Abstract
In sperm-dependent sexual/asexual mating systems, male mate choice is critical for understanding the mechanisms behind apparent stability observed in natural populations. The gynogenetic Amazon molly (Poecilia formosa) requires sperm from sexual males (e.g. Poecilia latipinna) to trigger embryogenesis, but inheritance is strictly maternal. Consequently, males should try to avoid or reduce the cost of mating with asexuals. We investigated male mate choice by documenting the presence of sperm in natural populations and found that a higher proportion of sexual females had sperm than asexuals. In addition, among those females that had sperm, sexuals had more sperm than asexuals. Our results hint at a role for male mate choice as a stabilizing factor in such systems.
Keywords: Amazon molly, male mate choice, mate preference, sperm transfer, gynogenesis, Poeciliidae
1. Introduction
Understanding the evolution and maintenance of sexual reproduction remains a major challenge in evolutionary biology (West et al. 1999). Asexual, all-female species produce twice as many daughters and thus should quickly outcompete sexuals in the short run (Maynard Smith 1978; Bell 1982; Barton & Charlesworth 1998). Some asexual species, however, need to mate with males of heterospecific sexual species, the sperm of which triggers embryogenesis; this mechanism is called gynogenesis (Beukeboom & Vrijenhoek 1998; Schlupp 2005). In our study system, a gynogenetic fish (Poecilia formosa) of hybrid origin relies on sperm from host males of the two parental species (Poecilia latipinna or Poecilia mexicana). Here, local extinction of the sexual species would also bring about the local extinction of the asexuals. How can such a sexual/asexual mating complex be stable?
Two main types of regulatory mechanisms have been proposed. Ecological disadvantages for asexuals could explain the stability of mixed sexual/asexual systems. One example would be the ‘Red Queen hypothesis’, which states that recombination produces genetically diverse offspring that are harder to target by pathogens than the clonal asexuals (Van Valen 1973). However, currently no evidence supports this explanation in P. latipinna and P. formosa as they do not differ significantly in parasite loads (Tobler & Schlupp 2005). Alternative to ecological disadvantages, the ‘behavioural regulation hypothesis’ assumes that male mate choice regulates the system (reviewed in Schlupp 2005). In laboratory experiments, both P. latipinna and P. mexicana males have been shown to prefer mating with conspecifics (reviewed in Schlupp 2005), and P. latipinna males prefer larger over smaller females regardless of species (Gumm & Gabor 2005). Obviously, male rejection of heterospecific females would be adaptive and mating preferences for conspecifics are both predicted by theory and described in other sexual/asexual mating complexes (McKay 1971; Løyning & Kirkendall 1996; Engeler & Reyer 2001). Previous laboratory studies were able to research male choice on the behavioural level, including differential sperm production (Aspbury & Gabor 2004) and sperm transfer (Schlupp & Plath 2005), but so far studies investigating male choice under natural conditions have been missing.
We conducted a field study testing for male mate choice in mixed populations of P. latipinna and P. formosa. We compared the amount of sperm found in the genital tracts of sexual and asexual females as a proxy for male mate choice. Based on a recent metapopulation model, male mate choice and sperm limitation of asexuals are two factors predicted to drive such species complexes towards stability (Kokko et al. 2008). Hence, this is the first study that links behavioural patterns observed in the laboratory with fitness-relevant sperm transfer in the wild.
2. Material and methods
(a) Study populations
Both species were collected at five sites in Texas (see the electronic supplementary material for locations) during five field trips in 2006 throughout their natural breeding season (March, June, July, August and September). They were kept at densities of 30–35 fishes in aerated Styrofoam coolers in approximately 5 l of water and transported to a nearby field laboratory for sperm analyses. We assessed the relative frequencies of mature males and females (sexual and asexual) during each field trip by conducting six standardized hauls using a standard seine (4 m long and 4 mm mesh width) and consistently covering the same area. For total frequencies for each month, please refer to the electronic supplementary material.
(b) Field experiment
At the beginning of our study (all field sites in March and two sites in June) we kept females and males at an approximate ratio of 10 : 1 together in the same cooler for approximately 60–240 min. This was an unintended experiment, but it gave males and females an opportunity to mate after being captured (series 1). To avoid these matings and focus only on the matings that happened in nature, all subsequent sperm measurements were conducted on females that were separated from males upon capture (series 2).
(c) Semen extraction and sperm counts
Semen extraction and sperm counts followed standard protocols (e.g. Evans et al. 2003; Schlupp & Plath 2005; see the electronic supplementary material).
(d) Statistical analyses
All tests were calculated separately for series-1 and series-2 sampling times using SPSS 11 (SPSS, Inc. 2002). In series 1, the frequency of females with sperm was compared between both types of females using logistic regression. In series 2, ‘time of sampling’ (as proxy for time of year) was included as another factor. To test for differences between species in amount of sperm flushed out, females without sperm in their genital tract were excluded from the analysis. Because data were not normally distributed even after attempting transformation, non-parametric U-tests were employed. Female standard length was compared between species using t-tests.
