Abstract
Geographical variation in birdsong is taxonomically widespread and behaviourally salient, with females often preferring local over non-local song. However, the benefits associated with this preference remain poorly understood. One potential explanation is that song may reflect a male's place of origin and thus allow females to obtain genes well adapted to the local environment. We studied naturally occurring variation in the degree to which the elements of a male's song repertoire matched those of the local population (‘syllable sharing’) in migratory song sparrows (Melospiza melodia melodia). Syllable sharing was correlated with genetic similarity to the local population, suggesting that song reflects population of origin. Males sharing more syllables also had larger testosterone-dependent traits, fewer blood-borne parasites and reduced indicators of stress. Our findings are consistent with locally good genes models. Alternatively, immigrants' condition may suffer due to unfamiliarity with the breeding site or inability to match song elements during territorial interactions. Females preferring ‘local-sounding’ males may thus obtain genetic and/or direct benefits for their offspring.
Keywords: genetic similarity, geographical variation, local adaptation, locally good genes, song
1. Introduction
Because songbirds learn their songs, usually during early life, birdsong often varies geographically (Kroodsma & Baylis 1982). Such variation can have important implications for mate choice and evolution. In many species, females prefer local songs over those recorded from more distant sites (Searcy & Nowicki 2005). However, the question of whether and how such preferences benefit females remains unanswered.
One potential adaptive explanation is that song advertises place of origin. Thus, females may obtain genes well adapted to the local environment (Nottebohm 1969; Reinhold 2004). However, despite much attention, the relationship between song and population of origin remains unclear (Searcy & Nowicki 2005). Second, local song may confer social advantages. In western song sparrows (Melospiza melodia morphna), song sharing predicts territory tenure (Beecher et al. 2000). Thus, local singers may be better able to defend territories and provision their offspring. Third, accurately producing local-sounding song may reflect song-learning ability (Searcy et al. 2002) which may in turn advertise quality. Females preferring local song may thus obtain direct and/or genetic benefits for their offspring. Alternatively, preferring local song may be a non-adaptive by-product of species recognition mechanisms.
We examined the relationships between song, physiological condition and genetic similarity to the local population in song sparrows (Melospiza melodia melodia). Male song sparrows have repertoires of many song types (5–13 in our study population), which are important in establishing territories and attracting mates. Even in migratory populations, female song sparrows prefer local songs over those recorded from as little as 34 km away (Searcy et al. 2002). Our primary goal was to determine whether a male's song similarity to the local population (proportion of shared song elements) reflects genetic similarity to this population and/or condition. Because song and condition might be associated either indirectly (e.g. through locally good genes effects) or directly (through social advantages to producing local-sounding song), we attempted to distinguish the effects of genetic similarity on condition from those of song similarity.
2. Material and methods
(a) Study population
We studied song, genetic similarity and condition in song sparrows breeding near Newboro, Ontario. The study site supports 30–40 breeding pairs, and is not physically isolated from other surrounding populations. Song sparrows in this area are migratory, but philopatry is high. Almost half of the breeding adults (presumably all those surviving) return to the site the following year. Each year, 5–15% of new recruits have been banded on the study site as nestlings, providing a minimum estimate of natal philopatry. This suggests the potential for considerable population genetic structuring (but see Zink & Dittmann 1993).
We captured 29 adult male song sparrows in mist nets or Potter traps, from April to June 2006. Each subject received a unique combination of coloured leg bands to permit field identification. All subjects defended territories and bred (or attempted to breed) at the study site.
(b) Song recording and analysis
We recorded the song repertoires of 19 subjects in spring 2006. For the remaining 10 subjects, repertoires were recorded in previous years. Adult song sparrows do not alter their repertoires (Nordby et al. 2002), so we were confident that these repertoires remained unchanged. We considered a repertoire sampled in full after recording 300 consecutive or 450 non-consecutive songs (Pfaff et al. 2007).
Whole song matching is very rare at our site (in 2006, only one song type was shared), as in other M. m. melodia populations (Harris & Lemon 1972; Hughes et al. 1998). However, males often share song elements with their neighbours. Partial song matching is used in territorial interactions (Anderson et al. 2005), suggesting that alternative forms of song matching exist (Burt et al. 2002). We estimated song similarity based on the proportion of shared syllables in a male's repertoire (‘syllable sharing’). We generated spectrograms in Syrinx (J. Burt; www.syrinxpc.com), classified song types by visual inspection and generated a catalogue of syllables across all song types. Similar to Podos et al. (1992), we defined a syllable as one or more traces on the spectrogram which always occurred together. Shared syllables were identified by two independent observers' consensus. We identified a total of 135 syllables (19–48 per male), and screened each male's repertoire for the presence of each syllable. We generated a pairwise matrix of syllable sharing, using Jaccard's similarity coefficient adjusted for differences in repertoire size (Tracy & Baker 1999), calculated as
where c is the number of syllables common to both birds' repertoires; a is the syllables in A's repertoire but not in B's; b is the syllables in B's repertoire but not in A's; and d is the difference between A's and B's syllable repertoires. For each male, we calculated the average Sj with all other subjects, as an index of syllable sharing with the local population.
