Abstract
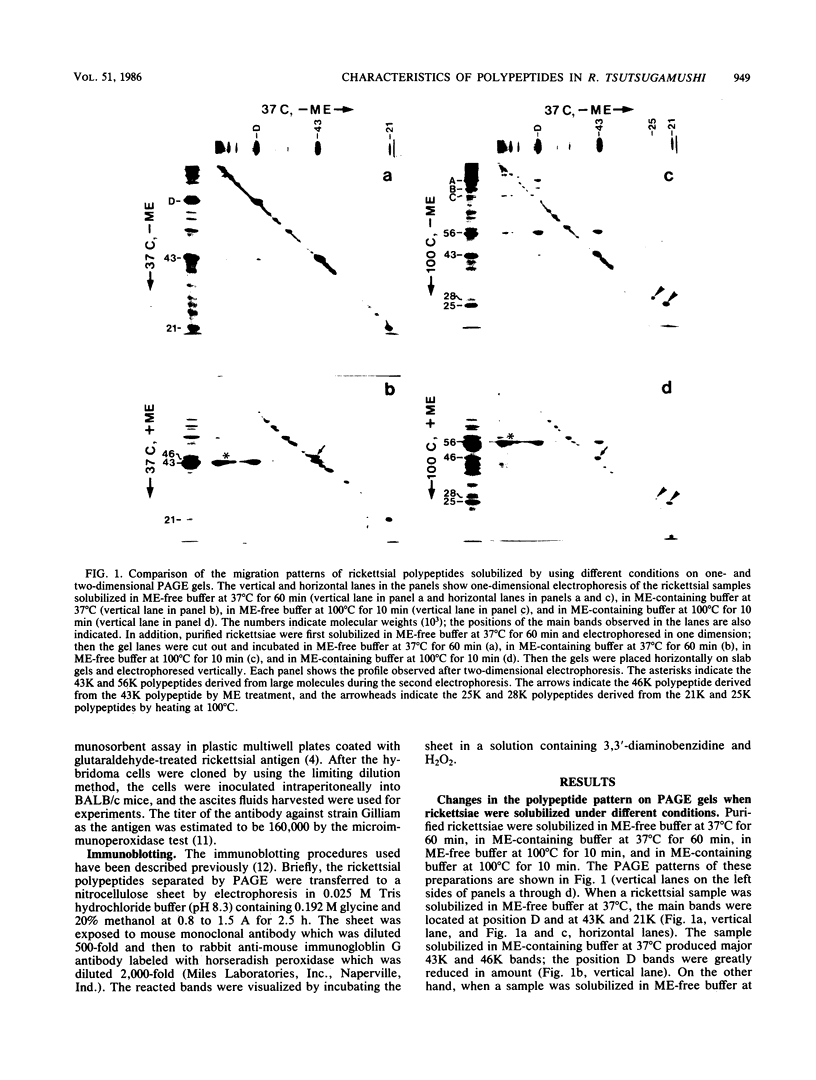
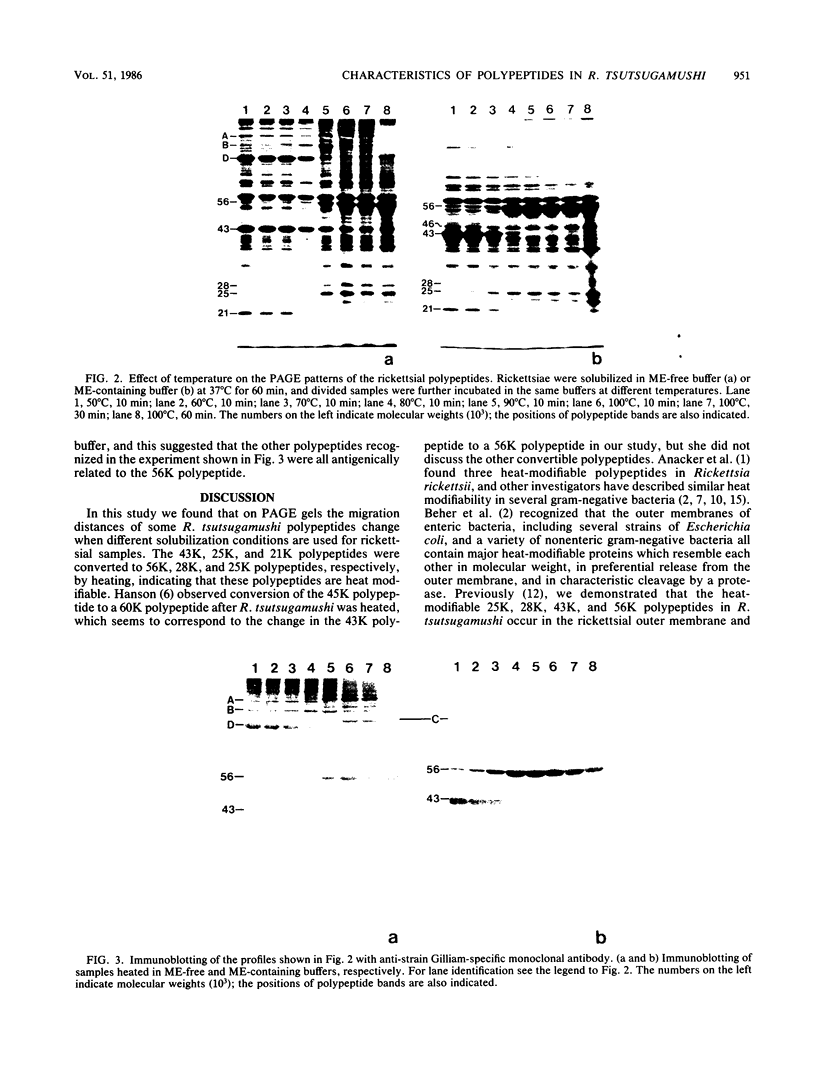
The polypeptide compositions and antigenic components of Rickettsia tsutsugamushi were analyzed by modifying the solubilization conditions prior to polyacrylamide gel electrophoresis and by using monoclonal antibodies in immunoblotting experiments. Several polypeptides were converted to larger or smaller molecules by using various conditions for rickettsial sample preparation. Solubilization of a sample in 2-mercaptoethanol-containing buffer resulted in conversion of high-molecular-weight polypeptides to smaller polypeptides and conversion of some of the 43-kilodalton (43K) polypeptide to a 46K polypeptide. The heat modifiability of selected polypeptides was shown by heating samples at 100 degrees C. A major polypeptide on the rickettsial surface which showed strain-specific antigenicity appeared at the 43K position in samples solubilized at 37 degrees C but moved to the 56K position after samples were heated at 100 degrees C. Immunoblotting with an anti-56K polypeptide monoclonal antibody demonstrated that the reactive antigens existed predominantly as the higher-molecular-weight polypeptides. These polypeptides were converted to 43K polypeptides at 37 degrees C or the 56K polypeptides at 100 degrees C by cleavage of disulfide linkages with 2-mercaptoethanol treatment.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., Philip R. N., Williams J. C., List R. H., Mann R. E. Biochemical and immunochemical analysis of Rickettsia rickettsii strains of various degrees of virulence. Infect Immun. 1984 Jun;44(3):559–564. doi: 10.1128/iai.44.3.559-564.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beher M. G., Schnaitman C. A., Pugsley A. P. Major heat-modifiable outer membrane protein in gram-negative bacteria: comparison with the ompA protein of Escherichia coli. J Bacteriol. 1980 Aug;143(2):906–913. doi: 10.1128/jb.143.2.906-913.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisberg B. L., Campbell J. M., Bozeman F. M. Antigenic diversity of rickettsia tsutsugamushi: epidemiologic and ecologic significance. J Hyg Epidemiol Microbiol Immunol. 