(e) Validation of the technique
To test for bias in the applied sperm-retrieval technique between types of females, a laboratory experiment was conducted; we found no differences (see the electronic supplementary material).
3. Results
(a) Mate choice and sperm transfer with males in the coolers
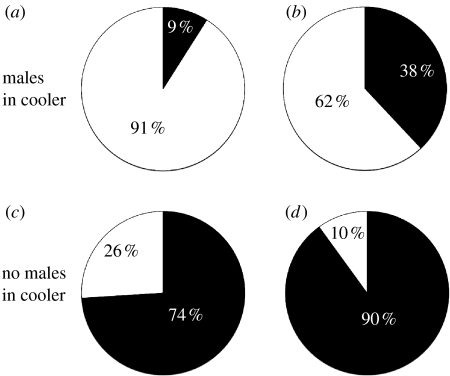
After being transferred from field sites to the laboratory together with males, a significantly higher proportion of sexual females had sperm in their genital tract (table 1; figure 1). Among those females that had sperm, P. latipinna females (total sperm, median: 783 333.5, IQR: 175 000, N=30) had significantly more sperm than P. formosa (median: 66 667, IQR: 141 667.3, N=28, U56=208.500, p<0.001), although P. formosa were on average larger (P. latipinna: 41.75±9.71 mm (mean±s.d.), N=51; P. formosa: 45.94±8.97 mm, N=72; t121=−2.473, p=0.015).
Table 1.
Logistic regression on the frequency of females with sperm in their genital tract.
| −2 log likelihood | B | s.e. | Wald | d.f. | p | |
|---|---|---|---|---|---|---|
| (a) Females with males in cooler (Χ12=9.033; p=0.003) | ||||||
| species | 79.773 | −1.804 | 0.679 | 7.053 | 1 | 0.008 |
| (b) Females without males in cooler (Χ12=15.696; p<0.001) | ||||||
| species | 219.747 | −1.148 | 0.349 | 10.808 | 1 | 0.001 |
| time of sampling | 219.747 | 23.453 | 3 | <0.001 | ||
Figure 1.
Pie charts depicting the percentage of females with sperm in their genital tract (white) to females without (black). (a,b) Females kept together with males prior to sperm analysis and (c,d) females kept separate from males prior to sperm analysis. (a,c) P. latipinna and (b,d) P. formosa.
(b) Mate choice and sperm transfer under natural conditions
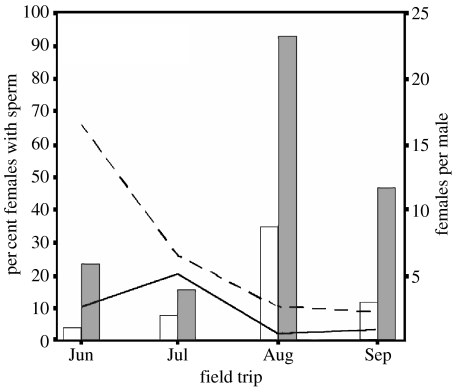
A significantly higher proportion of P. latipinna females had sperm in their genital tract than P. formosa when no males were in the coolers (table 1; figure 1). Time of year (time of sampling) also had a significant influence on the proportion of females with sperm (table 1; figure 2): during June 10.7±6.0% (mean±s.e.) of P. formosa had sperm, this number increased to 20.7±5.4% in July, but then dropped to 2.3±2.3 and 3.6±2.5% in August and September, respectively. In June 66.7±11.4% of P. latipinna had sperm, which decreased to 26.3±7.2, 10.5±7.2 and 8.9±4.3% in July, August and September, respectively (figure 2).
Figure 2.
Combined line and bar graphs. Lines represent the proportion of P. latipinna and P. formosa females with sperm in their genital tract on four separate field trips (all females were kept isolated from males prior to sperm analysis), and bars represent the total number of females per male in all populations combined on the day of sampling. Dashed line and open bars, P. latipinna; solid line and grey bars, P. formosa.
Among those females that had sperm in their genital tract, no significant difference was found between species in the total number of sperm retrieved (P. latipinna: median: 216 666.5, IQR: 800 000, N=36; P. formosa: median: 83 333, IQR: 107 666.5, N=21, U55=179.500; p=0.101). Again, P. formosa were larger (P. latipinna: 39.78±7.40 mm, N=120; P. formosa: 42.69±7.79 mm, N=185; t303=3.202; p=0.002).
(c) Species and sex ratio
Throughout our study, P. formosa was always the more common species. In addition, female P. latipinna outnumbered males (figure 2). However, an interesting shift occurred in August/September because males became disproportionally rare, while the ratio of female P. latipinna to P. formosa remained largely unchanged (figure 2).
