Abstract
Ultrasound contrast agents are gas filled microbubbles that enhance the ultrasound image. They behave similarly to red blood cells and cross all capillary beds; making contrast enhanced ultrasonography (CEU) a suitable technique to study vasculature and tissue blood flow. Ultrasound contrast agents have been found to be safe after intravenous injection. CEU has been used extensively in the field of cardiology. Currently, study of renal vasculature and renal blood flow requires complicated, time consuming and expensive techniques, which are not commonly used in clinical settings. CEU potentially may serve as a relatively noninvasive and safe technique for studying renal hemodynamics in health and disease. In this article we have reviewed the literature on the use of CEU in the study of kidney disease.
Contrast Enhanced Ultrasound (CEU)
Contrast ultrasonography has been used to improve ultrasound images. It has been used extensively in cardiology to assess myocardial perfusion during echocardiography.[1–6] This imaging technique utilizes stabilized gas bubbles to enhance the ultrasound image. Ultrasound contrast agents are microbubbles made of a protein, saccharide or lipid shells and a gas filled core. These agents usually contain a high molecular weight gas such as perfluorocarbons. [7;8] Microbubbles have a diameter between 1 and 6 µm, a dimension that permit unimpeded passage through all capillary beds.[9] The advantage of ultrasound contrast agents, in comparison to contrast agents used in magnetic resonance or computed tomography, is that the bubbles behave the same as the red blood cells and do not diffuse out of the vascular space.[2]
Ultrasound waves directed at the vasculature are scattered upon interaction with intravenously infused contrast agents resulting in a bright image of the vasculature. Therefore, using contrast agents with ultrasound permits detection of changes in the microcirculation, improves Doppler imaging in tissues with slow blood flow rates, and provides more detailed information on structure of vasculature during imaging in B-mode. Ultrasound contrast agents have been proven to be safe and free of hemodynamic effects. These characteristics make ultrasound contrast agents ideal for the study of tissue blood flow and microvascular as well as large vessels flow patterns and velocity. [2;10] The current generation of microbubbles have smaller diameters minimizing pulmonary capillary entrapment. This would allow intravenous rather than direct arterial injection of the contrast agent. [10]
To study tissue perfusion, microbubbles are administered as a constant intravenous infusion reaching a steady state within 1 – 2 minutes. Microbubbles in the tissue are then destroyed with a pulse of high energy ultrasound. Subsequent ultrasound imaging and the rate of replenishment of tissue with microbubbles provides information on the velocity of flow and total tissue perfusion. [11;12] Analyzing serial images obtained during this time, a graph is generated depicting changes in video (acoustic) intensity versus time after destruction of microbubbles in the tissue (Figure 1). The slope of this graph (β), represents the velocity of microbubbles (blood) and the plateau of the graph (A) represents tissue blood volume. Since flow of blood in a tissue is the volume of blood moving at a mean velocity, then tissue blood flow would be the product of the two (A × β).
Figure 1.
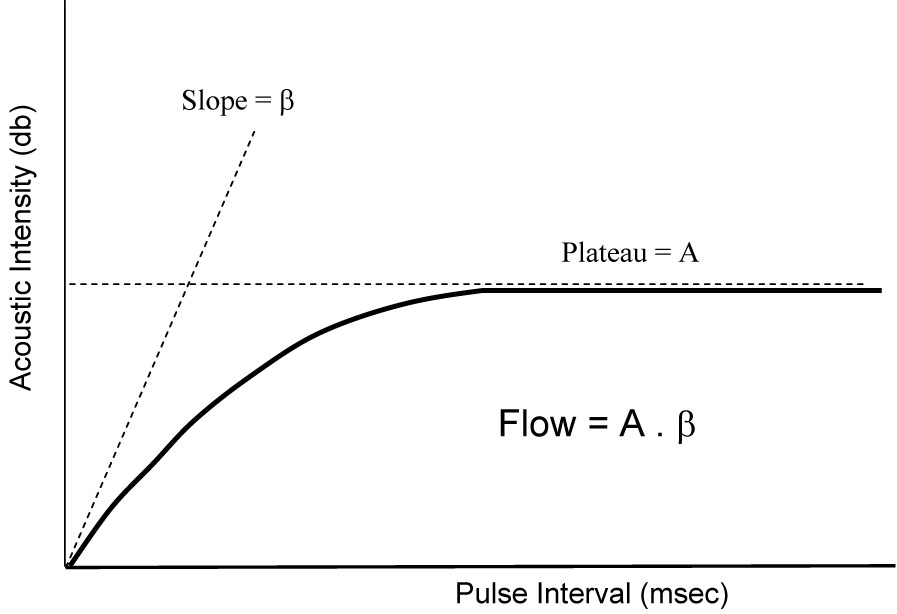
Changes in acoustic intensity versus pulse interval after destruction of microbubbles with high energy ultrasound in the tissue. β or slope of the curve representsthe velocity of blood in the tissue and A or plateau of the curve is tissue blood volume. The product of the two (A*β) represents tissue blood flow.