(c) Genetic analysis
We collected a small blood sample via brachial venepuncture from each subject within 3 min of capture for genetic and haematological analysis. We genotyped the birds at seven microsatellite loci: Escμ 1 (Hanotte et al. 1994); Pdoμ 5 (Griffith et al. 1999); Mme 2 and 7 (Jeffery et al. 2001); and Sosp 3, 13 and 14 (L. Keller 2006, personal communication). Amplification conditions are provided in the electronic supplementary material. We calculated Wang's (2002) relatedness coefficients for all pairs of subjects using Mark (K. Ritland; www.genetics.forestry.ubc.ca/ritland/programs.html), and calculated the average of all relatedness coefficients to estimate the degree to which each bird's genotype was representative of local allele frequencies (‘genetic similarity’).
(d) Physiological condition
The first time each male was captured, we measured the length of its cloacal protuberance (CP) to the nearest 0.5 mm using dial calipers. CP size is testosterone dependent and increases throughout the early breeding season, so we calculated the regression of the linear residual of CP against date to minimize seasonal effects. We also prepared thin-film blood smears, which were fixed in 100% methanol and treated with Wright–Giemsa stain. Smears were examined under a light microscope, using a 100× oil immersion objective lens to screen 10 000 erythrocytes. We noted the total number of haematozoan parasites (mostly genus Haemoproteus) and polychromatic (immature) erythrocytes, following Campbell (1995). We categorized 100 leucocytes per smear to calculate the heterophil : lymphocyte ratio (H/L). Both polychromasia and H/L are positively associated with stress (Maxwell 1993; Clinchy et al. 2004).
We entered each male's residual CP, parasite load, polychromasia and H/L into a principal components analysis (PCA) to create an index of physiological condition. Proportional data were arcsine transformed. PCA identified two components with eigenvalues over one, together explaining 73.9% of the observed variance (table 1). PC1, interpreted as indicating good condition, loaded positively with CP and negatively with parasite load, polychromasia and H/L.
Table 1.
Factor loadings for physiological condition in male song sparrows.
| variable | PC1 | PC2 |
|---|---|---|
| residual CP | 0.419 | −0.713 |
| parasite load | −0.606 | −0.652 |
| polychromasia | −0.912 | −0.121 |
| H/L | −0.745 | 0.277 |
| cumulative variance explained | 48.2% | 73.9% |
| eigenvalue | 1.930 | 1.026 |
(e) Statistics
We used simple correlations to examine the links between syllable sharing and genetic similarity, syllable sharing and condition, and genetic similarity and condition. For variables used in multiple analyses, we applied sequential Bonferroni corrections as needed. We used partial correlation to examine the associations between syllable sharing and condition, when the effects of genetic similarity were removed, and between genetic similarity and condition, when the effects of syllable sharing were removed. All data were normally distributed (Kolmogorov–Smirnov test) and all tests were two-tailed.
3. Results
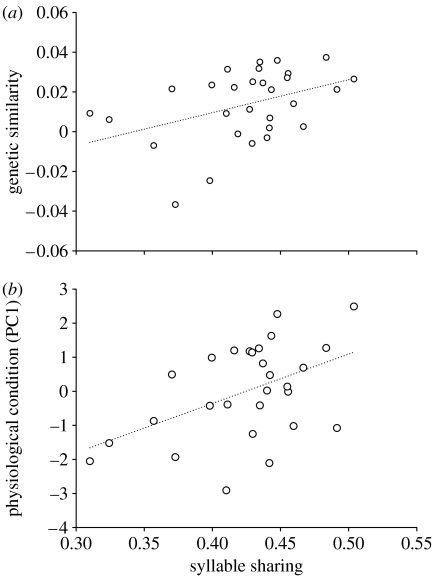
Syllable sharing was positively correlated with genetic similarity to the local population (Pearson's r=0.414, p=0.025; figure 1a) and with condition as assessed by PC1 (r=0.476, p=0.010; figure 1b) but not by PC2 (r=−0.334, p=0.083). Genetic similarity was positively related to PC1 (r=0.437, p=0.020) but not to PC2 (r=−0.165, p=0.401).
Figure 1.