1968;12(1):18–25. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Hindahl M. S., Iglewski B. H. Isolation and characterization of the Legionella pneumophila outer membrane. J Bacteriol. 1984 Jul;159(1):107–113. doi: 10.1128/jb.159.1.107-113.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Jones R. B. Disulfide-linked oligomers of the major outer membrane protein of chlamydiae. J Bacteriol. 1983 May;154(2):998–1001. doi: 10.1128/jb.154.2.998-1001.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Outer membrane proteins of Escherichia coli. I. Effect of preparative conditions on the migration of protein in polyacrylamide gels. Arch Biochem Biophys. 1973 Aug;157(2):541–552. doi: 10.1016/0003-9861(73)90673-5. [DOI] [PubMed] [Google Scholar]
- Suto T. [Rapid and sensitive serodiagnosis of tsutsugamushi disease by means of indirect immunoperoxidase reaction (author's transl)]. Rinsho Byori. 1982 Jan;30(1):10–17. [PubMed] [Google Scholar]
- Tamura A., Ohashi N., Urakami H., Takahashi K., Oyanagi M. Analysis of polypeptide composition and antigenic components of Rickettsia tsutsugamushi by polyacrylamide gel electrophoresis and immunoblotting. Infect Immun. 1985 Jun;48(3):671–675. doi: 10.1128/iai.48.3.671-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A., Takahashi K., Tsuruhara T., Urakami H., Miyamura S., Sekikawa H., Kenmotsu M., Shibata M., Abe S., Nezu H. Isolation of Rickettsia tsutsugamushi antigenically different from Kato, Karp, and Gilliam strains from patients. Microbiol Immunol. 1984;28(8):873–882. doi: 10.1111/j.1348-0421.1984.tb00743.x. [DOI] [PubMed] [Google Scholar]
- Tamura A., Urakami H., Tsuruhara T. Purification of Rickettsia tsutsugamushi by Percoll density gradient centrifugation. Microbiol Immunol. 1982;26(4):321–328. doi: 10.1111/j.1348-0421.1982.tb00181.x. [DOI] [PubMed] [Google Scholar]
- van Alphen L., Riemens T., Poolman J., Zanen H. C. Characteristics of major outer membrane proteins of Haemophilus influenzae. J Bacteriol. 1983 Aug;155(2):878–885. doi: 10.1128/jb.155.2.878-885.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]