4. Discussion
Our study resulted in two main findings. First, a higher proportion of P. latipinna than P. formosa had sperm in their genital tracts. This was true for series 1, in which matings were possible inside coolers, and, most importantly, also for series 2 where sperm transfer under natural conditions could be determined. Second, sexual P. latipinna had more sperm under all conditions (although not always statistically significant), even when males became exceedingly rare towards the end of the breeding season. These results are consistent with the predictions of a recent metapopulation model on the stability of this sexual/asexual mating complex (Kokko et al. 2008). Furthermore, our results are in agreement with behavioural studies in several other asexual/sexual mating systems, where males prefer sexual females over asexual females in the laboratory (reviewed in Schlupp 2005). Also, by interpreting pregnancy rates, asexual Poeciliopsis monacha-lucida were found to lack sperm more often than sexual P. lucida (McKay 1971). However, very few studies have directly looked at the role of sperm (but see Aspbury & Gabor (2004) and Schlupp & Plath (2005)).
We also found several other interesting patterns: P. formosa was more common than P. latipinna, and Amazon molly females were larger than their sexual counterparts during all field trips. Since under laboratory conditions P. latipinna males prefer larger females to smaller ones (Gumm & Gabor 2005), wild males apparently prefer to mate with P. latipinna females despite their smaller body size. These patterns are consistent with male mate choice operating in this system under natural conditions, and allow male mate choice to contribute to the stable coexistence of sexual and asexual forms by reducing fitness of P. formosa. However, we cannot rule out that other factors, like e.g. female competition for mating, play a role here (reviewed in Schlupp 2005).
Hubbs (1964) found that wild-caught P. formosa and P. latipinna females produce approximately the same number of eggs, but a higher proportion of these actually develop into embryos in P. latipinna. Theoretically, this difference in fecundity (i.e. number of developing embryos) could be explained by two scenarios: besides sperm limitation, lower fecundity in P. formosa could also be driven by other mechanisms, like developmental instability due to the accumulation of deleterious mutations in clonal, non-recombining asexuals (sensu Muller 1964). However, fecundity (i.e. number of offspring per female) of laboratory-reared P. formosa does not differ from that of equal-sized P. latipinna (I. Schlupp, A. Taebel-Hellwig & M. Tobler 2008, unpublished data), which further suggests that sperm limitation in nature due to male mate choice plays a substantial role in stability of this sexual/asexual mating complex.
The proportion of females with sperm fluctuated over the summer and decreased towards the end of the season in both species. Interestingly, the ratio of female P. latipinna to P. formosa did not change much in our study. Males, however, became proportionally rarer during the course of the breeding season. It has been suggested that male choice is negatively frequency dependent in this and other sexual/asexual mating systems (reviewed in Schlupp 2005), with males preferring the rare species and discriminating against asexuals only when conspecific females are rare. This question has been addressed in another poeciliid, Poeciliopsis, where several studies demonstrated that mating success of asexuals was inversely correlated with density (e.g. Moore & McKay 1971; Stenseth et al. 1985; but see Keegan-Rogers & Schultz 1988). Our data, however, do not support negative frequency dependence of male mate choice, since P. formosa was always the more common species. Rather our data mirror Løyning & Kirkendall's (1996) findings in the bark beetle Ips acuminatus, where males generally discriminate less when the ratio of males to females decreases.
In summary, compared with their sexual counterparts, only a small proportion of P. formosa successfully acquires sperm in syntopic populations. Furthermore, they most likely also receive less sperm than P. latipinna. Regardless of how much sperm is really needed to fertilize a whole clutch, this strongly suggests that wild P. formosa are sperm limited and underscores the role of male mate choice as a stabilizing factor in this system (see also Kokko et al. 2008). However, more research is needed to investigate how male mate choice influences life-history characteristics in this and other sexual/asexual mating complexes.
Acknowledgments
We thank the Texas Fish and Wildlife Department for collecting permits and also M. J. Ryan (UT Austin) for letting us use his laboratory. L. D. Devenport, J. C. Kelly, E. C. Marsh-Matthews and L. J. Weider kindly improved previous drafts of the manuscript. M. Tobler helped in the field and with the statistical analyses. This work represents partial fulfillment of the PhD requirements for R.R. and was partially funded by the University of Oklahoma and the Deutsche Forschungsgemeinschaft (to M.P.). The authors have adhered to the Guidelines for the Use of Animals in Research (R05-014 and R06-34).
