Abstract
Aggressive competition is an important aspect of social interactions, but conflict can be costly. Some animals are thought to minimize the costs of conflict by using conventional signals of agonistic ability (i.e. badges of status) to assess rivals. Although putative badges have been found in a range of taxa, little research has tested whether individuals use badges to assess potential rivals before they engage in aggressive contests. Here, choice trials were used to test how the variable black facial patterns in Polistes dominulus wasps are used during rival assessment. Focal wasps were given access to two patches of food, each guarded by a wasp whose facial pattern had been experimentally altered. Wasps chose food patches based on the facial pattern of the guard, preferring to challenge guards with facial patterns indicating a low level of quality, while avoiding guards with facial patterns indicating a high level of quality. Therefore, status badges play an important role during rival assessment; paper wasps use facial patterns alone to quickly assess the agonistic abilities of strangers.
Keywords: resource-holding potential, dominance, badge of status, melanin, condition dependent, sparrow
1. Introduction
Rival assessment reduces costly aggressive interactions by allowing individuals to avoid escalated contests with high-quality opponents. Assessment is often based on characteristics that are logically associated with fighting ability such as body size or call frequency (Parker 1974; Hurd 2006). In some taxa, individuals may use conventional signals to assess rivals. Conventional signals differ from other signals of fighting ability because the signals have no logical a priori connection with their bearer's fighting ability. For example, the colour of a karate belt conveys information about fighting ability, but there is no logical reason to assume that a person with a black belt will be a better fighter than a person with a yellow belt unless you know the convention. Conventional signals have also been termed ‘badges of status’ (or badges). The classic badge is the black throat patch in sparrows (Rohwer 1975; Whitfield 1987), though badges are also found in other taxa (Part & Qvarnstrom 1997; Pryke & Andersson 2003; Whiting et al. 2003; Tibbetts & Dale 2004).
It is not obvious how conventional signals could accurately convey information about fighting ability. As a result, there has been debate about whether badges are true signals of fighting ability or merely correlated with dominance (Whitfield 1987; Senar 2006). The critical test of badge signal value is whether receivers use badges to assess rivals before they interact. A few studies have shown that birds use conventional signals to choose among rivals (Great tit, Jarvi & Bakken 1984; Harris Sparrow, Rohwer 1985; Auklet, Jones 1990; Siskin, Senar & Camerino 1998), but, to our knowledge, there have been no studies in non-avian taxa.
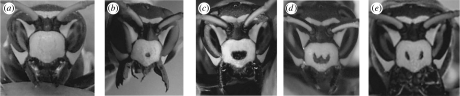
Polistes dominulus paper wasps have conspicuous black facial patterns that provide a good system for studying the role of badges during rival choice (figure 1). Previous work has shown that P. dominulus facial patterns are associated with size (Tibbetts & Dale 2004; Tibbetts 2006). Independently of size, facial patterns are associated with dominance (Tibbetts & Dale 2004) and condition (Tibbetts & Curtis 2007). The relevant aspect of P. dominulus facial patterns is the amount of disruption or ‘brokenness’ in the black facial coloration. The number of facial spots is a good proxy for brokenness, with ‘0’ spots indicating low quality and ‘2’ spots indicating high quality (figure 1). We tested whether P. dominulus use facial patterns to choose among potential opponents.
Figure 1.
(a–e) Portraits of five P. dominulus paper wasps illustrating some of the naturally occurring diversity in the size, shape and number of black facial spots. The wasps are arrayed from (a) lowest advertised quality (0 spots) to (e) highest advertised quality (2 spots).
2. Material and methods
The focal wasps were allowed to choose between two patches of food. Each food patch was guarded by a wasp with an experimentally manipulated facial pattern. All wasps were nest-founding females collected from the wild in early May. Most wasps were from single-foundress nests, though one pair of co-founding queens were used as focal wasps (their exclusion does not influence the results). Guards and focal wasps were collected from sites at least 4 km apart to ensure that they had not previously interacted. Every trial used a different focal wasp, with no duplication within or between experiments. All the focal wasps had a single spot, indicating an average level of quality.
