Abstract
Amazon river dolphins or botos (Inia geoffrensis Blainville) were observed carrying objects in 221 social groups over a 3-year study period. Sticks, branches and clumps of grass were taken from the water surface and often repeatedly thrashed or thrown. Lumps of hard clay were collected from the river bed and held in the mouth while the carrier rose slowly above the surface and submerged again. Carriers were predominantly adult males and less often subadult males. Adult females and young dolphins rarely carried objects. Groups of dolphins in which object carrying occurred were differentially large and comprised a greater proportion of adult males and adult females. Aggression, mostly between adult males, was significantly associated with object carrying. The behaviour occurred year-round, with peaks in March and July. A plausible explanation of the results is that object carrying by adult males is aimed at females and is stimulated by the number of females in the group, while aggression is targeted at adult males and is stimulated by object carrying in the group. We infer that object carrying in this sexually dimorphic species is socio-sexual display. It is either of ancient origin or has evolved independently in several geographically isolated populations.
Keywords: object carrying, Amazon river dolphin, boto, social behaviour, aquatic mammal
1. Introduction
Throughout their range in the Amazon and Orinoco river basins of South America, including geographically isolated populations in Brazil, Venezuela and Bolivia (Best & da Silva 1989), Amazon river dolphins or botos (Inia geoffrensis Blainville) have been observed apparently playing with objects. The behaviour is commonplace, but has hitherto not received scientific attention. In Mamirauá, a flooded rainforest reserve in the Brazilian Amazon, botos often carry objects in their mouth. These objects are of natural origin—branches and sticks, floating vegetation and lumps of hard clay—and are sometimes thrashed against the water surface or thrown with a violent head movement (figure 1a). Dolphins carrying objects (‘carriers’) sometimes swim in a ritualized manner, languidly spinning on their own axis with the head above water, mouth agape (figure 1b).
Figure 1.
Adult male botos (a) thrashing Paspalum repens, a hollow-stemmed macrophyte, against the water surface and (b) displaying a lump of clay from the river bed.
During a single day in 2003, three individually known botos were observed carrying objects, and the realization that all were adult males prompted speculation that this behaviour may not be play at all. This paper presents a quantitative analysis of the social and temporal context in which object carrying occurs, and seeks to explain its purpose and motivation.
2. Material and methods
Observations were made from small boats in the Mamirauá Reserve, Amazonas, Brazil. Observational effort was made on most days during a 36-month study period commencing 16 August 2003, and was uniform across the year. Identification of individuals, and knowledge of their sex and age, was based on freeze brands. Over half of the botos encountered during this study were of known sex and maturity.
Dolphins were patchily distributed within the study site, a 225 km2 area of river and flooded forest. Observers searched along waterways, stopping whenever dolphins were encountered to identify marked animals and assess group size and structure. A ‘group’ comprised all animals surfacing within the view of the observer, always within a radius of 200 m and usually within 50 m. Group membership and size in this species is labile, with no long-term association except between a mother and calf (A. R. Martin & V. M. F. da Silva 1994–2007, unpublished data). Owing to poor water clarity, observations were of activity above the surface. Aggressive behaviour was defined as lunging, leaping onto another animal and biting or striking another animal with the head or tail.
Observed dolphins were categorized as adult male, adult female, mother–calf pair, subadult, juvenile or calf. The boto has one of the highest degrees of sexual dimorphism in cetaceans (Martin & da Silva 2006), with adult males considerably larger, and usually much more pink, than adult females or any other class of dolphins. These differences are readily apparent to experienced observers in the field, normally allowing unequivocal discrimination between adult males and all other animals. Unmarked subadult males were occasionally incorrectly classed as ‘adult female’ if they were of similar size and colour.
3. Results
(a) Frequency of behaviour
Object carrying was observed in 221 boto groups (‘object groups’) out of 6026 groups (3.7%) encountered. The average observation time of each group was 27.8 (range 1–292) min, giving one object-carrying event per 12.6 hours of observation year-round or approximately one event in daylight per group per day. One carrier was observed in 189 groups, two to four in 31 groups and 11 in one group, but the incidence of multiple carriers may have been underestimated in some cases owing to the small proportion of time spent at the surface by botos.
(b) Status of carrier
Carriers were individually identified on 73 occasions, involving 35 different dolphins: 20 adult males (57%); nine subadult males (26%); three adult females (9%); and three juveniles (9%: two males and one female). All nine dolphins observed carrying objects three times or more were adult males, and 55 out of the 73 observations (75%) involved adult males.
In 146 observations of unmarked carriers, 143 (98%) were judged to be adult or subadult males, two to be adult females and one a juvenile.
(c) Type of object
Of the 274 objects identified, 110 (40%) were floating vegetation (usually Paspalum repens, a large and hollow-stemmed matting grass; figure 1a), 65 (24%) were branches or sticks and 99 (36%) were negatively buoyant lumps of hard clay (figure 1b). On all 23 occasions when a dolphin carrying clay was individually identified, it was an adult male. None of the objects were edible or usable in any other context.
