Abstract
Group B streptococcus (GBS) is one of the leading causes of neonatal infection; however the molecular mechanisms involved are not clearly known. Here we used high and low hemolytic GBS isolates and mutant GBS that lacks β-hemolysin expression and showed that GBS infection or exposure to GBS-hemolysin extract induces primary human trophoblast, placental fibroblast and JEG3 trophoblast cell line death, and that GBS-induced trophoblast death was β-hemolysin dependent. The fibroblasts and trophoblasts provide an innate immune barrier between fetal and maternal circulation in the placenta. These data suggest that GBS may disrupt this barrier to invade fetal circulation.
Keywords: Streptococcus agalactiae, placenta, trophoblast, Toll like receptors, apoptosis, cell death, preterm delivery, GBS, β-hemolysin, invasion, pregnancy
Introduction
GBS plays an important role in neonatal infection and preterm delivery [1]; however the microbial factors involved in the pathogenesis are not clearly known.
Trophoblasts are the outermost cell layer of the blastocyst. Once attached into the uterine wall, trophoblasts invade and proliferate into the decidua to develop the placenta and provide the barrier between the maternal and fetal circulation. Placental fibroblasts provide a supportive cellular network and play an important role for placental development. Currently there are no data on the effect of GBS infection on trophoblast or placental fibroblast survival.
GBS induced host cell death was suggested to be important for the initiation of infection, bacterial survival, and suppression of the host immune responses [2–4]. Here we hypothesized that GBS infection may induce cell death in the placenta and this may play a role in microbial invasion and ultimately fetal and maternal infection and preterm delivery.
We exposed the JEG3 human trophoblast cell line and primary human placental trophoblasts and fibroblasts to GBS isolates with various hemolytic activity and showed that GBS infection and exposure to GBS extract are toxic to human trophoblasts and fibroblasts and that GBS β-hemolysin plays the key role in GBS toxicity.
Materials and Methods
Cells Lines and Reagents
JEG3human trophoblast cell line was obtained from American Type Tissue Culture Collection (Manaaas, VA) and cultured in MEM (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum, 10 mM HEPES, 1 mM sodium pyruvate and 100 nM of penicillin/streptomycin (Invitrogen Life Technologies). Antibiotic free media was used during the attachment phase of the infection experiments.
Isolation and culture of primary trophoblasts
Trophoblasts were isolated from placentas obtained according to NIH, CSMC and Planned Parenthood IRB protocols. Briefly, tissue specimens were washed with cold HBSS (Invitrogen Life Technologies) to remove excess blood. Cells were scraped from the membranes, transferred to trypsin-EDTA (Invitrogen Life Technologies, Carlsbad, CA) digestion buffer and incubated at 37°C for 10 min with shaking. An equal volume of DMEM medium (Invitrogen Life Technologies) containing 10% FBS was added to inactivate the trypsin. This mixture was vortexed for 20 seconds, allowed to sediment, and the supernatant was collected. This was repeated twice and the collected supernatant was centrifuged at 1500 rpm for 10 min. Contaminating RBC were removed by resuspending the cellular pellet with HBSS, layering this over the same volume of Lymphocyte Separation Media (ICN Biomedicals, Aurora, OH), and centrifuging at 2000 rpm for 25 min. The cellular interface containing the trophoblast cells was collected and resuspended in DMEM supplemented with 10% normal human serum (Gemini Bio-Products, Woodland, CA) and cultured at 37°C/5% CO2 for three passages. This technique provides >98% pure trophoblast cells.
Isolation and culture of human placenta fibroblast
Primary fibroblasts were isolated from human placentas obtained from consenting fullterm-pregnant healthy women who underwent elective cesarean section according to the CSMC IRB protocol. Minced placenta was washed in sterile Phosphate Buffer Saline (PBS), trypsinized in 0.25 Trypsin (Gibco) at 37°C for 3 hours and centrifuged at 2000 rpm for 10 minutes at room temperature. The supernatant was removed and the cells were resuspended in Dulbecco Modified Essential Medium (DMEM) with 4 g/ml of glucose (Gibco), 20 % fetal Bovine serum and 1% antibiotics. The cells were plated in 100 mm Petri dishes and cultured at 37°C. The fibroblasts adhered at the bottom of the plate. The nonadherent nonfibroblast cells were washed and removed. Passage 10 cells were used for the studies.