CEU has been used extensively in cardiology for assessment of myocardial viability and the potential for myocardial recovery after a myocardial infarction [3;4;6], coronary flow reserve after percutaneous angioplasty [5] and also for the detection of coronary artery disease [1;13]. More recently, this technique has been utilized in many different clinical settings such as in the detection of breast cancer [14–16], intestinal ischemia [17;18], peripheral arterial disease [19], aortic disease [20] hepatic circulatory disease and hepatocellular carcinoma [21–25]. Other than diagnostic studies, CEU has been used as a vehicle for targeting genes and chemicals to specific tissues and as a means for the detection of certain tissue specific markers. [26] To achieve these goals microbubbles need to be modified. Lindner et al [27] demonstrated attachment of microbubbles to leukocytes adherent to the endothelial cells in presence of tissue inflammation. This attachment was more pronounced with cationic than anionic microbubbles. Later on, the same authors were able to show greater degree of microbubble attachment to leukocytes within the inflamed venules after incorporation of phosphatidylserine into the microbubble shell. [28] Ligands can be attached to the surface of microbubbles by either a direct covalent bond or through biotin-avidin linking. Monoclonal antibodies, peptides and vitamins could be attached to microbubble surface. It has been shown that ultrasonography using microbubbles can change permeability of cell membrane as well as that of the capillaries depending on the ultrasound settings. [26] Changing ultrasound parameters and local delivery of microbubbles can be used to maximize tissue delivery of drugs. Contrast enhanced ultrasound has been used in diagnostic imaging and as a therapeutic tool in atherosclerosis, thrombosis and malignancies. [26]
Ultrasound Contrast Agents (USCA)
As mentioned previously USCA are microbubbles containing gases with a diameter between 1 – 6 µm that remain in the intravascular space and behave similarly to red blood cells. [29] The size of these bubbles is an important factor. After intravenous injection, they need to pass through pulmonary capillaries (7 µm in diameter) and enter in to the arterial circulation for delivery to tissue.
Technical advances made over the last several years have focused on developing microbubbles with more stable shells and a core filled with gases with lower diffusibility to increase the half life of the microbubbles. The first generation of USCA contained a shell made of denatured albumin and a core filled with air (Albunex®, Molecular Biosystems, USA). Thereafter, combinations of saccharides and palmitic acid (acting as a surfactant) were used for the shell (Levovist®, Schering, Germany). More recent USCA contain perfluoro gases (Definity®, Bristol, Meyers, Squibb; SonoVue®, Bracco, Italy) [9;29] with lower diffusibility compared to air. [9]
Albunex®, the first approved contrast agent in the United States has a half life of less than a minute. Levovist® has a slightly better half life due to the addition of a sugar matrix as well as a small amount of palmitic acid to the shell, making it capable of multiple recirculations. Levovist® was the first contrast agent to be approved for radiology applications in the Europe and Canada. [7] Optison® (Molecular Biosystems & Mallinckrodt, USA) is approved in the U.S., Canada and Europe for cardiac indications. It is very similar to Albunex® except for replacing air with perfluorocarbon gas. This gas is eliminated unchanged by the lungs. It has a half-life greater than 5 minutes. SonoVue® is another type of perflurocarbon-based agent, which includes sulfur hexafluoride. The shell is stabilized with multiple surfactants, such as polyethylene glycol, phospholipids and palmitic acid. It is approved for cardiac and radiologic applications in Europe. [7] Definity® microbubbles are composed of octafluoropropane encapsulated in an outer lipid shell. Similar to SonoVue® and Optison®, it has a half-life longer than 5 minutes. Definity® has been approved in the United States for cardiac indications.
Extensive studies of these agents have demonstrated a significant safety profile with no evidence of toxicity to the kidneys, heart or liver. Minor adverse events, such as mild hypotension, dizziness, flushing and a transient rash have been reported in some studies. Rare cases of anaphylactic reactions have also been reported. Therefore close monitoring of subjects during imaging is recommended.
CEU in the study of kidney disease
Ultrasonography of the kidneys is limited because of the low resolution of the B-mode for kidneys and also because of low sensitivity of color Doppler images for smaller arteries and arterioles, especially in deep sections of the renal medulla. Other techniques, such as contrast enhanced computerized tomography and magnetic resonance, also have limitations related to their nature and their toxicities. CEU is potentially valuable tool in the study of both micro and macrovasculature within the kidneys and in the detection of renal artery stenosis, renal perfusion defects and possibly alterations in renal blood flow due to physiologic and pharmacologic stimuli. Another advantage of CEU over other techniques is its ability to provide information on regional tissue blood flow (see below). In addition, CEU may have other indications, such as the characterization of indeterminate lesions or acute or chronic rejection in renal transplantation. [30;31]
A controversial field in the study of pathogenesis of certain kidney diseases, especially diabetic kidney disease, is the role of hemodynamic changes, i.e. glomerular hyperfiltration and loss of glomerular autoregulation mechanism, as opposed to hormonal changes and inflammatory pathways. Autoregulation of GFR has been shown to be impaired or abolished in both type 1 and type 2 diabetes.[32;33] The remnant kidney animal model, in which 5/6 of the total renal parenchyma is removed, results in progressive sclerosis of renal glomeruli and proteinuria similar to what is seen in progressive human kidney disease.[34] This model demonstrates the role of glomerular hyperfiltration in development of glomerular sclerosis. However, there are multiple other pathogenic mechanisms that have been shown to play major roles in the development of pathologic changes in diabetic nephropathy. These include, activated renin angiotensin system, accumulation of advanced glycation endproducts, activation of protein kinase C, increased production of inflammatory cytokines and changes in production of growth factors among many other mechanisms. [35] Therefore, a noninvasive tool for monitoring of renal hemodynamic changes during early stages of renal disease and as it progresses to more advances stages will be a valuable tool for in vivo studies of these pathogenic mechanisms.
