Abstract
Mangrove forests are influenced by tidal flooding and ebbing for a period of approximately 12.4 hours (tidal cycle). Mangrove crickets (Apteronemobius asahinai) forage on mangrove forest floors only during low tide. Under constant darkness, most crickets showed a clear bimodal daily pattern in their locomotor activity for at least 24 days; the active phases of approximately 10 hours alternated with inactive phases of approximately 2 hours, which coincided with the time of high tide in the field. The free-running period was 12.56±0.13 hours (mean±s.d., n=11). This endogenous rhythm was not entrained by the subsequent 24 hours light–dark cycle, although it was suppressed in the photophase; the active phase in the scotophase continued from the active phase in the previous constant darkness, with no phase shift. The endogenous rhythm was assumed to be a circatidal rhythm. On the other hand, the activity under constant darkness subsequent to a light–dark cycle was more intense in the active phase continuing from the scotophase than from the photophase of the preceding light–dark cycle; this indicates the presence of circadian components. These results suggest that two clock systems are involved in controlling locomotor activity in mangrove crickets.
Keywords: circatidal rhythm, mangrove cricket, circadian component
1. Introduction
Intertidal zones are influenced by tidal flooding and ebbing for a period of approximately 12.4 hours (tidal rhythm), and many intertidal animals show a tidal rhythm in their daily activity. Moreover, the rhythms of some species persist even under constant conditions, with these endogenous circatidal rhythms having been most studied in crustaceans (Barnwell 1966; Enright 1972; Akiyama 1995).
For insects, endogenous tidal rhythms in daily activity have been examined in the following three species. The nocturnal beetle Thalassotrechus barbarae exhibits a diel rhythm of locomotor activity under constant conditions, i.e. a circadian rhythm (a free-running period of 23.9 hours); however, it is more active during the period corresponding to low tide than at other times of night, although this trend disappears after 3 days (Evans 1976). The salt-marsh collembolan Anurida maritima has a bimodal lunar-day rhythm that persists for at least 4 days under constant conditions (Foster & Moreton 1981). The larvae of the intertidal tiger beetle Callytron inspecularis show a circatidal rhythm in their burrow-plugging activity, although the free-running rhythm disappears after 3 days (Satoh et al. 2006). However, these observations and experiments have been preliminary in all three studies, and no study has revealed clear circatidal activity rhythms that persist for a long period in insects.
‘Mangrove’ is a generic term that refers to trees and shrubs common in the intertidal zones of tropical and subtropical areas. Insects on mangrove forest floors must know the time of high tide to escape submergence. Mangrove crickets (Apteronemobius asahinai) are distributed only on the floors of mangrove forests in China, Southeast Asia, and the Ryukyu archipelago of Japan. Since these common crickets are active during low tide and rest on mangrove stems during high tide, regardless of whether it is day or night, their locomotor activity is expected to show a clear endogenous rhythm synchronized with the tidal cycle (rather than the day–night cycle). In this study, we examined locomotor activity under constant conditions and under a light–dark cycle to determine the endogenous rhythm of the mangrove cricket.
2. Material and methods
For experiment 1, 24 adult male crickets were collected from the mangrove forest in Kin, Okinawa Prefecture, Japan (26°27′ N, 127°56′ E), and for experiment 2, 18 adult males were collected from the mangrove forest in Nago, Okinawa (26°33′ N, 128°2′ E). Crickets were individually housed in plastic Petri dish chambers (5 cm in diameter) immediately after collection. An infrared beam (EE SPW-321; Omron, Kyoto, Japan) was passed across each chamber, and the number of times the beam was interrupted was recorded at 6 min intervals on a personal computer. A small piece of dried food made from insect pellet (Oriental Yeast, Tokyo, Japan), agar, sorbic acid and propionic acid was placed at one end of each chamber and a water source was placed at the other end; the food and water were at opposite ends of a line at a diagonal to the infrared beam. The temperature was maintained at 25°C, and the photophase was provided by a fluorescent lamp (FL15D; Toshiba, Tokyo, Japan) with an irradiance of approximately 1.4 W m−2.
Activity recording started on the day following collection. In experiment 1, locomotor activity was recorded under constant darkness (DD) for 25 days and under a 12 L : 12 D cycle (LD) for the subsequent 22 days. The constant darkness was interrupted by turning on a light for approximately 2 hours on the last day of constant darkness to adjust the recording equipment. In experiment 2, locomotor activity was recorded under LD for 14 days and under DD for the subsequent 12 days. Under LD, nine crickets were kept on the lighting schedule with light on at 00.00 hours, and the other nine crickets on the reversed lighting schedule, i.e. light on at 12.00 hours. Rhythmicity was statistically determined via a chi-square periodogram analysis.