Choice trials were performed in a triangle-shaped arena (7 cm wide×6 cm long). At the narrow end of the arena there was a covered antechamber. At the opposite end of the arena, there were two sugar cubes. The wasps eat nectar in the wild and are reared on rock sugar in the laboratory. Food sharing among wasps occurs on and off nests. Wasps aggregate at rich food sources and access to food is influenced by dominance rank (Tibbetts & Reeve 2000; Dapporto et al. 2005). A freshly freeze-killed ‘guard’ wasp was positioned on top of each sugar cube. One of the authors (R.L.) chose pairs of guards that were similarly sized (within 10 mg) and had similar facial patterns and placed them in individual plastic containers. The other author (E.A.T.) randomly assigned guards to the high- or low-quality treatment before handling or observing the wasps. E.A.T. painted both the guards using a similar amount of the same colour of testors enamel paint, but the paint was applied to different areas of the clypeus to create guards with high- versus low-quality appearance. For example, in trials with a 1 versus 2 spot choice, both guards originally had 0 spots on their clypeus. The faces of both the guards were painted with black paint to produce one guard with one black spot and another guard with two black spots. In trials with a 0 versus 2 spot choice, both guards originally had one clypeal spot and both were painted with yellow paint to produce a 0 spot and a 2 spot guard. In trials with a 0 versus 1 spot choice, both guards originally had two clypeal spots. One wasp was painted with yellow paint to produce a 0 spot guard, while the other was painted with black paint to produce a 1 spot guard. By choosing similar pairs of guards and randomly assigning the experimental treatment, we ensured that the only consistent difference between the guards was the number of facial spots. Nine different pairs of guards were used.
The focal wasps were starved for 24 hours before the trial. Then they were placed in the closed antechamber for 5 min before they were released into the trial arena. We defined a choice as being made when the focal wasp opened her mandibles and began licking a sugar cube. The median time from release until eating was 1 min (range=4 s to 24 min). The focal wasps were given 30 min to make a choice because some individuals rested and/or groomed in the antechamber and/or trial arena before approaching the guards.
3. Results
The focal wasps were significantly more likely to eat at the food patch guarded by the wasp with a facial pattern advertising a low level of quality (39/48 trials) than at the food patch guarded by the wasp with a facial pattern advertising a high level of quality (9/48 trials; table 1, Pearson's Χ2=18.7, p=0<0.0001). Even though focal wasps strongly avoided guards advertising a high level of quality, the specific combination of guard facial patterns did not influence focal wasps' choices (table 1, Χ2=3.89, p=0.14). The focal wasps had no preference for the food patch on the right versus left of the arena (Χ2=0.33, p=0.56). Guard weight did not influence choices (t=0.5, p=0.6).
Table 1.
Polistes dominulus use facial patterns to assess potential opponents. (The focal wasps were more likely to approach a food patch guarded by a wasp with fewer black facial spots than a patch guarded by a wasp with more black facial spots (Pearson's Χ2=18.7, p=0<0.0001).)
| guard facial patterns | focal wasp choice | |
|---|---|---|
| low-quality guard | high-quality guard | |
| 0 versus 1 spot | 10 | 4 |
| 0 versus 2 spots | 17 | 5 |
| 1 versus 2 spots | 12 | 0 |
| total | 39 | 9 |
4. Discussion
The experimental results show that the variable black facial patterns in P. dominulus wasps function as badges that signal social status. Wasps avoid opponents with badges that signal higher quality, preferring to challenge opponents that signal lower quality. These results support the foundation of badge models by showing that receivers use badges to quickly assess the agonistic abilities of strangers. As a result, badges can reduce the costs of aggressive competition by allowing rapid rival assessment without overt aggression.
Given the long history of badge research, surprisingly few studies have shown that badges are used for rival assessment (Jarvi & Bakken 1984; Rohwer 1985; Senar & Camerino 1998). Research on the signal value of badges is particularly challenging because badge accuracy is thought to be maintained via increased aggression towards inaccurate signallers (i.e. social costs). As a result, receivers are expected to ‘probe’ signal accuracy instead of always ‘trusting’ that badges accurately reflect their bearer's abilities. This can produce apparent inconsistencies in experimental outcomes, as receiver responses to experimentally altered signals will vary based on whether receivers trust or probe signal accuracy. Consequently, receiver responses may vary depending on whether or not receivers interact with senders, the length of their interactions and the value of the resource being contested (Maynard-Smith & Harper 1988; Senar 2006).