Timely diagnosis of acute kidney injury (AKI) amenable to therapeutic intervention is critical. Thus far clinical trials have not conclusively shown beneficial effects in the treatment of AKI in part due to inability to differentiate prerenal azotemia from acute tubular necrosis. [36] Currently, the differentiation between acute tubular necrosis and prerenal azotemia is based upon urine output, urinary sodium excretion and rise in BUN and creatinine. These are considered poor markers of kidney function and fail to differentiate between the causes of AKI in many occasions. Assessing total and regional renal blood flow by CEU early in the course of AKI could be a valuable diagnostic and prognostic tool. Appropriate therapy can be delivered early and potentially attenuate or reverse AKI.
1. Renal Blood Flow
Schlosser et al [12] used CEU (Definity®) to study renal blood flow in pigs. In that study, CEU was used to study both macrovascular (main renal artery and larger vessels) and microvascular (renal cortical) blood flow. The authors found significantly higher blood velocities in the main vasculature compared to microvessels of the cortical region.[12]
In another study, Wei et al [11], used CEU (SonoVue®) to study changes in renal blood flow in response to flow limiting blockade in the renal arteries and infusion of dopamine in dogs. Tissue blood flow as obtained by CEU showed a significant rise after dopamine administration and a significant drop after occlusion of the main renal artery. These findings correlated very well with the renal artery flow obtained by a flow probe placed directly over the renal artery (r = 0.82, p< 0.001). [11]
Hosotani et al [37] compared renal blood flow obtained by CEU, radionuclide scanning using technetium-99m mercaptoacetyltriglycine (Tc-99m MAG3) and para-aminohippurate (PAH) in 16 patients with chronic kidney disease of various causes. To determine renal blood flow by CEU, Levovist® was injected intravenously and allowed to reach steady state. After destruction of microbubbles with a pulse of ultrasound images were obtained first at an interval (PI) of 10 cardiac cycles and then at PI of every cardiac cycle. Since the flow of blood is unchanged, less frequent pulsing would allow more time for replenishment of the tissue with microbubbles and as a result higher intensity of images compared to more frequent pulsing (every cardiac cycle). This reduction in image intensity after changing from 10 to 1 cardiac cycle (decline ratio, DR) in each kidney and average of the values for the left and right kidney were used as an estimate of split and total renal blood flow, respectively. [37] Using this technique, the authors were able to demonstrate a significant correlation (r = 0.69, p= 0.005) between average DR by CEU and renal blood flow determined by PAH clearance. The individual kidney DR also correlated well with MAG3 split renal blood flow values (r = 0.67, p< 0.005). [37]
Currently at the University of Virginia General Clinical Research Center we are conducting a study comparing CEU using Definity® , PAH clearance and renal artery Doppler flow to assess changes in renal blood flow in response to physiologic stimuli in healthy individuals. After intravenous injection of Definity®, a steady state is achieved in 1 to 2 minutes. As mentioned before, the microbubbles are destroyed in the tissue with a pulse of high energy ultrasound and then during continuous imaging, microbubble replenishment pattern is used to estimate renal blood flow. During this time, a nonlinear correlation between pulse interval and image intensity is used to determine blood velocity (slope of the reappearance curve), tissue blood volume (plateau of the graph) and the product of the two (A*β) to determine tissue blood flow. Using QLab software (Philips, USA), regions of interest are placed in the renal cortex, medulla and the whole kidney to determine regional and total renal blood flows. Subjects consume a meal with a high animal protein content (1.5 g/kg body weight) to increase renal blood flow. Ultrasound imaging using Definity® is repeated again 2 hours after ingestion of the high protein meal. The total duration of Definity® infusion for each set of images ranges between 3 – 5 minutes. To date 12 subjects have been studied and preliminary results suggest a good correlation between different modalities of determining RBF. Blood flow in the main renal artery as measured by Doppler ultrasound increased by a mean of 38%from baseline 2-hours after ingestion of a high protein meal. At the same time the changes from baseline in blood flow velocity (β), tissue blood volume (A) and tissue blood flow (A*β) were 16%, 36% and 56%, respectively. From 12 subjects enrolled, only one individual showed a brief and mild episode of flushing which resolved spontaneously.
