Abstract
Neuronal pentraxins (NPs) function in the extracellular matrix to bind α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. Three NPs have been described, neuronal activity-regulated pentraxin (Narp), which is regulated as an immediate early gene, NP1, and neuronal pentraxin receptor (NPR). Narp and NP1 enhance synaptogenesis and glutamate signaling by clustering AMPA receptors, whereas NPR contributes to removing AMPA receptors during group I metabotropic glutamate receptor-dependent long-term depression. Here, we examine mice with genetic deletions [knockout (KO)] of each NP to assess their contributions to cocaine-induced neuroplasticity. Consistent with a shared AMPA receptor clustering function for Narp and NP1, deletion of either NP caused similar behavioral alterations. Thus, although both Narp and NP1 deletion promoted cocaine-induced place preference, NPR deletion was without effect. In addition, although Narp and NP1 KO showed reduced time in the center of a novel environment, NPR KO mice spent more time in the center. Finally, although Narp and NP1 KO mice showed blunted locomotion after AMPA microinjection into the accumbens 3 weeks after discontinuing repeated cocaine injections, the AMPA response was augmented in NPR KO. Likewise, endogenous glutamate release elicited less motor activity in Narp KO mice. Consistent with reduced AMPA responsiveness after chronic cocaine in Narp KO mice, glutamate receptor 1 was reduced in the PSD fraction of Narp KO mice withdrawn from cocaine. These data indicate that NPs differentially contribute to cocaine-induced plasticity in a manner that parallels their actions in synaptic plasticity.
Neuronal pentraxins (NPs) are extracellular matrix proteins that are enriched in excitatory synapses, where they aggregate AMPA receptors (AMPARs) (O’Brien et al., 1999, 2002). Three different NPs have been described: neuronal activity regulated pentraxin (Narp; also termed NP2), NP1, and neuronal pentraxin receptor (NPR) (Kirkpatrick et al., 2000). Narp is an immediate early gene that is induced by synaptic activity, whereas NP1 and NPR are constitutively expressed (Xu et al., 2003). Narp and NP1 multimerize, thereby enhancing the clustering and synaptogenic activity of the individual proteins (Xu et al., 2003). Because Narp expression is dynamically regulated by synaptic activity (Berke et al., 1998; Reti and Baraban, 2000), it is proposed that the synergistic effect of forming multimers with NP1 gives neurons a mechanism to tune the degree of AMPAR clustering and synaptogenesis in response to synaptic activity (Xu et al., 2003).
In contrast to Narp and NP1, which are secreted proteins, NPR possesses a single transmembrane domain that confers a unique function. Group 1 metabotropic receptors (mGluR1/5) activate the extracellular metalloprotease ADAM 17 (TACE), which cleaves and releases NPR to promote endosome-dependent removal of AMPAR from the synapse. This regulated cleavage of NPR is essential for mGluR-induced long-term depression (Cho et al., 2008). Thus, NPs appear to have nearly opposing effects on AMPAR aggregation at synapses, with Narp and NP1 acting to enhance AMPAR clustering and NPR acting to remove AMPAR.
Cocaine induces changes in glutamate neurotransmission in nucleus accumbens that are linked to behavioral sensitization and addiction (Kalivas and O’Brien, 2008). For example, withdrawal from chronic cocaine increases the expression of the GluR1 subunit of AMPAR in accumbens (Boudreau and Wolf, 2005; Conrad et al., 2008) and correspondingly increases the ratio of AMPA to N-methyl-d-aspartate currents in accumbens medium spiny neurons (Kourrich et al., 2007). In addition, AMPA injected into the accumbens demonstrates behavioral cross-sensitization with cocaine and induces cocaine-seeking behavior in cocaine-experienced rats, whereas AMPA antagonists block cocaine-induced behavioral sensitization and seeking behavior (Pierce et al., 1996; Cornish and Kalivas, 2000; Di Ciano and Everitt, 2001).
The expression of Narp mRNA is transiently increased in striatum after acute cocaine (Berke et al., 1998), combined with Narp’s AMPAR clustering properties and the relevance of AMPAR in cocaine-induced neuroadaptations, identifies the Narp-NP1 complex as a candidate for regulating cocaine-induced neuroplasticity. Because previous studies failed to find changes in Narp in the accumbens after chronic cocaine administration (Lu et al., 2002; Reti et al., 2002), we made use of mice harboring deletions of individual NP genes to study the role of the NPs in behavioral adaptations produced by chronic cocaine administration and the capacity of cocaine to affect the response to AMPAR stimulation and the levels of glutamate receptors and subunits.
Materials and Methods
Experimental Subjects
The generation of NP KO and WT adult mice (8–10 weeks age; 129sv × C57BL/6 background) is described elsewhere (Bjartmar et al., 2006). Mice were bred from heterozygous mating pairs and genotyped at 4 to 5 weeks old. Narp KO mice were derived from one background, whereas the NPR and NP1 KOs were derived from another. Mice were group housed in an Association for Assessment and Accreditation of Laboratory Animal Care-approved animal facility (lights on, 7:00 AM; 23°C) with ad libitum food and water. All experiments were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and conducted according to specifications of the National Institutes of Health (Institute of Laboratory Animal Resources, 1996). The data from male and female were pooled for final analysis because no significant gender effect was found.
Surgical Procedures
One week after cocaine treatment, mice were anesthetized with ketamine (120 mg/kg i.p.) and xylazine (6 mg/kg i.p.) and mounted in a Kopf stereotax apparatus (David Kopf Instruments, Tujunga, CA) equipped with a Cunningham mouse adapter. Bilateral guide cannula (10 mm, 20 gauge; Small Parts, Inc., Miramar, FL) were positioned over the nucleus accumbens and secured with a light-cure dental resin (Kerr Corporation, Orange, CA). The stereotaxic coordinates employed were: AP, +1.1 mm; DL, ±1 mm, and DV, −2.2 mm (Franklin and Paxinos, 1997). The mice were allowed to recover for 1 week.