Receiver responses to badge alteration are context dependent in P. dominulus. During the choice trials described here, wasps behave as if they do not discern signal inaccuracy. They avoid rivals with facial patterns signalling a high level of quality even if the badge does not reflect its bearer's true behavioural dominance. However, prolonged behavioural interactions allow wasps to assess whether or not a badge accurately reflects its bearer's fighting ability. In longer-term interactions, wasps with experimentally altered badges suffer social costs; they receive much more aggression than controls and do not rise in status (Tibbetts & Dale 2004). Therefore, testing receiver responses to badges in multiple contexts is key to understanding both the meaning of the signal and the role of social costs in maintaining signal accuracy.
Paper wasp's use of a visual signal of agonistic ability is particularly interesting because most of the insect communication research focuses on other sensory modalities (Gerhardt & Huber 2002; Howard & Blomquist 2005). For example, there is a long history of research on chemical communication in social insects. Social insects use cuticular hydrocarbons (CHCs) to signal nest-mate identity and CHCs may also be involved in fertility and/or dominance signalling within nests (Sledge et al. 2001; Howard & Blomquist 2005; Monnin 2006; Dapporto et al. 2007). This study isolated the role of visual signals in Polistes communication by experimentally altering visual signals while leaving CHCs unaltered. Our results demonstrate that wasps can use visual signals alone to assess unfamiliar rivals.
Current evidence suggests that chemical and visual signals convey different messages and are used in different contexts. Badges are expected to be particularly valuable for assessing unfamiliar rivals (Rohwer 1975; Whitfield 1987), so wasps may primarily use visual signals during the early nest-founding phase when potential queens battle for dominance, colony membership is flexible and nest usurpation is common (Reeve 1991). In contrast, CHCs are associated with fertility and may primarily mediate interactions between queens and workers later in the colony cycle (Monnin 2006). Future experiments that independently manipulate CHCs and facial patterns are key for confirming the relative roles of chemical and visual signals during paper wasp communication.
Overall, the variable black facial patterns in P. dominulus wasps function as a badge of status used during rival assessment. Additional studies on badges in a variety of contexts and taxa are clearly needed. Nevertheless, these results indicate that badges can function as honest signals of agonistic ability and conventional signals may be an important aspect of social interactions in diverse taxa.
Acknowledgments
Thanks to A. Wantanabe for research assistance. B. Daley, J. Dale, R. Payne and three anonymous reviewers provided helpful comments. This research was supported by startup funds from the University of Michigan.
Supplementary Material
Video of a wasp chosing between guarded patches of food
References
- Dapporto L, Palagi E, Turillazzi S. Sociality outside the nest: helpers in pre-hibernating clusters of Polistes dominulus. Ann. Zool. Fenn. 2005;42:135–139. [Google Scholar]
- Dapporto L, Dani F.R, Turillazzi S. Social dominance molds cuticular and egg chemical blends in a paper wasp. Curr. Biol. 2007;17:R504–R505. doi: 10.1016/j.cub.2007.05.002. doi:10.1016/j.cub.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Gerhardt H, Huber F. University of Chicago Press; Chicago, IL: 2002. Acoustic communication in insects and anurans. [Google Scholar]
- Howard R.W, Blomquist G.J. Ecological, behavioral, and biochemical aspects of insect hydrocarbons. Annu. Rev. Entomol. 2005;50:371–393. doi: 10.1146/annurev.ento.50.071803.130359. doi:10.1146/annurev.ento.50.071803.130359 [DOI] [PubMed] [Google Scholar]