3. Results
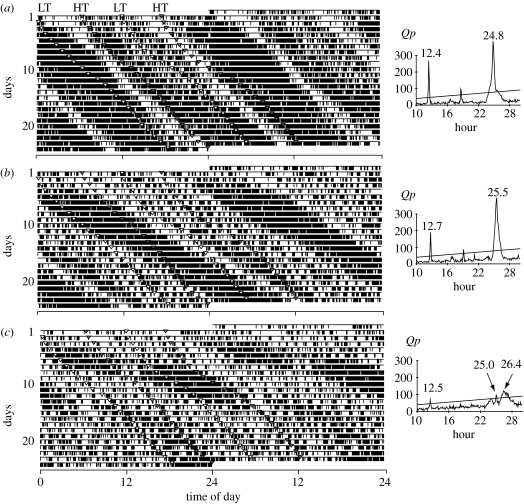
Eleven crickets showed a clear bimodal daily activity pattern under DD in experiment 1 (figure 1a,b). The activity rhythm consisted of active phases of approximately 10 hours alternating with inactive phases of approximately 2 hours. The free-running period (τ) was 12.56±0.13 hours (mean±s.d., n=11). During the first few days after collection, the active phase coincided with the time of low tide in the field, while the inactive phase coincided with the time of high tide. However, synchronization with the tidal cycle disappeared after the first few days in captivity because the period of the endogenous rhythm was slightly longer than that of the tidal cycle. In addition, intense activity alternating with weak activity was observed in some crickets (figure 1b). Intense activity was always observed during expected night-time for the first week. Seven crickets also showed a bimodal daily pattern, but the rhythm was not as clear (figure 1c). No cricket exhibited a unimodal daily pattern, i.e. circadian rhythmicity. The locomotor activity of 6 out of 24 crickets could not be recorded for an adequate period of time owing to technical problems or death of the crickets.
Figure 1.
(a–c) Three examples of the locomotor activity rhythm under DD for 24 days (presented by a double plotted actogram) and chi-square periodogram analyses (experiment 1). Arrows indicate times of expected low (LT; white) and high (HT; grey) tides in the field.
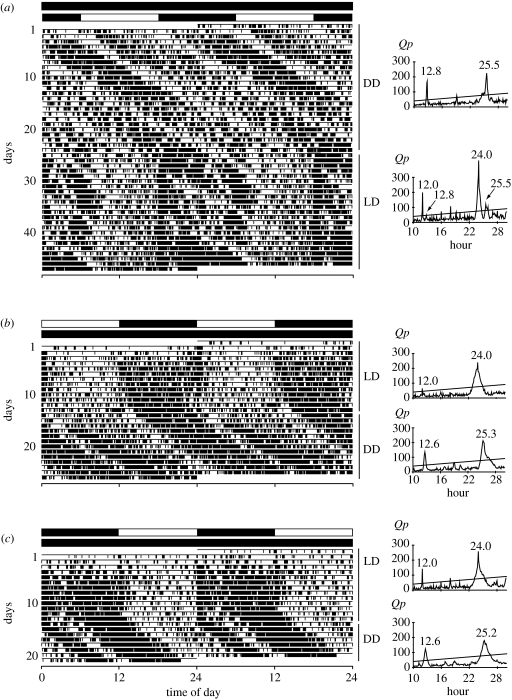
Under the subsequent LD, the endogenous rhythm with a period of approximately half a day did not become entrained to the light–dark cycle; the rhythm was expressed in the scotophase but suppressed in the photophase, and the active phase in the scotophase continued from the active phase in the previous constant darkness, with no phase shift (figure 2a). The maximum Qp value was at 24.0 hours, but many other peaks were also detected in the chi-square periodogram analysis under LD, including approximately 12 hours components. In experiment 2, the activity rhythm under LD was not clear even during the scotophase, but the bimodal daily activity pattern was observed under the subsequent DD (figure 2b,c). The activity was more intense in the active phase continuing from the scotophase than from the photophase of the preceding LD cycle; this indicates the presence of circadian components.
Figure 2.