Behavioral Tests
Cocaine Place Conditioning. The cocaine place conditioning procedure was described previously (Pacchioni et al., 2007). The place preference apparatus was a Plexiglas activity chamber (22 × 43 × 33 cm; AccuScan Instruments, Inc., Columbus, OH) with a black plastic enclosure (11 × 43 × 33; Harvard Apparatus Inc., Holliston, MA) that had black walls and ceiling, dividing the chamber into a black “closed” and a transparent “open” side. The procedure consisted of four phases: habituation, pretest, conditioning, and post-test. Habituation and pre- and post-tests were 15-min sessions where the mice had free access to both sides of the chamber. According to the time spent in each side of the chamber during pretest, mice were assigned to receive cocaine in the nonpreferred side and saline in preferred side during the conditioning phase. There were eight daily 15-min conditioning sessions (four saline and four cocaine), where the mouse was restricted to the respective side immediately after an i.p. injection. A separate group of animals received a saline injection before being restricted in each side of the chamber to facilitate the interpretation of the biased place conditioning paradigm results such as habituation to the non preferred side aversiveness (Bardo and Bevins, 2000). The post-test was conducted the day after the last conditioning session. Place conditioning was quantified as an increase in the time spent in the drug paired side during post-test compared with pretest and referred to as occupancy time (seconds).
Motor Activity. All animals were habituated to AccuScan activity boxes (22 × 43 × 33 cm) for 1 h. The locomotor activity was recorded during habituation and for 2 h immediately after the injection and estimated by distance traveled (centimeters).
Cocaine Behavioral Sensitization Paradigm. This paradigm consisted of seven daily injections (1 × 15 mg/kg i.p., 5 × 30 mg/kg i.p., 1 × 15 mg/kg i.p.). The motor activity was recorded after first and last injections of cocaine (15/mg/kg i.p.) or saline and at 3 weeks after finishing treatment.
Locomotor Response to Acute Caffeine. Each animal received all three injections (saline and caffeine at 10 and 30 mg/kg i.p.) separated by 7 days.
Microinjection of AMPA or (S)-3,5-Dihydroxyphenylglycine. An injection needle (33 gauge) was introduced into each guide cannula and extended 1.5 mm below the tip. Bilateral infusions of 0.25 μl/side were made over 90 s, and the injectors were removed 60 s later. Saline plus two doses of AMPA (0.1 and 0.3 nmol/side) (Bell and Kalivas, 1996) or (S)-3,5-dihydroxyphenylglycine (DHPG; 1 and 2.5 nmol/side) (Swanson et al., 2001) were infused into the accumbens. Separate groups of mice were used for the AMPA and DHPG studies. All mice randomly received all doses in a counterbalanced order. Each injection was separated by at least 3 days.
In Vivo Microdialysis Study
A microdialysis probe (24 gauge; 1–1.5 mm of active membrane) was lowered into the guide cannula and perfused with aCSF (5 mM glucose, 2.7 mM KCl, 140 mM NaCl, 1.4 mM CaCl2, 1.2 mM MgCl2, 0.15% phosphate-buffered saline, pH 7.4) at 2 μl/min. After 4 h, dialysis samples were collected every 20 min. Increasing concentrations of DHPG (0, 3, and 30 μM/side; Tocris Bioscience, Ellisville, MO) were infused into the accumbens through the probe for 60 min, as described previously for mice (Szumlinski et al., 2004). The last two samples of each drug concentration were averaged and used in statistical evaluation of the data.
Glutamate Measurement by High-Performance Liquid Chromatography with Fluorescent Detection
A precolumn derivatization of amino acids with o-phthalaldehyde was performed using a Gilson 231 XL autosampler (Gilson, Inc., Middleton, WI). Glutamate was separated via a reverse phase column (10 cm, 3 μm octadecylsilane; BAS Bioanalytical Systems, West Lafayette, IN) using the following mobile phase: 11% (v/v) acetonitrile, 100 mM NaH2PO4, and 0.1 mM EDTA, pH 6.0. Glutamate was detected using a Shimadzu 10RF-A fluorescence detector (Shimadzu, Kyoto, Japan; excitation wavelength, 340 nm; emission wavelength, 450 nm). For quantification, peak height was compared with an external standard curve.
Subcellular Fractionation
After 3 weeks of withdrawal from chronic cocaine or saline treatment, mice were decapitated. The ventral striatum was dissected from the brain as described elsewhere (Szumlinski et al., 2004), and subcellular fractionation was performed as described previously with minor modifications (Toda et al., 2006). In brief, fresh brain tissues were homogenized in cold buffer containing 0.32 M sucrose and 10 mM HEPES, pH 7.4. Homogenates were cleared two times at 1000g for 10 min to remove nuclei and large debris (P1). The resulting supernatants were concentrated at 12,000g for 20 min to obtain a crude membrane fraction (P2), which was rinsed twice (4 mM HEPES, 1 mM EDTA, pH 7.4, 20 min at 12,000g). Then, it was incubated (20 mM HEPES, 100 mM NaCl, 0.5% Triton X-100, pH 7.2) for 15 min and centrifuged at 12,000g for 20 min to pellet the synaptosomal membrane fraction (LP1). The supernatant was considered the non-postsynaptic density membrane fraction (non-PSD), sometimes referred to as the Triton-soluble fraction. The pellet was then solubilized (20 mM HEPES, 0.15 mM NaCl, 1% Triton X-100, 1% deoxycholic acid, 1% SDS, pH 7.5) for 1 h and centrifuged at 10,000g for 15 min. The supernatant contained the PSD or Triton-insoluble fraction. The integrity of non-PSD and PSD fractions was verified by immunoblotting for PSD-95, which was enriched in the PSD fraction, and synaptophysin, which was enriched in the non-PSD fraction (Supplemental Fig. 1). All buffers were supplemented with protease inhibitors cocktail (Complete mini tablets; Roche Diagnostics, Indianapolis, IN). Protein concentration was measured using the Bradford assay (Pierce Chemical, Rockford, IL).
Western Blotting
Western blotting for different subcellular fractions was conducted as described in detail elsewhere (Toda et al., 2006). Samples (5 μg/lane) were run in 10% Tris-acrylamide gels (Invitrogen, Carlsbad, CA) and transferred to polyvinylidene difluoride membranes. Overnight incubation with each primary antibody (Glur2, 1:250; BD Biosciences, San Jose, CA; Glur1, 1:500; Millipore Bioscience Research Reagents, Temecula, CA; mGluR5, 1:2000; Millipore Bioscience Research Reagents; and Narp, 1:2000, provided by P.F.W.) was followed by a secondary horseradish peroxidase-conjugated antibody (1:10,000 or 1:20,000; Upstate), and the reactivity was detected using Super-signal West Pico (Pierce Chemical). Densitometric analysis was performed with the Image J program (version 1.36b). Band intensities were measured by taking the integrated density of the pixels. Every gel has one lane loaded with an internal standard (accumbens P2 fraction from WT mice) to compare densities between gels. After normalizing to the internal standard, data were normalized to percentage change from respective controls. For the PSD subfraction, a control protein within each band, such as actin, was not used to normalize each lane. As described elsewhere, the PSD is very dynamic, making it difficult to insure the stability of a protein in this subfraction after gene deletion or pharmacological treatments (Toda et al., 2006).