- Hurd P.L. Resource holding potential, subjective resource value, and game theoretical models of aggressiveness signalling. J. Theor. Biol. 2006;241:639–648. doi: 10.1016/j.jtbi.2006.01.001. doi:10.1016/j.jtbi.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Jarvi T, Bakken M. The function of the variation in the breast stripe of the great tit Parus major. Anim. Behav. 1984;32:590–596. doi:10.1016/S0003-3472(84)80296-1 [Google Scholar]
- Jones I.L. Plumage variability functions for status signalling in least auklets. Anim. Behav. 1990;39:967–975. doi:10.1016/S0003-3472(05)80962-5 [Google Scholar]
- Maynard-Smith J.M, Harper D.G.C. The evolution of aggression—can selection generate variability? Phil. Trans. R. Soc. B. 1988;319:557–570. doi: 10.1098/rstb.1988.0065. doi:10.1098/rstb.1988.0065 [DOI] [PubMed] [Google Scholar]
- Monnin T. Chemical recognition of reproductive status in social insects. Ann. Zool. Fenn. 2006;43:515–530. [Google Scholar]
- Parker G.A. Assessment strategy and evolution of fighting behavior. J. Theor. Biol. 1974;47:223–243. doi: 10.1016/0022-5193(74)90111-8. doi:10.1016/0022-5193(74)90111-8 [DOI] [PubMed] [Google Scholar]
- Part T, Qvarnstrom A. Badge size in collared flycatchers predicts outcome of male competition over territories. Anim. Behav. 1997;54:893–899. doi: 10.1006/anbe.1997.0514. doi:10.1006/anbe.1997.0514 [DOI] [PubMed] [Google Scholar]
- Pryke S.R, Andersson S. Carotenoid-based status signalling in red-shouldered widowbirds (Euplectes axillaris): epaulet size and redness affect captive and territorial competition. Behav. Ecol. Sociobiol. 2003;53:393–401. doi:10.1007/s00265-003-0587-2 [Google Scholar]
- Reeve H.K. Polistes. In: Ross K.G, Matthews R.W, editors. The social biology of wasps. Comstock Publishing Associates; Ithaca, NY: 1991. pp. 99–148. [Google Scholar]
- Rohwer S. The social significance of avian winter plumage variability. Evolution. 1975;29:593–610. doi: 10.1111/j.1558-5646.1975.tb00853.x. doi:10.2307/2407071 [DOI] [PubMed] [Google Scholar]
- Rohwer S. Dyed birds achieve higher social-status than controls in Harris' sparrows. Anim. Behav. 1985;33:1325–1331. doi:10.1016/S0003-3472(85)80193-7 [Google Scholar]
- Senar J.C. Color displays as intrasexual signals of aggression and dominance. In: Hill G.E, McGraw K.J, editors. Bird coloration. Function and evolution. vol. 2. Harvard University Press; London, UK: 2006. pp. 87–136. [Google Scholar]
- Senar J.C, Camerino M. Status signalling and the ability to recognize dominants: an experiment with siskins (Carduelis spinus) Proc. R. Soc. B. 1998;265:1515–1520. doi:10.1098/rspb.1998.0466 [Google Scholar]
- Sledge M.F, Boscaro F, Turillazzi S. Cuticular hydrocarbons and reproductive status in the social wasp Polistes dominulus. Behav. Ecol. Sociobiol. 2001;49:401–409. doi:10.1007/s002650000311 [Google Scholar]
- Tibbetts E.A. Badges-of-status in worker and gyne Polistes dominulus wasps. Ann. Zool. Fenn. 2006;43:575–582. [Google Scholar]
- Tibbetts E.A, Curtis T.R. Rearing conditions influence quality signals but not individual identity signals in Polistes wasps. Behav. Ecol. 2007;18:602–607. doi:10.1093/beheco/arm013 [Google Scholar]
- Tibbetts E.A, Dale J. A socially enforced signal of quality in a paper wasp. Nature. 2004;432:218–222. doi: 10.1038/nature02949. doi:10.1038/nature02949 [DOI] [PubMed] [Google Scholar]
- Tibbetts E.A, Reeve H.K. Aggression and resource sharing among foundresses in the social wasp Polistes dominulus: testing transactional theories of conflict. Behav. Ecol. Sociobiol. 2000;48:344–352. doi:10.1007/s002650000240 [Google Scholar]
- Whitfield D.P. Plumage variability, status signaling and individual recognition in avian flocks. Trends Ecol. Evol. 1987;2:13–18. doi: 10.1016/0169-5347(87)90194-7. doi:10.1016/0169-5347(87)90194-7 [DOI] [PubMed] [Google Scholar]
- Whiting M.J, Nagy K.A, Bateman P.W. Evolution and maintenance of social status signalling badges: experimental manipulations in lizards. In: Fox S.F, McCoy J.K, Baird T.A, editors. Lizard social behavior. Johns Hopkins University Press; Baltimore, MD: 2003. pp. 47–82. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video of a wasp chosing between guarded patches of food