(d) Group size and structure
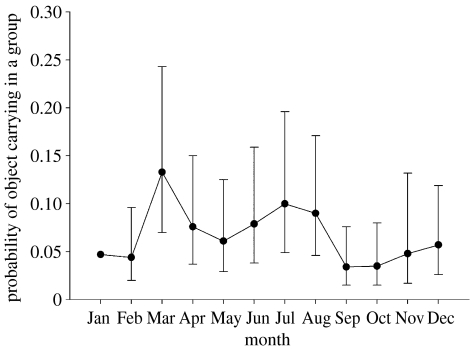
The occurrence of object carrying in a group was analysed in relation to the month and the number of animals in different age and sex classes present using a binary regression model (see electronic supplementary material). Carrying was more likely in groups with more adult males, as would be expected. After allowing for the number of adult males present, the probability of object carrying increased with the number of adult females (p=0.001) and varied between months (p<0.001), with higher values in June–August and a peak in March (figure 2). At least one probable adult female was observed in 194 out of 220 object groups (88.2%).
Figure 2.
Estimated monthly probability of object carrying being observed in a group after allowing for the effects of the number of adult males and females present.
Mean group size was 12.7 (n=220, s.e.=0.50) in groups with object carrying and 4.7 (n=5815, s.e.=0.06) in groups without object carrying.
(e) Aggression
Aggression was observed in 15 out of the 5815 non-object groups (0.3%) and in 22 out of the 210 object groups (10.5%) for which such data were available, a 40-fold difference (Χ12=346.7, p<0.001). With few exceptions, aggression was between adult males and was more likely in groups with proportionally more adult males. After allowing for the number of adult males present, aggression was more likely in object-carrying groups (p<0.001), but showed no relationship with the number of adult females (p=0.80).
4. Discussion
Observing river dolphins is analogous to peeping through an aquatic keyhole. Our inability to see below the water surface masks most of their behaviour from the human view, to the extent that copulation has never been unambiguously witnessed in over 13 000 hours of encounter time with botos in the long-term research project of which this study is a part. The interpretation of behaviour is therefore dependent on piecing together fragmentary evidence, and must be cognizant of the fact that most of these dolphins' lives are not accessible to human observers.
Despite these limitations, sufficient information on object carrying in botos has now been collected to permit conclusions about who does it, when and why. This behaviour is overwhelmingly characteristic of adult (less often subadult) males in large social groups, in the presence of adult females and other adult males. Juveniles were rarely observed carrying objects, contrary to expectations if this was play. Aggression between adult males was strongly associated with object carrying. Direct aggressive competition between males of this species for access to fertile females can be inferred from the high level of sexual size dimorphism, tooth rakes that literally cover the body of adult males and structural damage apparently caused by fighting (Mitani et al. 1996; Martin & da Silva 2006).
Contrary to our earlier assumption, the context of this behaviour indicates that its function is socio-sexual display, not play. All else being equal, it is apparently either triggered by an unusually large number of adult males and/or adult females in a group, or perhaps it attracts such animals into the group. A plausible explanation of the results is that object carrying is aimed at females and is stimulated by the number of females in the group, while aggression is aimed at other adult males and is stimulated by object carrying in the group.
The temporal distribution of carrying was not constant across the year, yet the objects themselves were always available. If object carrying is a sexual display, we might predict that it would be associated with any seasonal pattern in copulations and conceptions. We have no direct information on the relative frequency of conceptions from month to month in Inia, but births are weakly synchronized with a peak in September (A. R. Martin & V. M. F. da Silva 1994–2007, unpublished data based on ultrasound measurements of foetuses and field observations of neonates) and gestation length is likely to be between 11 and 15 months by analogy with other small odontocetes. The mode of carrying in July would indicate a gestation of 14 months if it reflects the peak of mating responsible for September births, and it is therefore consistent with this hypothesis. The mode of carrying in March is not mirrored by a known concentration of births 11–15 months later, but it may indicate that some females are cycling out of phase with the majority.
Object carrying as socio-sexual display has not been reported in any other aquatic mammal. One population of a marine dolphin (Tursiops aduncus) uses an object (a marine sponge) as a foraging tool, but here it is predominantly females that are involved and no display element is suspected (Krützen et al. 2005). Apart from modern humans, chimpanzees (Pan spp.) are the only terrestrial mammals in which objects (tree branches) have been linked to sexual display (Ingmanson 1996). The carrying of non-functional objects for display is similarly rare in other classes of animals, but is best known in birds, and here too it is adult males that are the carriers (e.g. black wheatear Oenanthe leucura: Moreno et al. 1994).