Histology
After concluding the microinjection or microdialysis experiments, mice were deeply anesthetized with pentobarbital (70 mg/kg i.p.) and perfused intracardially with 10% formalin. The brain was removed and stored in 10% formalin for a 1 week. Coronal sections (60 μm) were mounted and stained with Cresyl violet. Injection sites and probe placements were determined according to Franklin and Paxinos (1997) (Supplemental Fig. 2).
Data Analysis
Because of the different genetic backgrounds, Narp KO mice were compared with their WT littermates, whereas NP1 KO, NPR KO, and their WT littermates were analyzed separately. The data were statistically evaluated using two-, three-, or four-way ANOVA with main factors being genotype, drug treatment (saline or cocaine), time, and/or dose. When significant effects or interactions were identified, data were appropriately partitioned and evaluated using oneor two-way ANOVA followed by Bonferroni or least significant difference post hoc analyses to identify specific genotypic differences. Statistical comparisons of protein levels were made on data that were normalized to the chronic saline group in each genotype, and comparisons were made using an ANOVA or Student’s t test.
Results
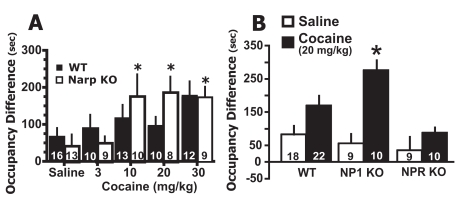
Deletion of NP Genes Differentially Affects Cocaine Place Preference. Figure 1A shows that in the Narp KO, 10, 20, and 30 mg/kg cocaine induced place conditioning relative to the saline control group (see Supplemental Table 1 for pre- and post-test scores). A two-way ANOVA revealed a significant effect of dose [F(1,100) = 12.75, p = 0.006] but no effect of genotype or interaction. Because of the significant effect of dose, each genotype was examined separately with a one-way ANOVA to determine which doses differed significantly from saline. Only in Narp KO was a significant effect of cocaine versus saline observed [F(4,48) = 3.75, p = 0.017]. Although 30 mg/kg cocaine in the WT appeared to induce place conditioning in comparison with saline control group, the one-way ANOVA did not reveal statistical significance. These data support a greater tendency for cocaine to induce place preference in KO than in WT mice.
Fig. 1.
Narp and NP1 KO mice show enhanced cocaine-induced conditioned place preference. A, cocaine induces place conditioning in Narp KO but not in WT mice. Cocaine place conditioning is expressed as mean ± S.E.M. occupancy difference in seconds (post-test time – pretest time). The number of determinations is shown in the bars. B, cocaine (20 mg/kg i.p.) elicited place preference in NP1 KO but not NPR KO or the NP1/NPR WT. *, p < 0.05 comparing saline-with cocaine-treated animals within each genotype using a Dunnett’s test (A) or Bonferroni post hoc test (B).
Akin to Narp KO, NP1 KO mice showed place preference to cocaine (20 mg/kg i.p.), whereas NPR KO mice showed no place preference to cocaine (Fig. 1B). The WT for the NP1 and NPR KO mice showed near significant place conditioning to cocaine relative to the saline group (p = 0.071). A two-way ANOVA revealed a significant effect of drug [F(1,57) = 15.83, p = 0.0002] and genotype [F(2,57) = 3.272, p = 0.045] but no interaction. A Bonferroni post hoc test revealed a significant effect of cocaine versus saline only in NP1 KO (p < 0.001).
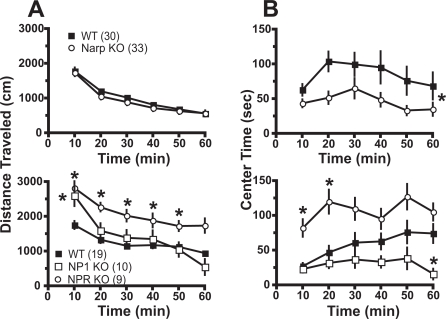
NP Gene Deletion Alters Open Field Behavior. Mice were placed into a novel environment (photocell apparatus), and behavior was estimated for 60 min, including locomotion (distance traveled) and the amount of time spent in the center of the box. Figure 2A shows that locomotor activity in response to a novel environment was not altered in Narp KO relative to WT. Except during the first 10 min, NP1 KOs were also not different from WT. However, NPR KO showed elevated locomotor activity in response to novelty across the first 50 min. Two-way ANOVA with repeated measures over time revealed an effect of time for Narp [F(5,305) = 121.20, p < 0.001] and NP1/NPR [F(5,175) = 83.90, p < 0.001], but only the NP1/NPR experiment showed a significant effect of genotype [F(2,175) = 8.90, p < 0.001] and an interaction between genotype and time [F(10,175) = 7.21, p < 0.001].
Fig. 2.
The deletion of NP genes altered spontaneous behavior in a novel open field. A, spontaneous locomotor activity, estimated by distance traveled, was not affected by Narp gene deletion, was greatly elevated in NPR KO, and was elevated during the first 10 min in NP1 KO. B, amount of time spent in the center of the open field was reduced in Narp and NP1 KO and increased in NPR KO. Data are shown as mean ± S.E.M., and the number of determinations in each group is shown in parentheses. *, p < 0.05, comparing KO with respective WT using a Bonferroni post hoc or main effect of genotype (Narp KO versus WT).
The amount of time spent in the center of the box is a measure of the level of anxiety (Prut and Belzung, 2003). The time spent in the center of the box was reduced in the Narp and NP1 KOs and elevated in the NPR KO relative to their respective WT (Fig. 2B), suggesting elevated anxiety levels in the Narp and NP1 KOs, whereas NPR KOs were less anxious than WT. Two-way ANOVA with repeated measures over time revealed an effect of genotype and time for Narp [genotype, F(1,225) = 5.74, p = 0.021; time, F(5,225) = 4.16, p = 0.001] and NP1/NPR [genotype, F(2,175) = 9.11, p < 0.001; time, F(5,175) = 6.01, p < 0.001], but only the NP1/NPR experiment showed a significant interaction between genotype and time [F(10,175) = 2.07, p = 0.029].