Syllable sharing is positively associated with (a) genetic similarity to the local population and (b) physiological condition (PC1).
When the effects of genetic similarity were removed, the partial correlation between syllable sharing and condition (PC1) approached significance (partial r=0.360, p=0.065). Removing the effects of syllable sharing resulted in no significant correlation between genetic similarity and PC1 (partial r=0.299, p=0.129).
4. Discussion
Our findings suggest that singing locally common song elements does indeed indicate a locally common genotype. This represents one of only a few demonstrated links between neutral-locus genotypes and geographical variation in song (Searcy & Nowicki 2005), and the first such finding (to our knowledge) in a system where song varies gradually among populations (Searcy & Nowicki 1999). If song reflects population of origin, this represents an important prerequisite for it being an isolating mechanism. Assignment tests, taking into account allele frequencies in surrounding populations, are clearly warranted.
Males producing locally common song elements were in better condition, with larger testosterone-dependent traits, fewer parasites and reduced indicators of physiological stress. We suggest several possible explanations for this finding. First, song and condition may be linked indirectly through dispersal, if each reflects population of origin. Philopatric males might outperform immigrants for several reasons, including locally good genes effects (Nottebohm 1969; Reinhold 2004), familiarity with the local area and/or inherent differences in quality. Second, the link between song and condition may be mediated through territorial interactions. Sharing song elements with rivals may affect the efficacy of territorial defence, and thereby condition. This possibility is supported somewhat by the nearly significant partial correlation between syllable sharing and condition, when controlling for genetic similarity. In M. m. morphna, song matching is clearly important to territoriality (Beecher et al. 2000). In M. m. melodia, similarly, locally and distantly recorded songs elicit different levels of aggression from territorial males (Harris & Lemon 1974; Searcy et al. 2002). However, the geographical scale at which this discrimination occurs may exceed typical dispersal distances (Zink & Dittmann 1993). Moreover, naturally occurring variation in whole and partial song sharing is not associated with territorial advantages in Pennsylvania M. m. melodia (Hughes et al. 2007). Third, local-sounding song may reflect song copying ability (Searcy et al. 2002), which may in turn advertise resistance to developmental stress (Nowicki et al. 1998). Thus, males that accurately produce local song elements may confer material and/or genetic benefits to their offspring.
Despite the potential advantages to preferring local song, to the extent that inbreeding depression occurs, females may face an important trade-off. Selecting local-sounding mates may provide direct and/or genetic benefits for their offspring, but at the same time increase inbreeding risk. The associations between song, genotype and condition suggest a clear advantage to local song preferences, but also raise questions as to the mechanisms underlying these relationships.
Acknowledgments
This research was approved by the University of Western Ontario's Animal Use Subcommittee.
We thank C. Hoggard, S. MacDougall-Shackleton, D. Mennill, L. Morton and M. Teeter for their assistance, J. Pfaff for recordings, L. Keller for primer sequences, four anonymous referees for their comments, Queen's University Biological Station for logistic support, and NSERC Canada and the Canada Foundation for Innovation for funding.
Supplementary Material
Microsatellite amplification conditions
References
- Anderson R.C, Searcy W.A, Nowicki S. Partial song matching in an eastern population of song sparrows, Melospiza melodia. Anim. Behav. 2005;69:189–196. doi:10.1016/j.anbehav.2004.02.019 [Google Scholar]
- Beecher M.D, Campbell S.E, Nordby J.C. Territory tenure in song sparrows is related to song sharing with neighbours, but not song repertoire size. Anim. Behav. 2000;59:29–37. doi: 10.1006/anbe.1999.1304. doi:10.1006/anbe.1999.1304 [DOI] [PubMed] [Google Scholar]
- Burt J.M, Bard S.C, Campbell S.E, Beecher M.D. Alternative forms of song matching in song sparrows. Anim. Behav. 2002;63:1143–1151. doi:10.1006/anbe.2002.3011 [Google Scholar]
- Campbell T.W. 2nd edn. Iowa State University Press; Ames, IO: 1995. Avian hematology and cytology. [Google Scholar]