2. Renal Artery Stenosis
Atherosclerosis is responsible for almost 90% of cases of renal artery stenosis. The incidence of atherosclerotic renal artery stenosis (ARAS) increases with age and in presence of peripheral arterial and coronary artery disease, diabetes, dyslipidemia and hypertension. The prevalence of ARAS varies depending upon the defining criteria, age, risk factors and imaging versus autopsy studies. [38] In an autopsy case series, ARAS was detected in more than 8% of individuals with diabetes and more than 10% of those with both hypertension and diabetes. [39] Although a high percentage (53%) of cases with severe degree of stenosis (80%) progress to occlusion [40], The rate of progression in cases with less severe stenosis is between 18% to 30% in 3 years. [41] The risk of renal atrophy in two years is between 5% and 20%, depending on the degree of stenosis at baseline. [42]
Many patients with ARAS are asymptomatic. Due to adverse events, such as risk of atheroembolic disease, contrast nephropathy, complete occlusion and restenosis associated with angioplasty, selection of the appropriate patient for diagnostic work up and treatment is critical. [38] Currently, digital subtraction renal angiography is considered the gold standard diagnostic test; however, it is seldom used because of its invasive nature and the risk of contrast nephropathy. This renders this test unsuitable in cases with reduced kidney function. Other imaging techniques include Doppler ultrasound, magnetic resonance angiography, carbon dioxide angiography, contrast enhanced computerized tomography and radionuclide scanning before and after an angiotensin converting enzyme inhibitor. These diagnostic modalities are each associated with practical and safety issues. Captopril renal scan is not recommended for the elderly, requires scanning at two separate occasions and is expensive. MR scanning using gadolinium in patients with kidney disease has been shown to be associated with the devastating condition, nephrogenic systemic fibrosis. [43;44] Although Duplex ultrasonography has been shown to have sensitivity and specificity as high as 98% in detection of ARAS in some series [45–49], being operator dependent, time consuming and associated with a higher than desired technical failure rates limits its use as well. Factors such as obesity, bowel gas, depth, tortuosity and number of renal arteries, and the presence of nephropathy limit the utility of this technique. [50;51]
More recently CEU has been utilized in diagnosis of ARAS. [52–54] In a European multi-center study by Claudon et al [52], 198 individuals suspicious of ARAS underwent Doppler ultrasound and Levovist®-enhanced Doppler ultrasound in random order before undergoing renal angiography. CEU resulted in a 20% increase in the number of renal arteries that were thought to be assessable by Doppler examination (from 63.9% to 83.8%, p = 0.001). These improvements were more obvious in obese individuals and those with reduced kidney function. [52] The feasibility of nonenhanced Doppler ultrasound varied significantly between centers and this variability was reduced after using the contrast agent. Enhanced Doppler ultrasound had a significantly higher degree of agreement with angiography than nonenhanced Doppler (70.2% vs. 50.3%, p = 0.001). [52] Similarly, in a study of patients suspicious of ARAS [53] after addition of a contrast agent (Levovist®), Missouris and colleagues demonstrated a 20 db increase in Doppler intensity. Addition of contrast agent improved sensitivity and specificity of Doppler ultrasound in diagnosis of ARAS from 85% to 94% and 79% to 88%, respectively. At the same time the examination time was cut almost in half. [53]
3. Kidney Transplantation
Schwenger and colleagues [55] compared color Doppler ultrasound and CEU in the diagnosis of chronic allograft nephropathy (CAN) in 26 outpatients with a kidney transplant and a stable renal function. While resistance and pulsatility indices obtained by Doppler ultrasound did not show any correlation with kidney function (serum creatinine or GFR), renal blood flow determined by CEU (A × β) demonstrated a significant correlation with serum creatinine (r = −0.62, p = 0.0004) and GFR (r = 0.49, p= 0.007). [55] Fifty percent of the study subjects had evidence of CAN on their biopsy samples. Among these individuals, no correlation between biopsy results and either serum creatinine or Doppler ultrasound findings was found. However, the value of renal blood flow determined by CEU was significantly lower for individuals with evidence of CAN on the biopsy than those without it (3.75 versus 8.25 dB/s, p< 0.001). Setting a threshold of 5.79 dB/s for renal blood flow, the authors found sensitivity, specificity and diagnostic accuracy of 82%, 64% and 73%, respectively for CEU in diagnosing CAN. [55]
Summary
CEU is a safe and relatively noninvasive imaging technique suitable for study of large and small vessels and determination of tissue perfusion. It is simple and easy to perform in any clinical setting. CEU has been used extensively in fields such as cardiology and hepatology. This technique is promising for studies of kidney structure and function because of its safety profile and its ability to provide information on single kidney as well as regional blood flow. It has been used to determine changes in RBF in response to physiologic and pharmacologic interventions and to study disease states such as renal artery stenosis and post kidney transplantation.
Figure 2.