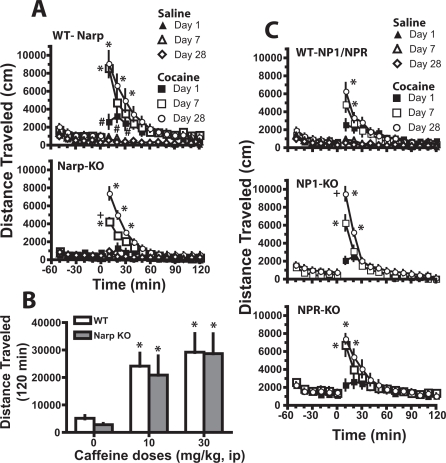
Deletion of NP Genes and Cocaine-Induced Locomotion and Behavioral Sensitization. Locomotor activity induced by acute cocaine and the development of behavioral sensitization to repeated cocaine was examined in the NPs KO and their respective WT mice by assessing the motor stimulant effect of cocaine on the first and last days of seven daily cocaine injections (days 1 and 7) and after a 3-week withdrawal period (day 28). Figure 3A shows that Narp gene deletion significantly reduced the acute motor response to cocaine relative to WT (e.g., day 1). Seven days of daily cocaine resulted in sensitized motor activity in both genotypes. However, in Narp KO, the response to cocaine on day 7 was lower than after 21 days of withdrawal (e.g., day 28), whereas the responses on days 7 and 28 were equivalent in WT mice. The majority of differences between treatment groups occurred during the first 20 min after cocaine injections. In contrast to cocaine, no significant differences in the response to saline injection were observed on any day or between genotypes. A four-way ANOVA revealed significant main effects of time [F(17,1989) = 94.65, p < 0.001], genotype [F(1,117) = 19.94, p < 0.001], injection day [F(2117) = 9.16, p < 0.001], and drug [F(1,117) = 107.76, p < 0.001]. In addition, all between-subjects main effects showed significant interactions with time [genotype, F(17,1989) = 3.86, p < 0.001; injection day, F(34,1989) = 12.94, p < 0.001; drug, F(17,1989) = 83.50, p < 0.001]. Therefore, the data were evaluated using multiple two-way ANOVA with repeated measures over time followed by a least significant difference test to determine time points where the genotypes differed when a significant interaction between main effects was found.
Fig. 3.
The deletion of NP genes altered the development, but not the expression, of cocaine-induced behavioral sensitization. Mice were injected once a day for 7 days with either saline or cocaine (1 × 15, 5 × 30, 1 × 15 mg/kg). A, Narp deletion reduced the acute response to cocaine and delayed the development of full sensitization relative to WT by showing reduced motor activity on day 7 versus day 28. The numbers of determinations in each group were WT/saline = 10, Narp-KO/saline = 10, WT/cocaine = 11, and Narp-KO/cocaine = 12. B, lack of effect by Narp gene deletion on caffeine-induced motor activity. n = 9 for Narp-KO and n = 6 for WT. C, NP1 deletion reduced the development of sensitization to daily cocaine relative to WT by showing reduced motor activity on day 7 versus day 28. The numbers of determinations in each group were WT/saline = 8, WT/cocaine = 10, NP1-KO/cocaine = 10, and NPR-KO/cocaine = 9. All data are expressed as mean ± S.E.M. distance traveled (centimeters). *, p < 0.05 comparing saline with cocaine- or caffeine-treated animals within each genotype. +, p < 0.05 comparing KO to corresponding WT; #, p < 0.05, comparing genotypes on day 1.
To determine whether the lower response to acute cocaine in Narp KO generalized to all psychomotor stimulants, locomotor activity was elicited by two different doses of caffeine (10 and 30 mg/kg i.p.). Figure 3B shows that Narp KO and WT showed equal motor stimulation at both doses of caffeine (time course data are shown in Supplemental Fig. 3). A two-way ANOVA with repeated measures over dose revealed a significant effect of dose [F(2,39) = 31.40, p < 0.001] but no effect of genotype or interaction.
Figure 3C shows that NP1/NPR WT, NP1 KO, and NPR KO mice all developed behavioral sensitization by 3 weeks of withdrawal from repeated cocaine compared with the 1st injection day. However, akin to the Narp KO, the NP1 KO showed less sensitization on day 7 than on day 28, whereas the sensitized WT and NPR KO motor responses were equivalent between days 7 and 28. No genotypic differences were identified in the motor response elicited by the first cocaine injection, and daily saline did not induce sensitization in the WT mice. A three-way ANOVA was conducted on the cocaine treatment data and revealed significant main effects of time [F(17,1292) = 172.45, p < 0.001], genotype [F(2,76) = 11.74, p < 0.001], and injection day [F(2,76) = 4.39, p = 0.016]. In addition, all between-subjects main effects showed significant interactions with time [genotype, F(34,1292) = 2.91, p = 0.001; injection day, F(34,1292) = 18.69, p < 0.001]. Therefore, the data were evaluated using multiple two-way ANOVA with repeated measures over time followed by a least significant difference test to determine time points where the genotypes differed. The fact that Narp and NP1 KO mice showed a full expression of sensitization on day 28 but not on day 7, whereas NPR KO and both WT mice showed it on day 7, suggests a delayed development of sensitization in Narp and NP1 KO compared with their WT.
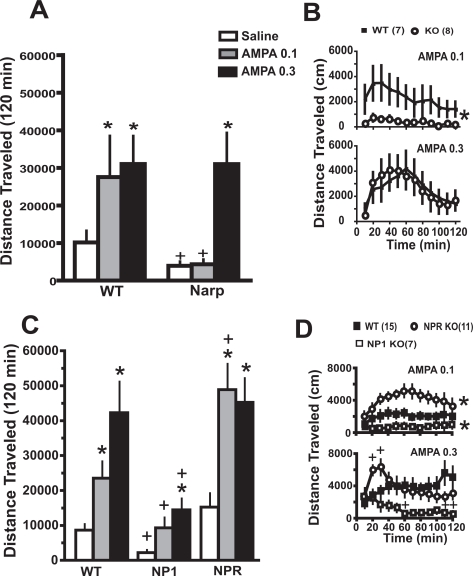
Deletion of NP Genes Differentially Affects the Motor Response to AMPA Injected into the Accumbens. Figure 4A shows that akin to previous reports in rats (Bell and Kalivas, 1996; Pierce et al., 1996), the motor stimulant response elicited by intra-accumbens administration of AMPA was augmented in mice pretreated 3 weeks earlier with daily cocaine relative to daily saline. Thus, neither dose of AMPA (0.1 and 0.3 nmol/side) elicited a motor stimulant response in WT or Narp KO pretreated with daily saline (Supplemental Table 3). Although both WT and Narp KO mice withdrawn from repeated cocaine showed enhanced motor activity at the highest dose of AMPA, only the WT showed elevated motor activity at the lower dose. This differential effect was especially apparent in the time course analysis (Fig. 4B). A three-way ANOVA showed significant main effects of AMPA dose [F(2,46) = 4.20, p = 0.021] and pretreatment [F(1,23) = 14.92, p < 0.001]. Thus, saline and cocaine pretreated animals were evaluated by separate two-way ANOVA with repeated measures over dose to identify genotypic differences, and in cocaine-pretreated mice, there were significant main effects of genotype [F(1,13) = 5.38, p = 0.037] and dose [F(2,26) = 6.19, p < 0.01]. A two-way ANOVA at each dose revealed a significant main effect of genotype only at 0.1 nmol AMPA [F(1,143) = 5.09, p = 0.042]; as expected, saline infusions did not induce locomotor activity under any condition (for temporal course of saline infusion in Narp KO and WT pretreated with daily cocaine, see Supplemental Fig. 4A).