Bacterial strains and culture conditions
We used wild-type clinical GBS isolates: highly encapsulated type III strain COH1; which has low hemolysin activity [5, 6] and highly hemolytic WT strain NCTC10/84 (1169-NT1), a serotype V isolated from the blood of a septic neonate [7]. For preparing the extract containing β-h/c (hemolysin/cytolysin) activity, the low hemolysin COH1, the highly hemolytic WT strain NCTC10/84 and the corresponding nonhemolytic, nonpigmented isogenic allelic exchange mutant NCTC:cylEΔcat (NCTC10/84M )were used [8].
Preparation of GBS Hemolysin Extracts
NCTC10/84 WT, COH1 and NCTC10/84M GBS strains were grown in Todd-Hewitt broth (THB, Difco) at 37°C overnight, diluted to 1/20 in 200mL of THB, and grown to mid-log phase (OD600=0.4=108cfu/mL). All strains grew equally well under the conditions used in these experiments. The bacteria (2×1010cfu) were harvested and incubated for 2 hours at 37°C in PBS containing 2% starch and 1% glucose. The supernatant was collected and filter sterilized, and cold 100% methanol was added (1:1) to precipitate the hemolysin. Extracts were incubated at −20°C overnight or at −80°C for 1 hour, and re-suspended in 2mL of PBS. The amounts of hemolysin extracts used in the exposure assays, 2, 1.5 and 0.5 µL corresponded to hemolysin extract from 2×107, 1.3×107 and 5×106 cfu respectively [2].
Hemolysin Activity Assay
Serial two-fold dilutions of 5µL of β-hemolysin extract with PBS were prepared, starting with a 1:40 dilution. An equal volume of 1% sheep erythrocytes in PBS was added. Solutions were incubated 1 hour at 37°C/5% CO2, then 30 minutes at 4°C. PBS glucose alone and lysed RBC in 1% SDS were used for negative and positive control respectively. Hemolytic titer of a given strain was determined by the reciprocal of the greatest dilution producing 50% of the hemoglobin release compared with the SDS positive control. The hemolytic titer of NCTC10/84 WT was 1280.
GBS infection
Assays for GBS infection was performed essentially as described before [2]. Briefly, GBS strains, COH1 and NCTC10/84 were grown in THB to mid-log phase (~ 108 CFU/ml; OD600=0.4), washed in PBS, resuspended in RPMI with 10% FBS, and used to infect trophoblast cell line and primary fibroblasts. Plates were centrifuged at 700g for 5 min to settle the bacteria on the monolayer surface then incubated at 37°C in 5% CO2 for 1 hour. To kill extracellular bacteria cells were washed 3x with sterile PBS and incubated with media containing gentamicin (100 ug/ml) and penicillin (5 ug/ml). The concentration and purity of each GBS inoculum was confirmed by quantitative culture on THB agar plates.
Lactate Dehydrogenase (LDH) Assay
Cell death was assessed by measuring the supernatant lactate dehydrogenase levels by performing colorimetric lactate dehydrogenase (LDH) assay (Roche Diagnostics) according to the manufacturer’s instructions. The data is reported as the percent of maximum LDH released upon Triton X-100 treatment of cells.
FACS analysis of apoptosis
Annexin V is a Ca2+-dependent protein with a strong affinity for phosphatidylserine (PS), which is externalized in the early stages of apoptosis. In non-apoptotic cells, PS is present only on the inner surface of the membrane and false positive results may occur if the membrane is damaged, as occurs in necrotic cells. Cell damage and false positive PS labeling can be assessed by staining with propidium iodide which does not enter an intact cell. We assessed apoptosis by incubating cells with FITC-conjugated annexin-V (Roche Molecular Biochemicals, Indianapolis, IN). Labeling procedures followed those suggested by the manufacturer's manual. Briefly, cells were resuspended in annexin labeling solution containing 10 mM HEPES (pH 7.4), 140 mM NaCl, 5 mM CaCl2, and fluorescein-conjugated annexin V for 15 min. After being washed twice with PBS, cell pellets were resuspended in PI (propidium iodine) (2 µg/ml) containing PBS and analyzed by flow cytometry. Apoptosis was presented as percent positive cells stained with Annexin-V. Necrotic cells were presented as the percent positive cells stained with PI.