Why is this apparently the only aquatic mammal to use objects for display? Perhaps because it can; probably no cetacean, seal or sirenian lives with more flotsam. But if access alone was the explanation, then the sympatric dolphin Sotalia fluviatilis and coastal species such as Tursiops or many seals could be expected to adopt the same behaviour. We suggest that this display is more likely to be an integral part of the boto's mating system, and, furthermore, that the extreme sexual dimorphism in body colour (greater in botos than in any other cetacean—many males are bright pink; Martin & da Silva 2006) also plays a role in sexual advertisement. The presence of adult females and multiple adult males in object groups may indicate some form of lek-like mate-choice process, perhaps analogous to the ‘floating leks’ proposed for humpback whales, in which the location of the display ground is not fixed as in many terrestrial taxa (Clapham 1996; Connor et al. 2000).
Similarities in reproductive behaviour between primates and cetaceans have been demonstrated by a number of recent studies and include group dynamics, cooperative relationships between males and the use of vocal displays (Connor et al. 2000). The discovery of object carrying as display in a cetacean, a characteristic previously thought to be restricted in mammals to higher primates, is further evidence of behavioural convergence between these two disparate mammalian orders, despite their contrasting ecologies.
Is this an example of cetacean culture? Possibly, but there is insufficient evidence on which to base a judgement at present. Culture in animals is generally considered to be learned behaviour rather than genetically inherited (e.g. Rendell & Whitehead 2001; Laland & Janik 2006), and it is currently unclear whether object carrying is learned. It is apparently not of recent origin, however. Botos have been observed carrying objects in populations geographically separated for millions of years (Banguera-Hinestroza et al. 2002), which suggests two scenarios: either the behaviour was ancestral to all populations and therefore of ancient origin, or it developed separately in these populations after separation. Both scenarios suggest that the behaviour is of fundamental importance to this species.
Acknowledgments
This work was carried out under successive permits granted to VMFdS by IBAMA (Brazilian National Environmental Agency) and satisfied its criteria on ethics and animal welfare.
We thank Instituto Mamirauá and the financial sponsors of Projeto Boto for their continued support of this long-term project, and the interns who collected data for this analysis.
Supplementary Material
Statistical methodology
References
- Banguera-Hinestroza E, Cardenas H, Ruiz-Garcia M, Marmontel M, Gaitan E, Vazquez R, Garcia-Vallejo F. Molecular identification of evolutionarily significant units in the Amazon river dolphin Inia sp. (Cetacea: Iniidae) J. Hered. 2002;95:312–322. doi: 10.1093/jhered/93.5.312. doi:10.1093/jhered/93.5.312 [DOI] [PubMed] [Google Scholar]
- Best, R. C. & da Silva, V. M. F. 1989 Amazon river dolphin, boto. Inia geoffrensis (de Blainville, 1817). In Handbook of marine mammals, vol. 4 (eds S. H. Ridgway & R. J. Harrison), pp. 1–23. London, UK: Academic Press.
- Clapham P.J. The social and reproductive biology of humpback whales: an ecological perspective. Mamm. Rev. 1996;26:27–49. [Google Scholar]
- Connor R.C, Read A, Wrangham R. Male reproductive strategies and social bonds. In: Mann J, Connor R.C, Tyack P, Whitehead H, editors. Cetacean societies: field studies of whales and dolphins. University of Chicago Press; Chicago, IL: 2000. pp. 247–269. [Google Scholar]
- Ingmanson E.J. Tool-using behavior in wild Pan paniscus: social and ecological considerations. In: Russon A.E, Bard K.A, Parker S.T, editors. Reaching into thought: the minds of the great apes. Cambridge University Press; Cambridge, UK: 1996. pp. 190–210. [Google Scholar]
- Krützen M, Mann J, Heithaus M.R, Connor R.C, Bejder L, Sherwin W.B. Cultural transmission of tool use in bottlenose dolphins. Proc. Natl Acad. Sci. USA. 2005;102:8939–8943. doi: 10.1073/pnas.0500232102. doi:10.1073/pnas.0500232102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laland K.N, Janik V.M. The animal cultures debate. Trends Ecol. Evol. 2006;21:542–547. doi: 10.1016/j.tree.2006.06.005. doi:10.1016/j.tree.2006.06.005 [DOI] [PubMed] [Google Scholar]
- Martin A.R, da Silva V.M.F. Sexual dimorphism and body scarring in the boto (Amazon river dolphin) Inia geoffrensis. Mar. Mamm. Sci. 2006;22:25–33. [Google Scholar]
- Mitani J.C, Gros-Louis J, Richards A.F. Sexual dimorphism, the operational sex ratio, and the intensity of male competition in polygynous primates. Am. Nat. 1996;147:966–980. doi:10.1086/285888 [Google Scholar]
- Moreno J, Soler M, Moller A.P, Linden M. The function of stone carrying in the black wheatear, Oenanthe leucara. Anim. Behav. 1994;47:1297–1309. doi:10.1006/anbe.1994.1178 [Google Scholar]
- Rendell L, Whitehead H. Culture in whales and dolphins. Behav. Brain Sci. 2001;24:309–324. doi: 10.1017/s0140525x0100396x. doi:10.1017/S0140525X0100396X [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Statistical methodology