Fig. 4.
Narp and NP1 gene deletion inhibit AMPA-induced motor activity. A, AMPA microinjection into the nucleus accumbens produced a dose-dependent increase in motor activity only in mice withdrawn from daily cocaine, and Narp KOs show reduced locomotor activity to the lowest dose of AMPA. The data are shown as mean ± S.E.M. total distance traveled (centimeters) during 2 h after AMPA. B, time course for AMPA-induced locomotion in WT and Narp KO. C, NP1 KO inhibits locomotor responding to AMPA relative to WT, whereas NPR KO shows enhanced responding. D, time course for AMPA-induced locomotion in WT, NP1 KO, and NPR KO. *, p < 0.05 compared with saline infusion within each genotype using a oneway ANOVA followed by a Bonferroni post hoc test (A and C). Main effect of KO versus WT (B and D). +, p < 0.05 comparing KO with corresponding WT using a oneway ANOVA followed by a Dunnett’s post hoc (A and C) or a least significant difference post hoc (D).
Figure 4C shows that after withdrawal from cocaine, the highest dose of AMPA elevated motor activity in both the NP1/NPR WT and NP1 KO but that the response in the NP1 KO was significantly blunted compared with the WT. In contrast, both doses of AMPA elicited motor activity in the NPR KO, indicating that at the lower dose, NPR deletion potentiated AMPA relative to WT. In addition, the motor response seen after acute microinjection of saline was blunted in NP1 KO relative to WT or NPR KO. The genotypic distinctions in AMPA-induced locomotion were also seen in the time course analysis (Fig. 4D); as expected, saline infusions did not induce locomotor activity under any condition (for temporal course of saline infusion in WT, NP1, and NPR KO pretreated with daily cocaine, see Supplemental Fig. 4B). A two-way ANOVA at each dose revealed significant main effects of genotype [F(2,62) = 7.66, p = 0.002] and dose [F(2,62) = 13.49, p < 0.001]. Two-way ANOVA at each dose revealed significant effects of time at both doses of AMPA, a main effect of genotype at 0.1 AMPA [F(2,330) = 26.57, p = 0.001], and a significant interaction between time and genotype at 0.3 AMPA [F(22,319) = 4.94, p < 0.001].
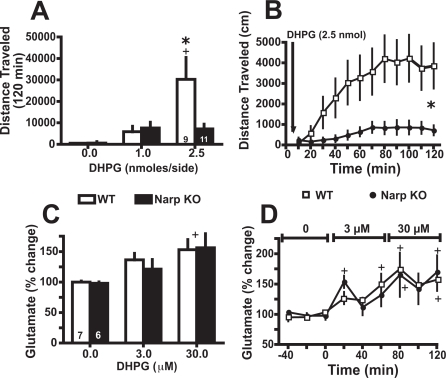
The Motor Response to Endogenously Released Glutamate Is Blunted in Narp KO. It was shown previously that stimulation of mGluR1 receptors releases synaptic glutamate (Schrader and Tasker, 1997; Swanson et al., 2001), and mGluR1-induced glutamate release into the accumbens promotes locomotor activity by stimulating AMPAR (Swanson et al., 2001). To determine whether, akin to AMPA, the motor response induced by endogenously released glutamate is also blunted by Narp gene deletion, the mGluR1/5 agonist DHPG was microinjected into the accumbens of WT and Narp KO mice. Figure 5A shows that DHPG produced a dose-dependent increase in motor activity in WT but had no significant effect in Narp KO mice. A two-way ANOVA revealed a significant effect of genotype [F(1,36) = 4.62, p = 0.041] and dose [F(2,36) = 26.84, p < 0.001] and an interaction between dose × genotype [F(2,36) = 13.4, p = 0.006]. This genotypic difference is clearly reflected in the time course of DHPG-induced motor activity (Fig. 5B). A three-way ANOVA revealed a significant effect of dose [F(2,54) = 10.1, p = 0.001] and significant interactions of genotype × dose [F(2,54) = 4.61, p = 0.001] and time × genotype × dose [F(22,594) = 3.21, p < 0.001]. Importantly, the genotypic distinction in DHPG-induced motor activity was not the result of a difference in the ability of DHPG to release glutamate. Thus, the dose-dependent increase in extracellular glutamate elicited by reverse dialysis of DHPG into the accumbens was equivalent in both genotypes (Fig. 5C). A twoway ANOVA revealed only a significant main effect of dose [F(2,11) = 31.06, p < 0.001]. The time course of DHPG induced changes in extracellular glutamate. A two-way ANOVA revealed only a significant effect of time [F(1,8) = 33.95, p < 0.001] (Fig. 5D).
Fig. 5.
Narp KOs show deficits in the motor response elicited by glutamate released endogenously after DHPG injection into the accumbens. A, dose-dependent induction of motor activity by DHPG in WT is absent in Narp KO mice. B, time course of DHPG-induced (2.5 nmol) locomotor activity revealed a marked genotypic difference. C, DHPG induced an equivalent dose-dependent release of glutamate between WT and Narp KO when reverse dialyzed into the accumbens. D, time course of DHPG-induced changes in extracellular glutamate. *, p < 0.05, comparing between genotypes. +, p < 0.05, comparing with baseline or saline injection within genotypes.
One mechanism whereby glutamate can regulate the clustering and internalization of NPs and AMPAR is via stimulation of mGluR5 (Cho et al., 2008). Therefore, the ventral striatum (including the nucleus accumbens) of WT and Narp KO was subfractionated, and the level of mGluR5 was measured by immunoblotting to determine whether alterations in mGluR5 could have influenced the actions of DHPG, which is an agonist at both mGluR1 and mGluR5. No genotypic difference was found between WT and Narp KO in the levels of mGluR5 monomer or dimmer in the PSD or non-PSD subfraction (Supplemental Fig. 5). The location of dialysis and injection cannulae in the nucleus accumbens is shown in Supplemental Fig. 2.