Statistical Analysis
The experiments were set up in triplicate and were repeated on at least three separate occasions. For LDH assays, the average LDH measurement of wells from each treatment was divided by the LDH value of the lysis control to calculate a percentage of total cell lysis LDH secreted, as recommended by the manufacturer. Error bars represent the 95% confidence intervals calculated using the standard deviation. Student’s t-test was used to compare the means between media and treatment groups. p-value <0.05 was reported as statistically significant.
Results
Exposure to GBS hemolysin extract leads to trophoblast death
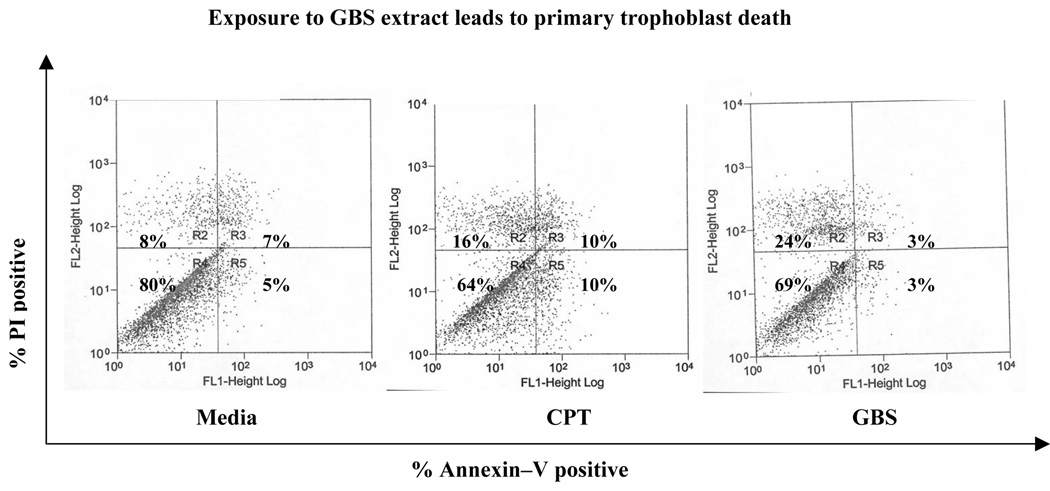
In order to test the effect of GBS on trophoblast survival, we isolated primary human placental trophoblasts by using well established methods, treated them with media, protein kinase C inhibitor calphostin (CPT, 4µM; as positive control for apoptosis) or GBS extract for 6 hours and assessed cell death by staining the cells with propidium iodide (PI, marker for necrosis) and Annexin-V (marker for apoptosis) respectively and performed flow cytometry. We observed that treatment with GBS hemolysin extract from NCTC10/84 induced a 3 fold increase in cell death in primary trophoblasts (increase from 8% PI positive media treated cells to 24% PI positive cells; Figure 1). These data suggest that GBS exposure induces trophoblast death.
Figure 1.