- Clinchy M, Zanette L, Boonstra R, Wingfield J.C, Smith J.N.M. Balancing food and predator pressure induces chronic stress in songbirds. Proc. R. Soc. B. 2004;271:2473–2479. doi: 10.1098/rspb.2004.2913. doi:10.1098/rspb.2004.2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith S.C, Stewart I.R.K, Dawson D.A, Owens I.P.F, Burke T. Contrasting levels of extra-pair paternity in mainland and island populations of the house sparrow (Passer domesticus): is there an ‘island effect’? Biol. J. Linn. Soc. 1999;68:303–316. [Google Scholar]
- Hanotte O, Zanon C, Pugh A, Greig C, Dixon A, Burke T. Isolation and characterization of microsatellite loci in a passerine bird: the reed bunting Emberiza schoeniclus. Mol. Ecol. 1994;3:529–530. doi: 10.1111/j.1365-294x.1994.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Harris M.A, Lemon R.E. Songs of song sparrows (Melospiza melodia): individual variation and dialects. Can. J. Zool. 1972;50:301–309. [Google Scholar]
- Harris M.A, Lemon R.E. Songs of song sparrows: reactions of males to songs of different localities. Condor. 1974;76:33–44. [Google Scholar]
- Hughes M, Nowicki S, Searcy W.A, Peters S. Song-type sharing in song sparrows: implications for repertoire function and song learning. Behav. Ecol. Sociobiol. 1998;42:437–446. doi:10.1007/s002650050458 [Google Scholar]
- Hughes M, Anderson R.C, Searcy W.A, Bottensek L.M, Nowicki S. Song type sharing and territory tenure in eastern song sparrows: implications for the evolution of song repertoires. Anim. Behav. 2007;73:701–710. doi:10.1016/j.anbehav.2006.09.013 [Google Scholar]
- Jeffery K.J, Keller L.F, Arcese P, Bruford M.W. The development of microsatellite loci in song sparrows, Melospiza melodia (Aves) and genotyping errors associated with good quality DNA. Mol. Ecol. Notes. 2001;1:11–13. doi:10.1046/j.1471-8278.2000.00005.x [Google Scholar]
- Kroodsma, D. E. & Baylis, J. R. 1982 A world survey of evidence for vocal learning in birds. In Acoustic communication in birds, vol. 2 (eds D. E. Kroodsma & E. H. Miller), pp. 311–337. New York, NY: Academic Press.
- Maxwell M. Avian blood leukocyte responses to stress. Worlds Poult. Sci. J. 1993;49:34–42. doi:10.1079/WPS19930004 [Google Scholar]
- Nordby J.C, Campbell S.E, Beecher M.D. Adult song sparrows do not alter their song repertoires. Ethology. 2002;108:39–50. doi:10.1046/j.1439-0310.2002.00752.x [Google Scholar]
- Nottebohm F. The song of the chingolo, Zonotrichia capensis, in Argentina: description and evaluation of a system of dialects. Condor. 1969;71:29–315. doi:10.2307/1366306 [Google Scholar]
- Nowicki S, Peters S, Podos J. Song learning, early nutrition and sexual selection in songbirds. Am. Zool. 1998;38:179–190. [Google Scholar]
- Pfaff J.A, Zanette L, MacDougall-Shackleton S.A, MacDougall-Shackleton E.A. Song repertoire size varies with HVC volume and is indicative of male quality in song sparrows (Melospiza melodia) Proc. R. Soc. B. 2007;274:2035–2040. doi: 10.1098/rspb.2007.0170. doi:10.1098/rspb.2007.0170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podos J, Peters S, Rudnicky T, Marler P, Nowicki S. The organization of song repertoires in song sparrows: themes and variations. Ethology. 1992;90:89–106. [Google Scholar]
- Reinhold K. Modeling a version of the good genes hypothesis: female choice of locally adapted males. Org. Divers. Evol. 2004;4:157–163. doi:10.1016/j.ode.2003.10.002 [Google Scholar]
- Searcy W.A, Nowicki S. Functions of song variation in song sparrows. In: Konishi M, Hauser M, editors. The design of animal communication. MIT Press; New York, NY: 1999. pp. 577–595. [Google Scholar]
- Searcy W.A, Nowicki S. Princeton University Press; Princeton, NJ: 2005. The evolution of animal communication: reliability and deception in signaling systems. [Google Scholar]
- Searcy W.A, Nowicki S, Hughes M, Peters S. Geographic song discrimination in relation to dispersal distances in song sparrows. Am. Nat. 2002;159:221–230. doi: 10.1086/338509. doi:10.1086/338509 [DOI] [PubMed] [Google Scholar]
- Tracy T.T, Baker M.C. Geographic variation in syllables of house finch songs. Auk. 1999;116:666–676. [Google Scholar]
- Wang J.L. An estimator for pairwise relatedness using molecular markers. Genetics. 2002;160:1203–1215. doi: 10.1093/genetics/160.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink R.M, Dittmann D.L. Gene flow, refugia, and evolution of geographic variation in the song sparrow (Melospiza melodia) Evolution. 1993;47:717–729. doi: 10.1111/j.1558-5646.1993.tb01228.x. doi:10.2307/2410178 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microsatellite amplification conditions