Because the stimulation of mGluR in the nucleus accumbens by DHPG induced the same glutamate release in both genotypes, the decreased locomotor response induced by intra-accumbens DHPG in Narp KO suggests a deficit in AMPAR signaling. However, when AMPA is injected, the deficit in AMPA signaling is evident only after a cocaine treatment but not after saline treatment.
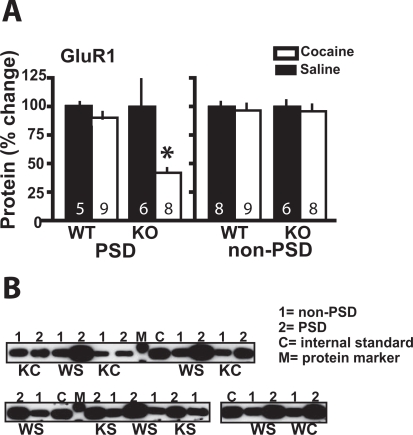
Cocaine Decreases the Expression of GluR1 in PSD of Narp KO. Narp clusters AMPAR by interacting with subunits GluR1–4, and consistent with previous reports (Lu et al., 2002; Reti et al., 2002), withdrawal from chronic cocaine did not affect the level of Narp in the PSD or non-PSD subfractions of WT mice (Supplemental Fig. 6). The levels of GluR1 and GluR2 were also measured in WT and Narp KO at 3 weeks of withdrawal from daily cocaine or saline administration. No differences between genotypes were measured in chronic saline-pretreated mice, and chronic cocaine had no effect on GluR1 or GluR2 measured in the PSD of WT mice (Fig. 6A; Supplemental Table 2). However, Fig. 6A shows that withdrawal from chronic cocaine significantly reduced the levels of GluR1 in the PSD subfraction of Narp KO but not WT mice [t(13) = 2.58, p = 0.022]. In contrast, chronic cocaine did not affect the level of GluR1 in the non-PSD subfraction of either genotype. No effects of cocaine were found on the levels of GluR2 in the PSD or non-PSD subfractions (Supplemental Table 2). Figure 6B illustrates representative immunoblots showing the reduction in GluR1 in the PSD subfraction of Narp KO after withdrawal from chronic cocaine. Note that only in the Narp KO pretreated with chronic cocaine did the densities in the PSD fraction approximate those in the non-PSD fraction. Otherwise, in all other-treatment groups, there is substantially more GluR1 in the PSD than the non-PSD fraction.
Fig. 6.
Withdrawal from chronic cocaine reduces GluR1 in the Narp KO but not WT mice. A, levels of GluR1 are reduced after withdrawal from chronic cocaine in the PSD subfraction of Narp KO but not WT mice. GluR1 was not reduced by cocaine in the non-PSD subfraction. B, representative immunoblots of GluR1. C, internal standard control (see Materials and Methods); 1, non-PSD, 2, PSD; Sa, chronic saline; Co, chronic cocaine; M, protein standard; WS, WT/saline; WC, WT/cocaine; KS, KO/saline; KC, KO/cocaine. *, p < 0.05, comparing chronic cocaine with saline treatments within each genotype.
Discussion
Our findings suggest opposing actions of Narp and NP1 versus NPR in cocaine-induced plasticity that parallel their differential actions in synaptogenesis and excitatory transmission. Narp and NP1 are able to multimerize and promote clustering of AMPAR (O’Brien et al., 2002; Xu et al., 2003), whereas NPR is thought to bind to the Narp/NP1/AMPA clusters and promote internalization of the clustered receptors (Cho et al., 2008). Consistent with a shared AMPAR clustering function for Narp and NP1, deletion of either NP caused similar alterations in spontaneous and cocaine-induced behavior and desensitized the motor response elicited by stimulating AMPAR in the nucleus accumbens. Thus, although both Narp and NP1 deletion promoted cocaine-induced place preference, NPR deletion was without effect. Narp or NP1 deletion inhibited AMPA-induced locomotion, whereas NPR deletion potentiated the motor effect of AMPA. In addition, although Narp and NP1 KO showed reduced time in the center of a novel environment, NPR KO mice spent more time in the center. As reported previously, withdrawal from chronic cocaine potentiates the motor stimulant effect of intra-accumbens AMPA (Pierce et al., 1996), and the fact that this potentiation was inhibited in Narp KO is consistent with the marked down-regulation of GluR1 in the ventral striatal PSD in Narp KO mice withdrawn from chronic cocaine administration. Taken together, these data implicate the NPs as potential regulators of the behavioral and cellular adaptations produced by chronic cocaine.
Neuronal Pentraxins and Cocaine-Induced Behavioral Adaptations. Cocaine administration initiates reward learning causing animals to develop a preference for the location where cocaine is administered relative to a location where a more neutral saline injection is given (Kelley, 2004; Tzschentke, 2007). The ability to make this association was potentiated in Narp and NP1 KO mice. Previous work revealed that Narp KO mice were unimpaired in acquiring conditioned reinforcement and Pavlovian-instrumental transfer in food-rewarded learning (Johnson et al., 2007) and morphine place conditioning (Crombag et al., 2008). By showing augmented cocaine-rewarded learning, the present findings are consistent with the lack of impairment by Narp gene deletion on reward learning and indicate interactions with cocaine-specific learning. A specific interaction with cocaine was indicated by the fact that Narp KO showed an impaired locomotor response to acute cocaine administration but normal caffeine-induced locomotion. Interestingly, although the acute response was impaired, the capacity to develop sensitized motor behavior with repeated administration appeared largely intact. However, akin to the similarities between the Narp and NP1 KO genotypes in cocaine place preference, both genotypes showed delayed development of sensitized motor behavior.
The co-occurrence of augmented place preference but delayed sensitization was surprising given the literature supporting the role of sensitization of incentive learning in chronic cocaine-treated animals (Robinson and Berridge, 2001). One possible explanation for this apparent dissociation may be the inability of Narp or NP1 KO to regulate AMPAR in response to cocaine. Thus, the capacity of AMPAR stimulation to induce behavioral activation was markedly reduced in the Narp and NP1 KO mice, and some studies have indicated that decreased GluR1 in the nucleus accumbens is associated with increased cocaine reward (Sutton et al., 2003), whereas others show that blocking AMPAR inhibits cocaine-induced behavioral sensitization (Vanderschuren and Kalivas, 2000). Furthermore, the overexpression of WT GluR1 in accumbens reduced cocaine-induced place conditioning (Kelz et al., 1999); recently, Bachtell et al. (2008) showed that overexpressing a WT-GluR1 in accumbens increases AMPA-induced locomotion after cocaine treatment but decreases cocaine sensitization and reinstatement. In addition, the overexpression of GluR1 holding a single point mutation (Q582E) in the pore region that reduces synaptic current through AMPAR decreases AMPA-induced locomotion after a cocaine treatment but increases cocaine sensitization and reinstatement. In the present study, Narp and NP1 KO showed similar responses to the overexpression of the mutant GluR1 in the Bachtell et al. (2008) study, suggesting that in the absence of pentraxins, the function of AMPAR may be decreased, which is also suggested by the lower DHPG-induced activity in Narp KO and by the reduced AMPA-induced activity in Narp/NP1 KO.