Primary human trophoblasts were treated with media, calphostin (CPT) or NCTC10/84 βH/C extract for six hours. Cell death was assessed by Annexin-V and propidium iodide staining using flow cytometry. Data shown is representative of two independent experiments.
Infection with GBS leads to JEG3 trophoblast cell death
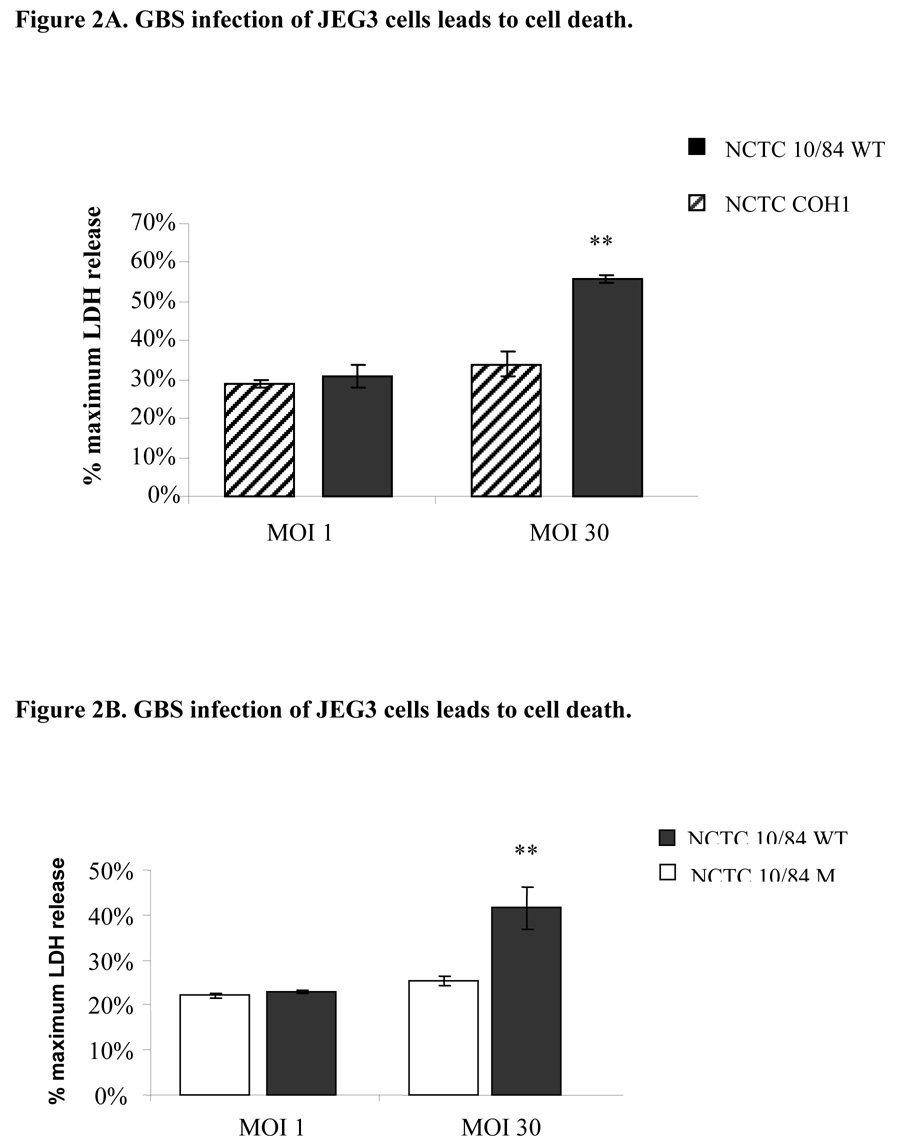
After observing that GBS extract induces primary trophoblast death, we moved to cell line experiments. We infected JEG3 trophoblast cells with highly hemolysin NCTC10/84, low hemolysin COH1 or mutant GBS strain that does not express hemolysin (NCTC10/84M) isolates for 1-hour as described above and measured the supernatant lactate dehydrogenase release to assess cell death. We observed that only infection with NCTC10/84 induced trophoblast death in a dose dependent manner (Figure 2A and 2B).
Figure 2.
Trophoblast monolayers (50,000 cells/well) were infected with highly hemolytic (NCTC10/84), low hemolytic GBS (COH1; Figure 2A) or non-hemolytic mutant NCTC10/84M strains (Figure 2B), and cell death was assessed after 1 hour by measuring the supernatant LDH release. Multiplicity of infection of 1 (5×104 cfu) and 30 (1.5×106 cfu) were used. The data shown is the representative of three separate experiments. Error bars represent the +/−95% confidence intervals of the mean of eight wells. ** P < 0.001 compared with low hemolytic GBS (COH1; Figure 2A) and ** P < 0.001 compared with mutant GBS (NCTC10/84M; Figure 2B).
