 10.1017/s1092852900017806. [DOI] [PubMed] [Google Scholar]
- 12.Kent GP, Schefft BK, Howe SR, Szaflarski JP, Yeh HS, Privitera MD. The effects of duration of intractable epilepsy on memory function. Epilepsy Behav. 2006 Nov;9(3):469–477. doi: 10.1016/j.yebeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Hermann BP, Wyler AR, Richey ET, Rea JM. Memory function and verbal learning ability in patients with complex partial seizures of temporal lobe origin. Epilepsia. 1987 Sep–Oct;28(5):547–554. doi: 10.1111/j.1528-1157.1987.tb03687.x. [DOI] [PubMed] [Google Scholar]
- 14.Testa SM, Schefft BK, Privatera MD, Yeh HS. Warrington's recognition memory for faces: interpretive strategy and diagnostic utility in temporal lobe epilepsy. Epilepsy Behav. 2004 Apr;5(2):236–243. doi: 10.1016/j.yebeh.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Vannest J, Szaflarski JP, Privitera MD, Schefft BK, Holland SK. Medial temporal fMRI activation reflects memory lateralization and memory performance in patients with epilepsy. Epilepsy Behav. 2008 Apr;12(3):410–418. doi: 10.1016/j.yebeh.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Janszky J, Ollech I, Jokeit H, Kontopoulou K, Mertens M, Pohlmann-Eden B, et al. Epileptic activity influences the lateralization of mesiotemporal fMRI activity. Neurology. 2004 Nov 23;63(10):1813–1817. doi: 10.1212/01.wnl.0000145563.53196.01. [DOI] [PubMed] [Google Scholar]
- 17.Tomlinson L, Stirling N, Merrifield E, Reynolds EH. Recognition memory in treated epileptic patients. Acta Neurol Scand Suppl. 1981;89:43–50. doi: 10.1111/j.1600-0404.1981.tb02361.x. [DOI] [PubMed] [Google Scholar]
- 18.Szaflarski JP, Holland SK, Jacola LM, Lindsell C, Privitera MD, Szaflarski M. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy Behav. 2008 Jan;12(1):74–83. doi: 10.1016/j.yebeh.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayakar P, Bernal B, Santiago Medina L, Altman N. False lateralization of language cortex on functional MRI after a cluster of focal seizures. Neurology. 2002 Feb 12;58(3):490–492. doi: 10.1212/wnl.58.3.490. [DOI] [PubMed] [Google Scholar]
- 20.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 21.Duewell S, Wolff SD, Wen H, Balaban RS, Jezzard P. MR imaging contrast in human brain tissue: assessment and optimization at 4 T. Radiology. 1996;199(3):780–786. doi: 10.1148/radiology.199.3.8638005. [DOI] [PubMed] [Google Scholar]
- 22.Wansapura JP, Holland SK, Dunn RS, Ball WS., Jr NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging. 1999;9(4):531–538. doi: 10.1002/(sici)1522-2586(199904)9:4<531::aid-jmri4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 23.Baxter L, Blum D, Johnson S. Memory performance on the intracarotid amobarbital procedure correlates with degree of lateralized functional magnetic resonance imaging (fMRI) activation during memory encoding. Epilepsia. 2001;42 Suppl. 7:77. [Google Scholar]
- 24.Schmithorst VJ, Dardzinski BJ, Holland SK. Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging. 2001 Jun;20(6):535–539. doi: 10.1109/42.929619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thevenaz P, Unser M, editors. IEEE Trans Image Processing. 1998. A pyramid approach to sub-pixel registration based on intensity. [DOI] [PubMed] [Google Scholar]
- 26.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- 27.Seidenberg M, Hermann B, Haltiner A, Wyler A. Verbal recognition memory performance in unilateral temporal lobe epilepsy. Brain Lang. 1993 Feb;44(2):191–200. doi: 10.1006/brln.1993.1013. [DOI] [PubMed] [Google Scholar]
- 28.Hermann BP, Wyler AR, Steenman H, Richey ET. The interrelationship between language function and verbal learning/memory performance in patients with complex partial seizures. Cortex. 1988 Jun;24(2):245–253. doi: 10.1016/s0010-9452(88)80033-9. [DOI] [PubMed] [Google Scholar]
- 29.Mayeux R, Brandt J, Rosen J, Benson DF. Interictal memory and language impairment in temporal lobe epilepsy. Neurology. 1980 Feb;30(2):120–125. doi: 10.1212/wnl.30.2.120. [DOI] [PubMed] [Google Scholar]
- 30.Dupont S, Samson Y, Van de Moortele PF, Samson S, Poline JB, Adam C, et al. Delayed verbal memory retrieval: a functional MRI study in epileptic patients with structural lesions of the left medial temporal lobe. Neuroimage. 2001 Nov;14(5):995–1003. doi: 10.1006/nimg.2001.0908. [DOI] [PubMed] [Google Scholar]
- 31.Dupont S, Samson Y, Van de Moortele PF, Samson S, Poline JB, Hasboun D, et al. Bilateral hemispheric alteration of memory processes in right medial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2002 Nov;73(5):478–485. doi: 10.1136/jnnp.73.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dupont S, Van de Moortele PF, Samson S, Hasboun D, Poline JB, Adam C, et al. Episodic memory in left temporal lobe epilepsy: a functional MRI study. Brain. 2000;123(Pt 8):1722–1732. doi: 10.1093/brain/123.8.1722. [DOI] [PubMed] [Google Scholar]
- 33.Cendes F, Andermann F, Dubeau F, Matthews PM, Arnold DL. Normalization of neuronal metabolic dysfunction after surgery for temporal lobe epilepsy. Evidence from proton MR spectroscopic imaging. Neurology. 1997 Dec;49(6):1525–1533. doi: 10.1212/wnl.49.6.1525. [DOI] [PubMed] [Google Scholar]
- 34.Quigg M, Bertram EH, Jackson T, Laws E. Volumetric magnetic resonance imaging evidence of bilateral hippocampal atrophy in mesial temporal lobe epilepsy. Epilepsia. 1997 May;38(5):588–594. doi: 10.1111/j.1528-1157.1997.tb01144.x. [DOI] [PubMed] [Google Scholar]
- 35.Janszky J, Jokeit H, Heinemann D, Schulz R, Woermann FG, Ebner A. Epileptic activity influences the speech organization in medial temporal lobe epilepsy. Brain. 2003 Sep;126(Pt 9):2043–2051. doi: 10.1093/brain/awg193. [DOI] [PubMed] [Google Scholar]
- 36.Morgan VL, Gore JC, Szaflarski JP. Temporal clustering analysis: What does it tell us about the resting state of the brain? Med Sci Monit. 2008 in print. [PMC free article] [PubMed] [Google Scholar]
- 37.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007 Oct 1;37(4):1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 97–9. [DOI] [PubMed] [Google Scholar]
- 38.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007 Sep;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 39.Jansen JF, Aldenkamp AP, Marian Majoie HJ, Reijs RP, de Krom MC, Hofman PA, et al. Functional MRI reveals declined prefrontal cortex activation in patients with epilepsy on topiramate therapy. Epilepsy Behav. 2006 Jun 20; doi: 10.1016/j.yebeh.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Oyegbile TO, Dow C, Jones J, Bell B, Rutecki P, Sheth R, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004 May 25;62(10):1736–1742. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]