It should be noted that other studies examining the rewarding effects of cocaine using conditioned place preference have yielded mixed results regarding the involvement of AMPAR. Furthermore, studies using AMPAR antagonists showed inhibition (Maldonado et al., 2007) or no effect (Cervo and Samanin, 1995) on cocaine place conditioning. Similar discrepancies were found using GluR1 KO mice where Dong et al. (2004) showed blunted cocaine-induced conditioned place preference in KO mice, but Mead et al. (2005) showed no differences in conditioned place preference between KO and WT mice. Notwithstanding, it is important to acknowledge that because the gene deletions were constitutive KO, it is not possible to rule out possible developmental influences of the missing protein that may underlie the differential effects on cocaine-induced neuroadaptations.
It is also important to note the possibility that the different levels of anxiety in the Narp, NP1, and NPR KO compared with WT may interfere with the interpretation of the biased place conditioning procedure. Thus, it is possible that habituation to the anxiety-provoking effects of the nonpreferred side may contribute to the increase in time spent in the nonpreferred side associated with repeated cocaine administration. However, exposure of the Narp and NP1 KO mice to the nonpreferred side after saline injections increased occupancy time similar to WT, and this increase was significantly lower than cocaine-induced increase in occupancy time in the Narp and NP1 KO genotypes.
Neuronal Pentraxins and AMPA Receptor Plasticity. Although not quantified for all genotypes, the reduction in GluR1 in the PSD subfraction from the ventral striatum of Narp KO only after withdrawal from cocaine reveals an interesting interaction between cocaine regulation of AMPAR and Narp. Previous studies show an increase in GluR1 after withdrawal from chronic cocaine that is associated with the development of behavioral sensitization (Boudreau and Wolf, 2005). Although behavioral correlations were not evaluated in the present study, the absence of Narp altered the effect of cocaine on AMPAR expression in the PSD fraction. Thus, only in the absence of Narp were the levels of GluR1 significantly reduced in the PSD subfraction after chronic cocaine, whereas in WT mice, the decrease after cocaine is just a trend. At present, it is not clear whether the presumed reduced capacity to cluster AMPAR in Narp KO results in less GluR1 after chronic cocaine because of a change in the trafficking of GluR1 into or out of the PSD subfraction or a change in synthesis of AMPAR subunits. However, the lack of change in GluR1 in the non-PSD fraction of either genotype indicates that major changes in synthesis were not present.
It is tempting to speculate that the functional segregation of NPs in synaptogenesis and excitatory transmission may underlie the differential effects of individual NP gene deletion on AMPA-induced motor activity, cocaine-induced place preference, and behavioral sensitization. Thus, the presumed deficit in AMPAR clustering associated with Narp or NP1 gene deletion would be predicted to impair AMPAR signaling. Conversely, NPR KO mice show reduced mGluR5-dependent long-term depression because of reduced internalization in NPR KO (Cho et al., 2008), which would be predicted to potentiate AMPA-induced responses. Correspondingly, Narp and NP1 deletions inhibited AMPA-induced locomotion, and NPR deletion augmented the motor response. In addition, cocaine place preference was enhanced, and the development of behavioral sensitization was retarded in Narp and NP1 KO, whereas place preference and behavioral sensitization were either inhibited or not affected in NPR KO. As discussed above, this segregation of cocaine phenotypes to specific genotypes is consistent with portions of the behavioral literature regarding a role for AMPAR in differentially regulating cocaine-induced reward and locomotion.
Conclusions
This study demonstrates a differential interaction between the various NPs and cocaine. The behavioral distinctions between the NPs correspond to previously reported functional categories in that deletion of the NP genes responsible for clustering AMPAR, Narp and NP1, resulted in a behavioral phenotype distinct from deletion of NPR, which is responsible for the internalization of clustered AMPAR. Moreover, it was shown that Narp may contribute to the regulation of AMPAR by cocaine because GluR1 levels in the PSD were reduced by a combination of withdrawal from chronic cocaine and Narp gene deletion. These data support the possibility that the NPs influence the excitatory neuroplasticity produced in the accumbens by chronic cocaine administration that is thought to contribute to addiction.
Acknowledgments
We thank Patricia B. Steinner for technical assistance in the completion of the experiments.