Infection with GBS leads to primary human placental fibroblast death
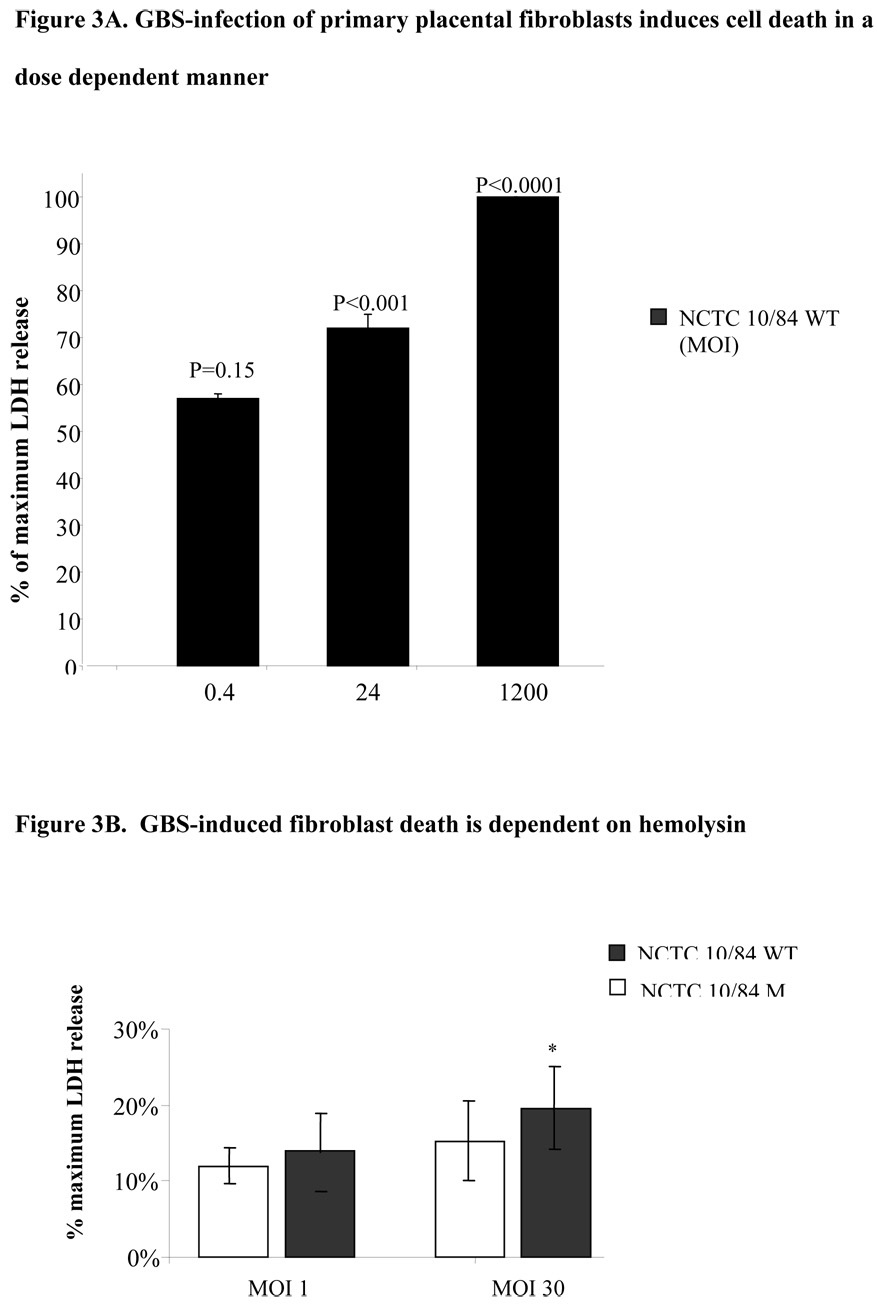
We isolated primary human placental fibroblasts as described above, infected them with GBS and assessed cell death by supernatant LDH assay. We observed that infection with highly hemolytic NCTC10/84 GBS strain leads to primary fibroblast death; whereas nonhemolytic mutant GBS does not (Figure 3B). These data suggest that pore forming exotoxin, β-hemolysin, which mediates GBS induced macrophage cell death [10], may play role in the GBS induced cell death in the reproductive tract.
Figure 3.
Primary placental fibroblast monolayers (50,000 cells/well) were infected with various MOI of highly hemolytic (NCTC10/84) for 1 hour and cell death was assessed by LDH assay (Figure 3A); in parallel experiments primary human fibroblasts were infected with either highly hemolytic (NCTC10/84) or mutant GBS (NCTC10/84M) strains that do not express hemolysin and cell death was assessed after 1 hour by measuring the supernatant LDH release. Multiplicity of infection of 1 (5×104 cfu) and 30 (1.5×106 cfu) were used (Figure 3B). The data shown is the representative of two separate experiments. Error bars represent the +/−95% confidence intervals of the mean of eight wells. *P < 0.05 compared with mutant GBS.