(a) An example of the locomotor activity rhythm under DD for 25 days and under LD for the subsequent 22 days, and associated periodogram analyses (experiment 1). Note that constant darkness was interrupted by turning on a fluorescent lamp for approximately 2 hours during the last day of constant darkness (within the period shown by shaded region). (b,c) An example of the locomotor activity rhythm under LD for 14 days and under DD for the subsequent 12 days, and associated periodogram analyses (experiment 2). Note that the data after day 22 in (c) were excluded from the analysis because a technical problem disrupted the recording. Open square, photophase; filled square, scotophase.
4. Discussion
Mangrove crickets showed a clear and persistent bimodal daily pattern in their locomotor activity under constant conditions. The period of this free-running rhythm, which was estimated to average 12.6 hours, approximated that of the tidal cycle and the active phase coincided with expected low tide times in the field. This evidence strongly suggests that the endogenous rhythm of mangrove crickets is circatidal. By contrast, bimodal activity rhythms controlled by the circadian clock have been observed in some insects, such as the fly Drosophila melanogaster (Helfrich-Förster 2000), the mosquito Aedes aegypti (Taylor & Jones 1969), the cockroach Leucophaea maderae (Wiedenmann 1980) and the burying beetle Nicrophorus quadripunctatus (Nisimura et al. 2005). However, the endogenous rhythm of the mangrove cricket is obviously not a bimodal circadian rhythm or some modification thereof because this rhythm was not entrained by a light–dark cycle. The exposure to the light–dark cycle did not cause any phase shifts in the rhythm, which continued from the previous constant darkness. We therefore conclude that the endogenous rhythm of the mangrove cricket is a circatidal rhythm. The distinctness and persistence of the circatidal rhythm in mangrove crickets is unique among insects in which circatidal rhythm has been studied. Furthermore, the activity under DD of some crickets was more intense when it corresponded to a night low tide than to a day low tide in experiment 1, and the activity under DD was more intense in the active phase continuing from the scotophase than from the photophase of the preceding artificial light–dark cycles, regardless of its relationship to the solar time in experiment 2. We therefore conclude that a circadian clock still exists in mangrove crickets. Note, however, that this circadian clock does not control the endogenous rhythm, but modifies its intensity by inhibiting activity during daytime.
Terrestrial insects in intertidal zones are specialized to avoid drowning during high tides. For example, the beetle Dicheirotrichus gustavi is active only on nights during neap tides, when high tides are so low that its habitat is not submerged, and it remains underground all day during spring tides (Treherne & Foster 1977; Foster 1983). A more accomplished strategy is to develop an endogenous tidal rhythm to synchronize the daily activity with low tides. The endogenous tidal clock in mangrove crickets allows the ‘anticipation’ of high tide and allows the crickets to move to mangrove stems before inundation. Additionally, this endogenous rhythm appears to be beneficial in predator avoidance—mangrove crickets may need to become inactive during high tide so that they do not jump on the water surface, where they risk both drowning and being caught by fish arriving on the mangrove forest floor with the tide. To obtain these benefits, mangrove crickets must synchronize their endogenous rhythm accurately with the tidal cycle in the field. Endogenous circatidal rhythms are entrained by environmental cues related to the tidal cycle, e.g. periodic changes in water turbulence (Enright 1965; Ehlinger & Tankersley 2006), hydrostatic pressure (Akiyama 2004), inundation (Holmström & Morgan 1983), salinity (Taylor & Naylor 1977) and temperature (Naylor 1963). In addition, moonlight can entrain the circatidal rhythm (Saigusa 1988). For insects, only Satoh et al. (2006) have demonstrated that the rhythm can be synchronized by cyclic submergence in the case of tiger beetles. Elucidating the zeitgeber of the circatidal rhythm in mangrove crickets is a future research topic.
Acknowledgments
We thank Dr K. Tomioka and Dr S. Shiga for their valuable advice. This work was partly supported by a grant-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (no. 19770018) and from Fujiwara Natural History Foundation. This study was also supported by the twenty-first century COE programme of the University of the Ryukyus.