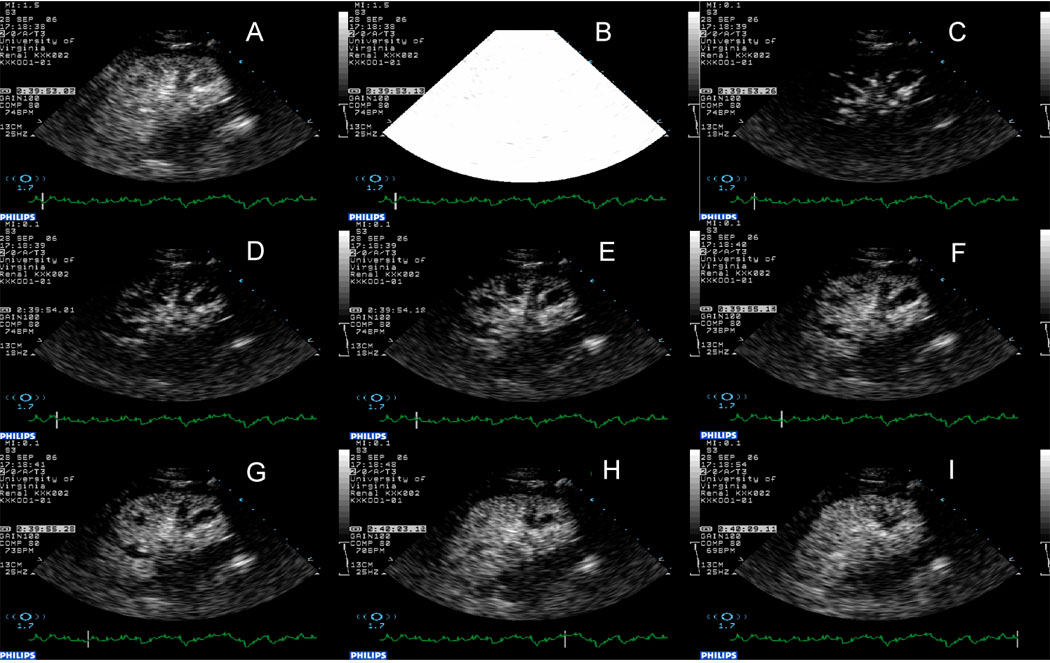
Assessment of renal blood flow using CEU. Panel A, steady state; Panel B, destruction of microbubbles in the tissue using high energy ultrasound. Panels C through I, replenishment of the tissue with microbubbles. Note, almost immediate appearance of microbubbles in the main arteries followed by cortex and finally the medulla.
Acknowledgement
This work was supported in part by funds from the National Institutes of Health to the University of Virginia General Clinical Research Center, M01 RR00847 and DK 074616, RO1DK56223, RO1 DK62324, RO1 DK065957.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaul S, Senior R, Dittrich H, Raval U, Khattar R, Lahiri A. Detection of coronary artery disease with myocardial contrast echocardiography: comparison with 99mTc-sestamibi single-photon emission computed tomography. Circulation. 1997;96:785–792. [PubMed] [Google Scholar]
- 2.Lindner J, Wei K. Contrast Echocardiography. Curr Probl Cardiol. 2002;27:449–520. doi: 10.1067/mcd.2002.129364. (abstract) [DOI] [PubMed] [Google Scholar]
- 3.Swinburn JM, Lahiri A, Senior R. Intravenous myocardial contrast echocardiography predicts recovery of dysynergic myocardium early after acute myocardial infarction. J Am Coll Cardiol. 2001;38:19–25. doi: 10.1016/s0735-1097(01)01317-1. [DOI] [PubMed] [Google Scholar]
- 4.Ragosta M, Camarano G, Kaul S, Powers ER, Sarembock IJ, Gimple LW. Microvascular integrity indicates myocellular viability in patients with recent myocardial infarction. New insights using myocardial contrast echocardiography. Circulation. 1994;89:2562–2569. doi: 10.1161/01.cir.89.6.2562. [DOI] [PubMed] [Google Scholar]
- 5.Lepper W, Hoffmann R, Kamp O, Franke A, de Cock CC, Kuhl HP, Sieswerda GT, Dahl J, Janssens U, Voci P, Visser CA, Hanrath P.Assessment of myocardial reperfusion by intravenous myocardial contrast echocardiography and coronary flow reserve after primary percutaneous transluminal coronary angioplasty Circulation 20001012368–2374. Notes: in patients with acute myocardial infarction. [DOI] [PubMed] [Google Scholar]
- 6.Balcells E, Powers ER, Lepper W, Belcik T, Wei K, Ragosta M, Samady H, Lindner JR. Detection of myocardial viability by contrast echocardiography in acute infarction predicts recovery of resting function and contractile reserve. J Am Coll Cardiol. 2003;41:827–833. doi: 10.1016/s0735-1097(02)02962-5. [DOI] [PubMed] [Google Scholar]
- 7.Jakobsen JA, Correas JM. Ultrasound contrast agents and their use in urogenital radiology: status and prospects. Eur Radiol. 2001;11:2082–2091. doi: 10.1007/s003300000817. [DOI] [PubMed] [Google Scholar]
- 8.Jakobsen JA. Ultrasound contrast agents: clinical applications. Eur Radiol. 2001;11:1329–1337. doi: 10.1007/s003300100964. [DOI] [PubMed] [Google Scholar]
- 9.Correas JM, Bridal L, Lesavre A, Mejean A, Claudon M, Helenon O. Ultrasound contrast agents: properties, principles of action, tolerance, and artifacts. Eur Radiol. 2001;11:1316–1328. doi: 10.1007/s003300100940. [DOI] [PubMed] [Google Scholar]
- 10.Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of Definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr. 2002;15:396–403. doi: 10.1067/mje.2002.117290. [DOI] [PubMed] [Google Scholar]
- 11.Wei K, Le E, Bin JP, Coggins M, Thorpe J, Kaul S. Quantification of renal blood flow with contrast-enhanced ultrasound. J Am Coll Cardiol. 2001;37:1135–1140. doi: 10.1016/s0735-1097(00)01210-9. [DOI] [PubMed] [Google Scholar]
- 12.Schlosser T, Pohl C, Veltmann C, Lohmaier S, Goenechea J, Ehlgen A, Koster J, Bimmel D, Kuntz-Hehner S, Becher H, Tiemann K. Feasibility of the flash-replenishment concept in renal tissue: which parameters affect the assessment of the contrast replenishment? Ultrasound Med Biol. 2001;27:937–944. doi: 10.1016/s0301-5629(01)00397-0. [DOI] [PubMed] [Google Scholar]