β-hemolysin mediates GBS induced trophoblast cell death
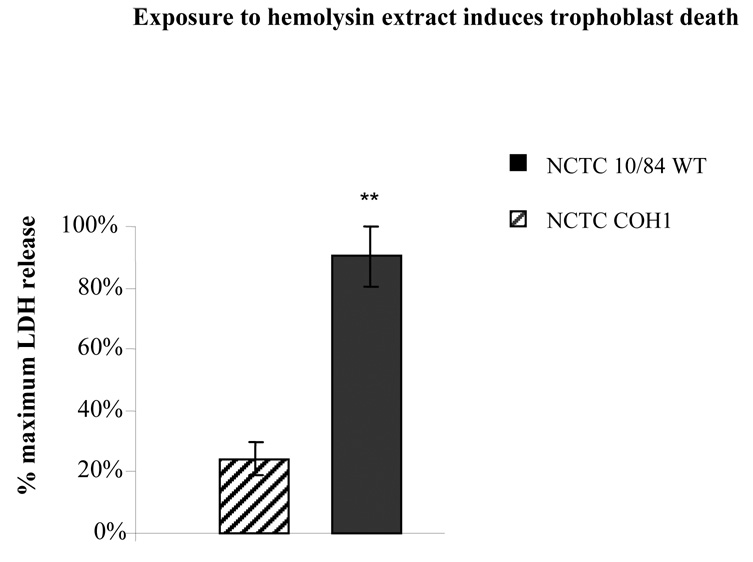
In order to assess the role of hemolysin in GBS induced cell death we isolated hemolysin extracts from GBS strains as described above and treated JEG3 cells with various concentrations of these extract for 1hr. The cell death was assessed by measuring the supernatant LDH release. We observed that hemolysin extract from wild type GBS NCTC10/84 induced cell death whereas low hemolysin GBS strain did not (Figure 4).
Figure 4.
Trophoblast monolayers (50,000 cells/well) were exposed to βH/C hemolysin extracts from 2×107 cfu of either highly hemolytic (NCTC10/84) or low hemolytic GBS (COH1) strains and death was assessed after 1 hour by measuring the supernatant LDH release. The data shown is the mean of three separate experiments. Error bars represent the +/− 95% confidence intervals of the mean of the three experiments (N=3). Each experiment was performed in six wells. ** P < 0.001 compared with low hemolytic GBS (COH1).
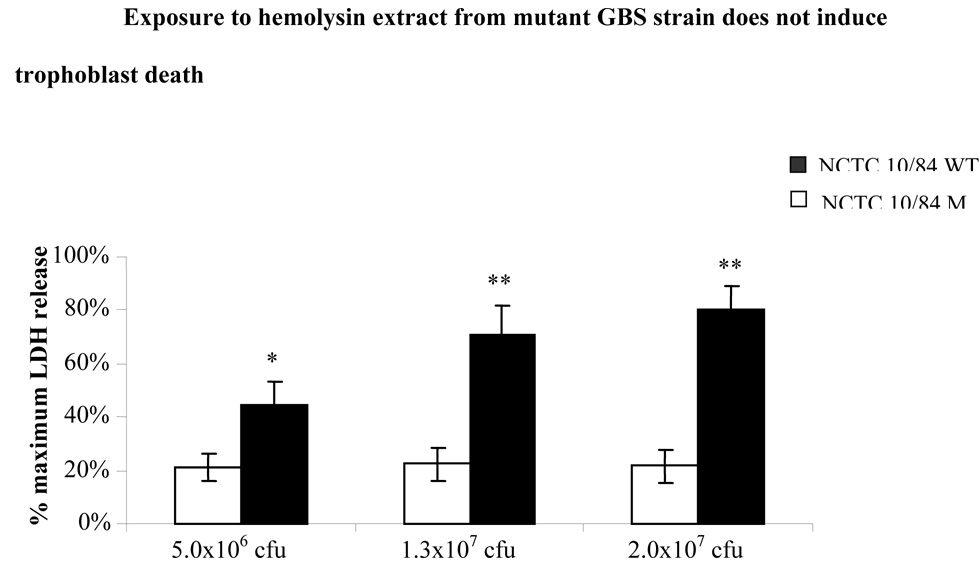
In parallel experiments, we isolated hemolysin extract from mutant GBS NCTC10/84 (NCTC10/84M) which does not express hemolysin, and compared NCTC10/84M hemolysin extract induced cell death with that induced by wild type NCTC10/84 as assessed by supernatant LDH assay. We observed that while treatment with wild type GBS extract induced trophoblast death in a dose dependent manner, treatment with extract from β-hemolysin deleted NCTC10/84 mutant was not toxic in JEG3 cells (Figure 5). These data suggest that β-hemolysin plays a key role in GBS induced trophoblast death.
Figure 5.