ABBREVIATIONS:
- NP
neuronal pentraxin
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPAR
AMPA receptor
- Narp
neuronal activity-regulated pentraxin
- NPR
neuronal pentraxin receptor
- mGluR
group I metabotropic glutamate receptor
- GluR
glutamate receptor subunit
- KO
knockout
- WT
wild type
- DHPG
(S)-3,5-dihydroxyphenylglycine
- PSD
postsynaptic density fraction
- ANOVA
analysis of variance
Footnotes
This study was supported by United States Public Health Service [Grants DA03906 and DA015851].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
References
- Bachtell RK, Choi KH, Simmons DL, Falcon E, Monteggia LM, Neve RL, Self DW. Role of GluR1 expression in nucleus accumbens neurons in cocaine sensitization and cocaine-seeking behavior. Eur J Neurosci. 2008;27:2229–2240. doi: 10.1111/j.1460-9568.2008.06199.x. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bell K, Kalivas PW. Context-specific cross-sensitization between systemic cocaine and intra-accumbens AMPA infusion in the rat. Psychopharmacology. 1996;127:377–383. doi: 10.1007/s002130050101. [DOI] [PubMed] [Google Scholar]
- Berke JD, Paletzki RF, Aronson GJ, Hyman SE, Gerfen CR. A complex program of striatal gene expression induced by dopaminergic stimulation. J Neurosci. 1998;18:5301–5310. doi: 10.1523/JNEUROSCI.18-14-05301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjartmar L, Huberman AD, Ullian EM, Rentería RC, Liu X, Xu W, Prezioso J, Susman MW, Stellwagen D, Stokes CC, et al. Neuronal pentraxins mediate synaptic refinement in the developing visual system. J Neurosci. 2006;26:6269–6281. doi: 10.1523/JNEUROSCI.4212-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res. 1995;673:242–250. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- Cho RW, Park JM, Wolff SB, Xu D, Hopf C, Kim JA, Reddy RC, Petralia RS, Perin MS, Linden DJ, et al. mGluR1/5-dependent long-term depression requires the regulated ectodomain cleavage of neuronal pentraxin NPR by TACE. Neuron. 2008;57:858–871. doi: 10.1016/j.neuron.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Glutamate transmission in the nucleus accumbens mediates relapse in cocaine addiction. J Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Dickson M, Dinenna M, Johnson AW, Perin MS, Holland PC, Baraban JM, Reti IM. Narp deletion blocks extinction of morphine place preference conditioning. Neuropsychopharmacology. 2008 doi: 10.1038/npp.2008.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P, Everitt BJ. Dissociable effects of antagonism of NMDA and AMPA/KA receptors in the nucleus accumbens core and shell on cocaine-seeking behavior. Neuropsychopharmacology. 2001;25:341–360. doi: 10.1016/S0893-133X(01)00235-4. [DOI] [PubMed] [Google Scholar]
- Dong Y, Saal D, Thomas M, Faust R, Bonci A, Robinson T, Malenka RC. Cocaine-induced potentiation of synaptic strength in dopamine neurons: behavioral correlates in GluRA(−/−) mice. Proc Natl Acad Sci U S A. 2004;101:14282–14287. doi: 10.1073/pnas.0401553101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin BJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego, CA: 1997. [Google Scholar]
- Institute of Laboratory Animal Resources . Guide for the Care and Use of Laboratory Animals. 7th ed. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council; Washington, DC: 1996. [Google Scholar]
- Johnson AW, Crombag HS, Takamiya K, Baraban JM, Holland PC, Huganir RL, Reti IM. A selective role for neuronal activity regulated pentraxin in the processing of sensory-specific incentive value. J Neurosci. 2007;27:13430–13435. doi: 10.1523/JNEUROSCI.4320-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Chen J, Carlezon WA, Jr, Whisler K, Gilden L, Beckmann AM, Steffen C, Zhang YJ, Marotti L, Self DW, et al. Expression of the transcription factor deltaFosB in the brain controls sensitivity to cocaine. Nature. 1999;401:272–276. doi: 10.1038/45790. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick LL, Matzuk MM, Dodds DC, Perin MS. Biochemical interactions of the neuronal pentraxins: neuronal pentraxin (NP) receptor binds to taipoxin and taipoxin-associated calcium-binding protein 49 via NP1 and NP2. J Biol Chem. 2000;275:17786–17792. doi: 10.1074/jbc.M002254200. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Marinelli M, Xu D, Worley PF, Wolf ME. Amphetamine and cocaine do not increase Narp expression in rat ventral tegmental area, nucleus accumbens or prefrontal cortex, but Narp may contribute to individual differences in responding to a novel environment. Eur J Neurosci. 2002;15:2027–2036. doi: 10.1046/j.1460-9568.2002.02036.x. [DOI] [PubMed] [Google Scholar]
- Maldonado C, Rodríguez-Arias M, Castillo A, Aguilar MA, Miñarro J. Effect of memantine and CNQX in the acquisition, expression and reinstatement of cocaine-induced conditioned place preference. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:932–939. doi: 10.1016/j.pnpbp.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Mead AN, Brown G, Le Merrer J, Stephens DN. Effects of deletion of gria1 or gria2 genes encoding glutamatergic AMPA-receptor subunits on place preference conditioning in mice. Psychopharmacology. 2005;179:164–171. doi: 10.1007/s00213-004-2071-8. [DOI] [PubMed] [Google Scholar]
- O’Brien R, Xu D, Mi R, Tang X, Hopf C, Worley P. Synaptically targeted Narp plays an essential role in the aggregation of AMPA receptors at excitatory synapses in cultured spinal neurons. J Neurosci. 2002;22:4487–4498. doi: 10.1523/JNEUROSCI.22-11-04487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Xu D, Petralia RS, Steward O, Huganir RL, Worley P. Synaptic clustering of AMPA receptors by the extracellular immediate-early gene product Narp. Neuron. 1999;23:309–323. doi: 10.1016/s0896-6273(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Pacchioni AM, Vallone J, Melendez RI, Shih A, Murphy TH, Kalivas PW. Nrf2 gene deletion fails to alter psychostimulant-induced behavior or neurotoxicity. Brain Res. 2007;1127:26–35. doi: 10.1016/j.brainres.2006.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- Reti IM, Baraban JM. Sustained increase in Narp protein expression following repeated electroconvulsive seizure. Neuropsychopharmacology. 2000;23:439–443. doi: 10.1016/S0893-133X(00)00120-2. [DOI] [PubMed] [Google Scholar]
- Reti IM, Reddy R, Worley PF, Baraban JM. Prominent Narp expression in projection pathways and terminal fields. J Neurochem. 2002;82:935–944. doi: 10.1046/j.1471-4159.2002.01051.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Schrader LA, Tasker JG. Presynaptic modulation by metabotropic glutamate receptors of excitatory and inhibitory synaptic inputs to hypothalamic magnocellular neurons. J Neurophysiol. 1997;77:527–536. doi: 10.1152/jn.1997.77.2.527. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schmidt EF, Choi KH, Schad CA, Whisler K, Simmons D, Karanian DA, Monteggia LM, Neve RL, Self DW. Extinction-induced upregulation in AMPA receptors reduces cocaine-seeking behaviour. Nature. 2003;421:70–75. doi: 10.1038/nature01249. [DOI] [PubMed] [Google Scholar]
- Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci. 2001;21:9043–9052. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Dehoff MH, Kang SH, Frys KA, Lominac KD, Klugmann M, Rohrer J, Griffin W, 3rd, Toda S, Champtiaux NP, et al. Homer proteins regulate sensitivity to cocaine. Neuron. 2004;43:401–413. doi: 10.1016/j.neuron.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Toda S, Shen HW, Peters J, Cagle S, Kalivas PW. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J Neurosci. 2006;26:1579–1587. doi: 10.1523/JNEUROSCI.4132-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Xu D, Hopf C, Reddy R, Cho RW, Guo L, Lanahan A, Petralia RS, Wenthold RJ, O’Brien RJ, Worley P. Narp and NP1 form heterocomplexes that function in developmental and activity-dependent synaptic plasticity. Neuron. 2003;39:513–528. doi: 10.1016/s0896-6273(03)00463-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.