- 13.Sobkowicz B, Tomaszuk-Kazberuk A, Malyszko J, Kalinowski M, Hryszko T, Kralisz P, Dobrzycki S, Malyszko J, Mysliwiec M, Musial WJ. Value of the real-time myocardial contrast echocardiography for risk stratification and for the detection of significant coronary stenosis in patients with end-stage renal disease. Nephrol Dial Transplant. 2007;22:668–669. doi: 10.1093/ndt/gfl575. [DOI] [PubMed] [Google Scholar]
- 14.Forsberg F, Piccoli CW, Merton DA, Palazzo JJ, Hall AL. Breast Lesions: Imaging with Contrast-enhanced Subharmonic US Initial Experience. Radiology. 2007 doi: 10.1148/radiol.2443061588. [DOI] [PubMed] [Google Scholar]
- 15.van Esser S, Veldhuis WB, van Hillegersberg R, van Diest PJ, Stapper G, ElOuamari M, Borel Rinkes IH, Mali WP, van den Bosch MA. Accuracy of contrast-enhanced breast ultrasound for pre-operative tumor size assessment in patients diagnosed with invasive ductal carcinoma of the breast. Cancer Imaging. 2007;7:63–68. doi: 10.1102/1470-7330.2007.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassano E, Rizzo S, Bozzini A, Menna S, Bellomi M. Contrast enhanced ultrasound of breast cancer. Cancer Imaging. 2006;6:4–6. doi: 10.1102/1470-7330.2006.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hata J, Kamada T, Haruma K, Kusunoki H. Evaluation of bowel ischemia with contrast-enhanced US: initial experience. Radiology. 2005;236:712–715. doi: 10.1148/radiol.2362040299. [DOI] [PubMed] [Google Scholar]
- 18.Hamada T, Yamauchi M, Tanaka M, Hashimoto Y, Nakai K, Suenaga K. Prospective evaluation of contrast-enhanced ultrasonography with advanced dynamic flow for the diagnosis of intestinal ischaemia. Br J Radiol. 2007 doi: 10.1259/bjr/59793102. [DOI] [PubMed] [Google Scholar]
- 19.Leiner T, Kessels AG, Nelemans PJ, Vasbinder GB, de Haan MW, Kitslaar PE, Ho KY, Tordoir JH, van Engelshoven JM. Peripheral arterial disease: comparison of color duplex US and contrast-enhanced MR angiography for diagnosis. Radiology. 2005;235:699–708. doi: 10.1148/radiol.2352040089. [DOI] [PubMed] [Google Scholar]
- 20.Abdulmalik A, Cohen G. The Use of Echocardiographic Contrast-Enhanced Rapid Diagnosis of Ruptured Aortic Dissection with Transthoracic Echocardiography. J Am Soc Echocardiogr. 2007 doi: 10.1016/j.echo.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Lu MD, Yu XL, Li AH, Jiang TA, Chen MH, Zhao BZ, Zhou XD, Wang JR. Comparison Of Contrast Enhanced Ultrasound And Contrast Enhanced Ct Or Mri In Monitoring Percutaneous Thermal Ablation Procedure In Patients With Hepatocellular Carcinoma: A Multi-Center Study In China. Ultrasound Med Biol. 2007 doi: 10.1016/j.ultrasmedbio.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Chen MH, Wu W, Yang W, Dai Y, Gao W, Yin SS, Yan K. The use of contrast-enhanced ultrasonography in the selection of patients with hepatocellular carcinoma for radio frequency ablation therapy. J Ultrasound Med. 2007;26:1055–1063. doi: 10.7863/jum.2007.26.8.1055. [DOI] [PubMed] [Google Scholar]
- 23.Ridolfi F, Abbattista T, Marini F, Vedovelli A, Quagliarini P, Busilacchi P, Brunelli E. Contrast-enhanced ultrasound to evaluate the severity of chronic hepatitis C. Dig Liver Dis. 2007 doi: 10.1016/j.dld.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 24.Albrecht T, Oldenburg A, Hohmann J, Skrok J, Hoffmann CW, Schettler S, Wolf KJ. Imaging of liver metastases with contrast-specific low-MI real-time ultrasound and SonoVue. Eur Radiol. 2003;13 Suppl 3:N79–N86. doi: 10.1007/s00330-003-0012-2. [DOI] [PubMed] [Google Scholar]