Trophoblast monolayers (50,000 cells/well) were exposed to various amounts of βH/C hemolysin extracts from 2×107 cfu, 1.3×107 cfu or 5×106 cfu of either highly hemolytic (NCTC10/84) or nonhemolytic GBS (NCTC10/84M) strains. Cell death was assessed after 1 hour by measuring the supernatant LDH release. The data shown is the mean of three separate experiments. Error bars represent the +/− 95% confidence intervals of the mean of the three experiments (N=3). Each experiment was performed in six wells. *P < 0.05 compared with nonhemolytic GBS (NCTC10/84M) strain extract. ** P < 0.001 compared with nonhemolytic GBS (NCTC10/84M) strain extracts.
Discussion
Although GBS infection is one of the most common causes of newborn sepsis, the microbial factors involved in the pathogenesis are not clearly known.
GBS infection is known to induce cell death in many systems. GBS-induced apoptosis appears to represent an important strategy used by the bacterium that limit inflammation during infection and facilitate bacterial escape from normal host immune defenses. In brain microvascular endothelial cells [2], macrophages [3], pulmonary and umbilical endothelial cells [4, 7] and pulmonary epithelial cells [8]; which constitute the innate barriers against microorganisms, GBS infection leads to cell death. Trophoblast cells are epithelial in origin; the single cell trophoblast layer in fertilized embryo develop into the placenta to provide the fetal-maternal innate immune barrier. Here we show for the first time that GBS infection and exposure to GBS components induce trophoblast death. Although highly pathogenic, CHO1 strain did not but high hemolysin activity NCTC10/84 strain induced trophoblast death. These data suggest that GBS hemolysin may play the key role in GBS penetration through the placental trophoblast barrier to cause neonatal disease and chorioamnionitis.
GBS attaches to and invades a variety of human tissues, including embryonic, fetal, and adult buccal epithelial cells [9], lung epithelial and endothelial cells [10] and brain microvascular endothelial cells [11]. In placenta, GBS attaches to amnion and chorion cells, however it invades only the latter [12]. We observed that GBS attaches to and invades the trophoblasts (data not shown).
We observed that 6 hr GBS extract exposure of trophoblasts led to PI positive cell death and that apoptosis was not significant as assessed by the low percent positive cells stained for Annexin V. This may be because at 6 hours most primary trophoblasts are already necrotic. Indeed in cell line experiments 1 hr-treatment with GBS extract was sufficient to induce cell death and LDH release.
Fettucciari and colleagues have shown that in macrophages surface-bound GBS was able to trigger apoptosis [3]. In parallel, exposure to GBS cell wall components was sufficient to induce chemokine expression in the decidual cells, and in trophoblasts exposure to heat inactivated GBS modulated pro- and anti-inflammatory cytokine expression [13]. These data suggest that GBS cell wall or cytoplasmic components may mediate host cell toxicity.
β-hemolysin appears to play the key role in GBS-induced macrophage and epithelial death (3, 4, 7, 8, 14, 15). We observed that there was not a significant cell death in trophoblasts infected with hemolysin deleted mutant GBS NCTC10/84M even at higher MOI. These data suggest that β-hemolysin plays the key role for GBS-induced trophoblast death. Ulett and colleagues have shown that β-hemolysin is dispensable for GBS toxicity in infected macrophages [16]. However in their experiments they used a GBS strain that produces little hemolysin and most likely unveiled a secondary and less potent mechanism for toxicity.
It could be speculated that processes that lead to bacterial lysis and β-hemolysin release, such as the antibiotic treatment of colonized women, may potentially lead to trophoblast toxicity and disruption of the placental integrity. A similar phenomenon has been observed with the use of bacteriolytic antibiotics in meningitis where the release of bacterial components upon antibiotic treatment augments the host inflammatory response which in turn contributes to the pathophysiology of brain injury [17]. Consequently studies are needed to understand the effect of GBS-antibiotic prophylaxis on trophoblast barrier and potential for invasion with other colonizing bacteria such as E coli.
GBS infection plays a role in intrauterine demise, preterm delivery and neonatal infection. Our data demonstrate that GBS-induces trophoblast death. We propose that GBS-induced trophoblast cell death may play a role in GBS invasion of the fetus.
Acknowledgments
This work was supported by NIH NCRR GCRC Grant (M01-RR00425) and March of Dimes grant(#6-FY06-329) to OE. Dr. George Liu was supported in part by Burroughs Welcome Career Award.