References
- Akiyama T. Circatidal swimming activity rhythm in a subtidal cumacean Dimorphostylis asiatica (Crustacea) Mar. Biol. 1995;123:251–255. doi:10.1007/BF00353616 [Google Scholar]
- Akiyama T. Entrainment of the circatidal swimming activity rhythm in the cumacean Dimorphostylis asiatica (Crustacea) to 12.5-hour hydrostatic pressure cycles. Zool. Sci. 2004;21:29–38. doi: 10.2108/0289-0003(2004)21[29:EOTCSA]2.0.CO;2. doi:10.2108/zsj.21.29 [DOI] [PubMed] [Google Scholar]
- Barnwell F.H. Daily and tidal patterns of activity in individual fiddler crab (Genus Uca) from the Woods Hole region. Biol. Bull. 1966;130:1–17. doi: 10.2307/1539948. doi:10.2307/1539948 [DOI] [PubMed] [Google Scholar]
- Ehlinger G.S, Tankersley R.A. Endogenous rhythms and entrainment cues of larval activity in the horseshoe crab Limulus polyphemus. J. Exp. Mar. Biol. Ecol. 2006;337:205–214. doi:10.1016/j.jembe.2006.06.035 [Google Scholar]
- Enright J.T. Entrainment of a tidal rhythm. Science. 1965;147:864–867. doi: 10.1126/science.147.3660.864. doi:10.1126/science.147.3660.864 [DOI] [PubMed] [Google Scholar]
- Enright J.T. A virtuoso isopod. Circa-lunar rhythms and their tidal fine structure. J. Comp. Physiol. 1972;77:141–162. doi:10.1007/BF00693603 [Google Scholar]
- Evans W.G. Circadian and circatidal locomotory rhythms in the intertidal beetle Thalassotrechus barbarae (Horn): Carabidae. J. Exp. Mar. Biol. Ecol. 1976;22:79–90. doi:10.1016/0022-0981(76)90110-6 [Google Scholar]
- Foster W.A. Activity rhythms and the tide in a saltmarsh beetle Dicheirotrichus gustavi. Oecologia. 1983;60:111–113. doi: 10.1007/BF00379328. doi:10.1007/BF00379328 [DOI] [PubMed] [Google Scholar]
- Foster W.A, Moreton R.B. Synchronization of activity rhythms with the tide in a saltmarsh collembolan Anurida maritima. Oecologia. 1981;50:265–270. doi: 10.1007/BF00348049. doi:10.1007/BF00348049 [DOI] [PubMed] [Google Scholar]
- Helfrich-Förster C. Differential control of morning and evening components in the activity rhythm of Drosophila melanogaster—sex-specific differences suggest a different quality of activity. J. Biol. Rhythms. 2000;15:135–154. doi: 10.1177/074873040001500208. [DOI] [PubMed] [Google Scholar]
- Holmström W.F, Morgan E. Laboratory entrainment of the rhythmic swimming activity of Corophium volutator (Pallas) to cycles of temperature and periodic inundation. J. Mar. Biol. Assoc. UK. 1983;63:861–870. [Google Scholar]
- Naylor E. Temperature relationships of the locomotor rhythm of Carcinus. J. Exp. Biol. 1963;40:669–679. [Google Scholar]
- Nisimura T, Numata H, Yoshioka E. Effect of temperature on circadian rhythm controlling the crepuscular activity of the burying beetle Nicrophorus quadripunctatus Kraatz (Coleoptera: Silphidae) Entomol. Sci. 2005;8:331–338. doi:10.1111/j.1479-8298.2005.00132.x [Google Scholar]
- Saigusa M. Entrainment of tidal and semilunar rhythms by artificial moonlight cycles. Biol. Bull. 1988;174:126–138. doi:10.2307/1541779 [Google Scholar]
- Satoh A, Momoshita H, Hori M. Circatidal rhythmic behaviour in the coastal tiger beetle Callytron inspecularis in Japan. Biol. Rhythm Res. 2006;37:147–155. doi:10.1080/09291010500429939 [Google Scholar]
- Taylor B, Jones M.D.R. The circadian rhythm of flight activity in the mosquito Aedes aegypti (L.): the phase-setting effects of light-on and light-off. J. Exp. Biol. 1969;51:59–70. doi: 10.1242/jeb.51.1.59. [DOI] [PubMed] [Google Scholar]
- Taylor C.A, Naylor E. Entrainment of the locomotor rhythm of Carcinus by cycles of salinity change. J. Mar. Biol. Assoc. UK. 1977;57:273–277. [Google Scholar]
- Treherne J.E, Foster W.A. Diel activity of an intertidal beetle, Dicheirotrichus gustavi Crotch. J. Anim. Ecol. 1977;46:127–138. doi:10.2307/3951 [Google Scholar]
- Wiedenmann G. Two peaks in the activity rhythm of cockroaches controlled by one circadian pacemaker. J. Comp. Physiol. 1980;137:249–254. doi:10.1007/BF00657120 [Google Scholar]