- 25.Hohmann J, Albrecht T, Hoffmann CW, Wolf KJ. Ultrasonographic detection of focal liver lesions: increased sensitivity and specificity with microbubble contrast agents. Eur J Radiol. 2003;46:147–159. doi: 10.1016/s0720-048x(02)00053-0. [DOI] [PubMed] [Google Scholar]
- 26.Ferrara K, Pollard R, Borden M. Ultrasound microbubble contrast agents: fundamentals and application to gene and drug delivery. Annu Rev Biomed Eng. 2007;9:415–447. doi: 10.1146/annurev.bioeng.8.061505.095852. [DOI] [PubMed] [Google Scholar]
- 27.Lindner JR, Coggins MP, Kaul S, Klibanov AL, Brandenburger GH, Ley K. Microbubble persistence in the microcirculation during ischemia/reperfusion and inflammation is caused by integrin- and complement-mediated adherence to activated leukocytes. Circulation. 2000;101:668–675. doi: 10.1161/01.cir.101.6.668. [DOI] [PubMed] [Google Scholar]
- 28.Lindner JR, Dayton PA, Coggins MP, Ley K, Song J, Ferrara K, Kaul S. Noninvasive imaging of inflammation by ultrasound detection of phagocytosed microbubbles. Circulation. 2000;102:531–538. doi: 10.1161/01.cir.102.5.531. [DOI] [PubMed] [Google Scholar]
- 29.Cosgrove D. Ultrasound contrast agents: an overview. Eur J Radiol. 2006;60:324–330. doi: 10.1016/j.ejrad.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Correas JM, Claudon M, Tranquart F, Helenon AO. The kidney: imaging with microbubble contrast agents. Ultrasound Q. 2006;22:53–66. [PubMed] [Google Scholar]
- 31.Nilsson A. Contrast-enhanced ultrasound of the kidneys. Eur Radiol. 2004;14 Suppl 8:P104–P109. [PubMed] [Google Scholar]
- 32.Parving HH, Kastrup H, Smidt UM, Andersen AR, Feldt-Rasmussen B, Christiansen JS. Impaired autoregulation of glomerular filtration rate in type 1 (insulin-dependent) diabetic patients with nephropathy. Diabetologia. 1984;27:547–552. doi: 10.1007/BF00276965. [DOI] [PubMed] [Google Scholar]
- 33.Christensen PK, Hansen HP, Parving HH. Impaired autoregulation of GFR in hypertensive non-insulin dependent diabetic patients. Kidney Int. 1997;52:1369–1374. doi: 10.1038/ki.1997.463. [DOI] [PubMed] [Google Scholar]
- 34.Fogo AB. Progression and potential regression of glomerulosclerosis. Kidney Int. 2001;59:804–819. doi: 10.1046/j.1523-1755.2001.059002804.x. [DOI] [PubMed] [Google Scholar]
- 35.Schrijvers BF, De Vriese AS, Flyvbjerg A. From hyperglycemia to diabetic kidney disease: the role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr Rev. 2004;25:971–1010. doi: 10.1210/er.2003-0018. [DOI] [PubMed] [Google Scholar]
- 36.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven't worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2:356–365. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- 37.Hosotani Y, Takahashi N, Kiyomoto H, Ohmori K, Hitomi H, Fujioka H, Aki Y, Fukunaga M, Yuasa S, Mizushige K, Kohno M. A new method for evaluation of split renal cortical blood flow with contrast echography. Hypertens Res. 2002;25:77–83. doi: 10.1291/hypres.25.77. [DOI] [PubMed] [Google Scholar]
- 38.Zalunardo N, Tuttle KR. Atherosclerotic renal artery stenosis: current status and future directions. Curr Opin Nephrol Hypertens. 2004;13:613–621. doi: 10.1097/00041552-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Sawicki PT, Kaiser S, Heinemann L, Frenzel H, Berger M. Prevalence of renal artery stenosis in diabetes mellitus--an autopsy study. J Intern Med. 1991;229:489–492. doi: 10.1111/j.1365-2796.1991.tb00382.x. [DOI] [PubMed] [Google Scholar]
- 40.Tollefson DF, Ernst CB. Natural history of atherosclerotic renal artery stenosis associated with aortic disease. J Vasc Surg. 1991;14:327–331. [PubMed] [Google Scholar]
- 41.Caps MT, Perissinotto C, Zierler RE, Polissar NL, Bergelin RO, Tullis MJ, Cantwell-Gab K, Davidson RC, Strandness DE., Jr Prospective study of atherosclerotic disease progression in the renal artery. Circulation. 1998;98:2866–2872. doi: 10.1161/01.cir.98.25.2866. [DOI] [PubMed] [Google Scholar]