We thank Dr.Victor Nizet (UCSD, San Diego, CA) for the wild type and knock out Streptococcus isolates.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baker C, Edwards M. Group B streptococcal infections. In: Remington J, Klein J, editors. Infectious diseases of the fetus and newborn infant. Vol. 742. Philadelphia, Pa: W. B. Saunders; 1990. pp. 742–811. [Google Scholar]
- 2.Doran KS, Liu GY, Nizet V. Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signaling pathways in brain endothelium and contributes to development of meningitis. J Clin Inves. 2003;112:736–744. doi: 10.1172/JCI17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fettucciari K, Rosati E, Scaringi L, et al. Group B Streptococcus induces apoptosis in macrophages. J Immunol. 2000;165:3923–3933. doi: 10.4049/jimmunol.165.7.3923. [DOI] [PubMed] [Google Scholar]
- 4.Gibson RL, Nizet V, Rubens CE. Group B streptococcal beta-hemolysin promotes injury of lung microvascular endothelial cells. Pediatr Res. 1999;45:626–634. doi: 10.1203/00006450-199905010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Wessels MR, Benedi V-J, Kasper DL, Heggen LM, Rubens CE. Type III capsule and virulence of group B streptococci. In: Dunny GM, Cleary PP, McKay LL, editors. Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington, D.C: American Society for Microbiology; 1991. pp. 219–223. [Google Scholar]
- 6.Gibson RL, Lee MK, Soderland C, Chi EY, Rubens CE. Group B streptococci invade endothelial cells: type III capsular polysaccharide attenuates invasion. Infect. Immun. 1993;61:478–485. doi: 10.1128/iai.61.2.478-485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nizet V, Gibson RL, Chi EY, Framson PE, Hulse ML, Rubens CE. Group B streptococcal β-hemolysin expression is associated with injury of lung epithelial cells. Infect. Immun. 1996;64:3818–3826. doi: 10.1128/iai.64.9.3818-3826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pritzlaff CA, Chang JC, Kuo SP, Tamura GS, Rubens CE, Nizet V. Genetic basis for the beta-haemolytic/cytolytic activity of group B Streptococcus. Mol Microbiol. 2001;39:236–247. doi: 10.1046/j.1365-2958.2001.02211.x. [DOI] [PubMed] [Google Scholar]
- 9.Teti G, Tomasello F, Chiofalo MS, Orefici G, Mastroeni P. Adherence of group B streptococci to adult and neonatal epithelial cells mediated by lipoteichoic acid. Infect Immun. 1987;55:3057–3064. doi: 10.1128/iai.55.12.3057-3064.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubens CE, Smith S, Hulse M, Chi EY, van Belle G. Respiratory epithelial cell invasion by group B streptococci. Infect. Immun. 1992;60:5157–5163. doi: 10.1128/iai.60.12.5157-5163.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nizet V, Kim KS, Stins M, Jonas M, Chi EY, Nguyen D, Rubens CE. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 1997;65:5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winram SB, Jonas M, Chi E, Rubens CE. Characterization of group B streptococcal invasion of human chorion and amnion epithelial cells In vitro. Infect Immun. 1998;66:4932–4941. doi: 10.1128/iai.66.10.4932-4941.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudley DJ, Edwin SS, Van Wagoner J, Augustine NH, Hill HR, Mitchell MD. Regulation of decidual cell chemokine production by group B streptococci and purified bacterial cell wall components. Am J Obstet Gynecol. 1997;177:666–672. doi: 10.1016/s0002-9378(97)70162-5. [DOI] [PubMed] [Google Scholar]
- 14.Liu GY, Doran KS, Lawrence T, Turkson N, Puliti M, Tissi L, Nizet V. Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. Proc Natl Acad Sci U S A. 2004;101:14491–14496. doi: 10.1073/pnas.0406143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchlewicz BA, Duncan JL. Properties of a hemolysin produced by group B streptococci. Infect. Immun. 1980;30:805–809. doi: 10.1128/iai.30.3.805-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulett GC, Bohnsack JF, Armstrong J, Adderson EE. Beta-hemolysinin-dependent induction of apoptosis of macrophages infected with serotype III group B streptococcus. J Infect Dis. 2003;1888:1049–1053. doi: 10.1086/378202. [DOI] [PubMed] [Google Scholar]
- 17.Grandgirard D, Schurch C, Cottagnoud P, Leib SL. Prevention of brain injury by the nonbacteriolytic antibiotic daptomycin in experimental pneumococcal meningitis. Antimicrob Agents Chemother. 2007;51:2173–2178. doi: 10.1128/AAC.01014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]