Supplementary Material
Study populations; semen extraction and sperm counts; statistical analyses; validation of the sperm-flushing technique
References
- Aspbury A.S, Gabor C.R. Discriminating males alter sperm production between species. Proc. Natl Acad. Sci. USA. 2004;101:15 970–15 973. doi: 10.1073/pnas.0405653101. doi:10.1073/pnas.0405653101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton N.H, Charlesworth B. Why sex and recombination? Science. 1998;281:1986–1990. doi:10.1126/science.281.5385.1986 [PubMed] [Google Scholar]
- Bell G. University of California Press; Berkeley, CA: 1982. The masterpiece of nature: the evolution and genetics of sexuality. [Google Scholar]
- Beukeboom L.W, Vrijenhoek R.C. Evolutionary genetics and ecology of sperm-dependent parthenogenesis. J. Evol. Biol. 1998;11:755–782. doi:10.1007/s000360050117 [Google Scholar]
- Engeler B, Reyer H.-U. Choosy females and indiscriminate males: mate choice in mixed populations of sexual and hybridogenetic water frogs (Rana lessonae, Rana esculenta) Behav. Ecol. 2001;12:600–606. doi:10.1093/beheco/12.5.600 [Google Scholar]
- Evans J.P, Pierotti M, Pilastro A. Male mating behavior and ejaculate expenditure under sperm competition risk in the eastern mosquitofish. Behav. Ecol. 2003;14:268–273. doi:10.1093/beheco/14.2.268 [Google Scholar]
- Gumm J.M, Gabor C.R. Asexuals looking for sex: conflict between species and mate-quality recognition in sailfin mollies (Poecilia latipinna) Behav. Ecol. Sociobiol. 2005;58:558–565. doi:10.1007/s00265-005-0957-z [Google Scholar]
- Hubbs C.L. Interactions between a bisexual fish species and its gynogenetic sexual parasite. Bull. Tex. Mem. Mus. 1964;8:1–72. [Google Scholar]
- Keegan-Rogers V, Schultz R.J. Sexual selection among clones of unisexual fish (Poeciliopsis: Poeciliidae): genetic factors and rare-female advantage. Am. Nat. 1988;132:846–868. doi:10.1086/284893 [Google Scholar]
- Kokko H, Heubel K.U, Rankin D.J. How populations persist when asexuality requires sex: the spatial dynamics of coping with sperm parasites. Proc. R. Soc. B. 2008;275:817–825. doi: 10.1098/rspb.2007.1199. doi:10.1098/rspb.2007.1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løyning M.K, Kirkendall L.R. Mate discrimination in a pseudogamous bark beetle (Coleoptera: Scolytidae): male Ips acuminatus prefer sexual to clonal females. Oikos. 1996;77:336–344. doi:10.2307/3546074 [Google Scholar]
- Maynard Smith J.M. Cambridge University Press; Cambridge, UK: 1978. The evolution of sex. [Google Scholar]
- McKay F.E. Behavioral aspects of population dynamics in unisexual–bisexual Poeciliopsis (Pisces: Poeciliidae) Ecology. 1971;52:778–790. doi:10.2307/1936025 [Google Scholar]
- Moore W.S, McKay F.E. Coexistence in unisexual–bisexual species complexes of Poeciliopsis (Pisces: Poeciliidae) Ecology. 1971;52:791–799. doi:10.2307/1936026 [Google Scholar]
- Muller H.J. The relation of recombination to mutational advance. Mutat. Res. 1964;1:2–9. doi: 10.1016/0027-5107(64)90047-8. [DOI] [PubMed] [Google Scholar]
- Schlupp I. The evolutionary ecology of gynogenesis. Annu. Rev. Ecol. Evol. Syst. 2005;36:399–417. doi:10.1146/annurev.ecolsys.36.102003.152629 [Google Scholar]
- Schlupp I, Plath M. Male mate choice and sperm allocation in a sexual/asexual mating complex of Poecilia (Poeciliidae, Teleostei) Biol. Lett. 2005;1:169–171. doi: 10.1098/rsbl.2005.0306. doi:10.1098/rsbl.2005.0306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenseth N.C, Kirkendall L.R, Moran N. On the evolution of pseudogamy. Evolution. 1985;39:294–307. doi: 10.1111/j.1558-5646.1985.tb05667.x. doi:10.2307/2408363 [DOI] [PubMed] [Google Scholar]
- Tobler M, Schlupp I. Parasites in sexual and asexual mollies (Poecilia, Poeciliidae, Teleostei): a case for the Red Queen? Biol. Lett. 2005;1:166–168. doi: 10.1098/rsbl.2005.0305. doi:10.1098/rsbl.2005.0305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Valen L. A new evolutionary law. Evol. Theory. 1973;1:1–30. [Google Scholar]
- West S.A, Lively C.M, Read A.F. A pluralist approach to sex and recombination. J. Evol. Biol. 1999;12:1003–1012. doi:10.1046/j.1420-9101.1999.00119.x [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Study populations; semen extraction and sperm counts; statistical analyses; validation of the sperm-flushing technique