- 42.Caps MT, Zierler RE, Polissar NL, Bergelin RO, Beach KW, Cantwell-Gab K, Casadei A, Davidson RC, Strandness DE., Jr Risk of atrophy in kidneys with atherosclerotic renal artery stenosis. Kidney Int. 1998;53:735–742. doi: 10.1046/j.1523-1755.1998.00805.x. [DOI] [PubMed] [Google Scholar]
- 43.Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242:647–649. doi: 10.1148/radiol.2423061640. [DOI] [PubMed] [Google Scholar]
- 44.Perazella MA, Rodby RA. Gadolinium use in patients with kidney disease: a cause for concern. Semin Dial. 2007;20:179–185. doi: 10.1111/j.1525-139X.2007.00269.x. [DOI] [PubMed] [Google Scholar]
- 45.Hansen KJ, Tribble RW, Reavis SW, Canzanello VJ, Craven TE, Plonk GW, Jr, Dean RH. Renal duplex sonography: evaluation of clinical utility. J Vasc Surg. 1990;12:227–236. doi: 10.1067/mva.1990.22791. [DOI] [PubMed] [Google Scholar]
- 46.Strandness DE., Jr Duplex imaging for the detection of renal artery stenosis. Am J Kidney Dis. 1994;24:674–678. doi: 10.1016/s0272-6386(12)80230-7. [DOI] [PubMed] [Google Scholar]
- 47.Olin JW, Piedmonte MR, Young JR, DeAnna S, Grubb M, Childs MB. The utility of duplex ultrasound scanning of the renal arteries for diagnosing significant renal artery stenosis. Ann Intern Med. 1995;122:833–838. doi: 10.7326/0003-4819-122-11-199506010-00004. [DOI] [PubMed] [Google Scholar]
- 48.van der Hulst VP, van Baalen J, Kool LS, van Bockel JH, van Erkel AR, Ilgun J, Pattynama PM. Renal artery stenosis: endovascular flow wire study for validation of Doppler US. Radiology. 1996;200:165–168. doi: 10.1148/radiology.200.1.8657905. [DOI] [PubMed] [Google Scholar]
- 49.Drelich-Zbroja A, Jargiello T, Drelich G, Lewandowska-Stanek H, Szczerbo-Trojanowska M. Renal artery stenosis: value of contrast-enhanced ultrasonography. Abdom Imaging. 2004;29:518–524. doi: 10.1007/s00261-003-0125-8. [DOI] [PubMed] [Google Scholar]
- 50.Desberg AL, Paushter DM, Lammert GK, Hale JC, Troy RB, Novick AC, Nally JV, Jr, Weltevreden AM. Renal artery stenosis: evaluation with color Doppler flow imaging. Radiology. 1990;177:749–753. doi: 10.1148/radiology.177.3.2243982. [DOI] [PubMed] [Google Scholar]
- 51.Postma CT, van Aalen J, de Boo T, Rosenbusch G, Thien T. Doppler ultrasound scanning in the detection of renal artery stenosis in hypertensive patients. Br J Radiol. 1992;65:857–860. doi: 10.1259/0007-1285-65-778-857. [DOI] [PubMed] [Google Scholar]
- 52.Claudon M, Plouin PF, Baxter GM, Rohban T, Devos DM. Renal arteries in patients at risk of renal arterial stenosis: multicenter evaluation of the echo-enhancer SH U 508A at color and spectral Doppler US. Levovist Renal Artery Stenosis Study Group. Radiology. 2000;214:739–746. doi: 10.1148/radiology.214.3.r00fe02739. [DOI] [PubMed] [Google Scholar]
- 53.Missouris CG, Allen CM, Balen FG, Buckenham T, Lees WR, MacGregor GA. Non-invasive screening for renal artery stenosis with ultrasound contrast enhancement. J Hypertens. 1996;14:519–524. [PubMed] [Google Scholar]
- 54.Lencioni R, Pinto S, Cioni D, Bartolozzi C. Contrast-Enhanced Doppler Ultrasound of Renal Artery Stenosis: Prologue to a Promising Future. Echocardiography. 1999;16:767–773. doi: 10.1111/j.1540-8175.1999.tb00148.x. [DOI] [PubMed] [Google Scholar]
- 55.Schwenger V, Korosoglou G, Hinkel UP, Morath C, Hansen A, Sommerer C, Dikow R, Hardt S, Schmidt J, Kucherer H, Katus HA, Zeier M. Real-time contrast-enhanced sonography of renal transplant recipients predicts chronic allograft nephropathy. Am J Transplant. 2006;6:609–615. doi: 10.1111/j.1600-6143.2005.01224.x. [DOI] [PubMed] [Google Scholar]


