Abstract
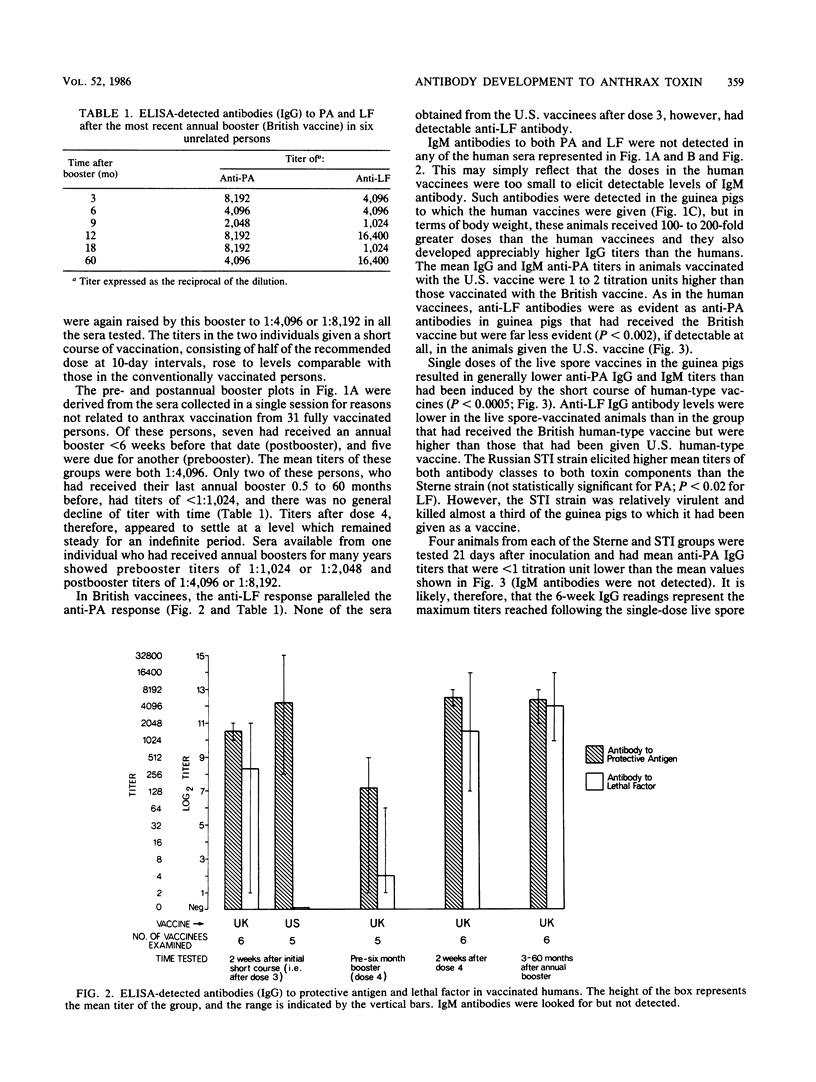
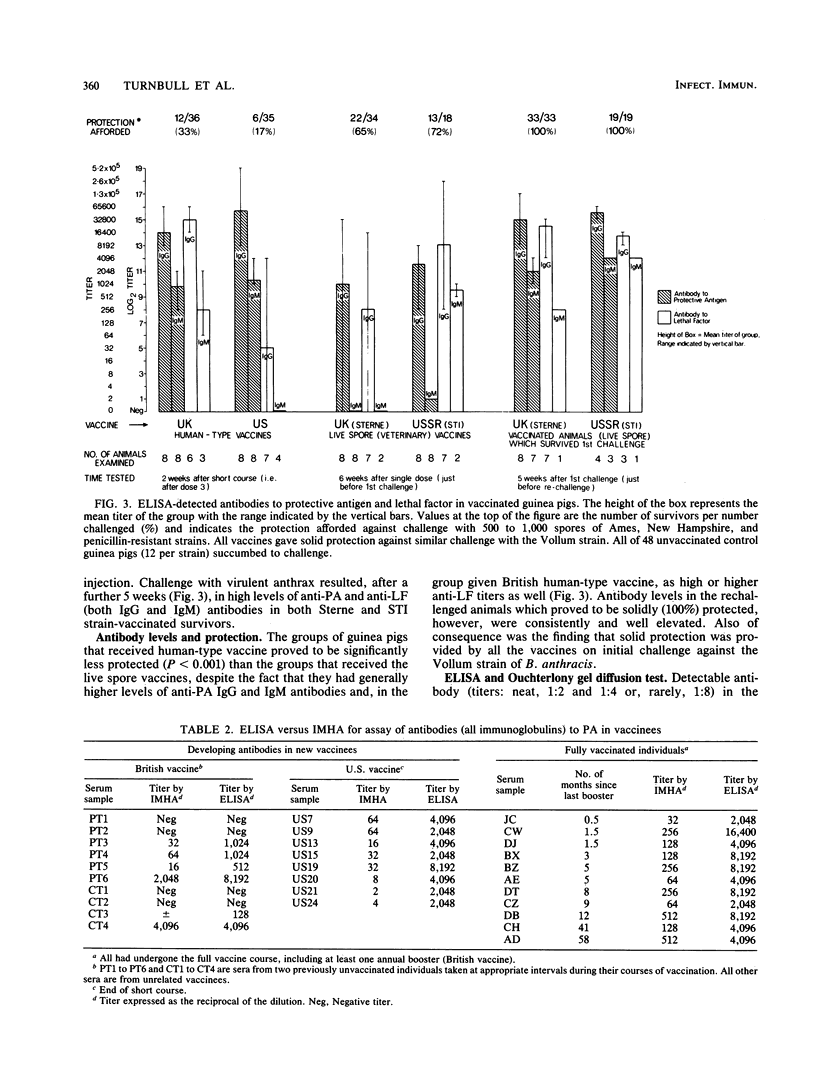
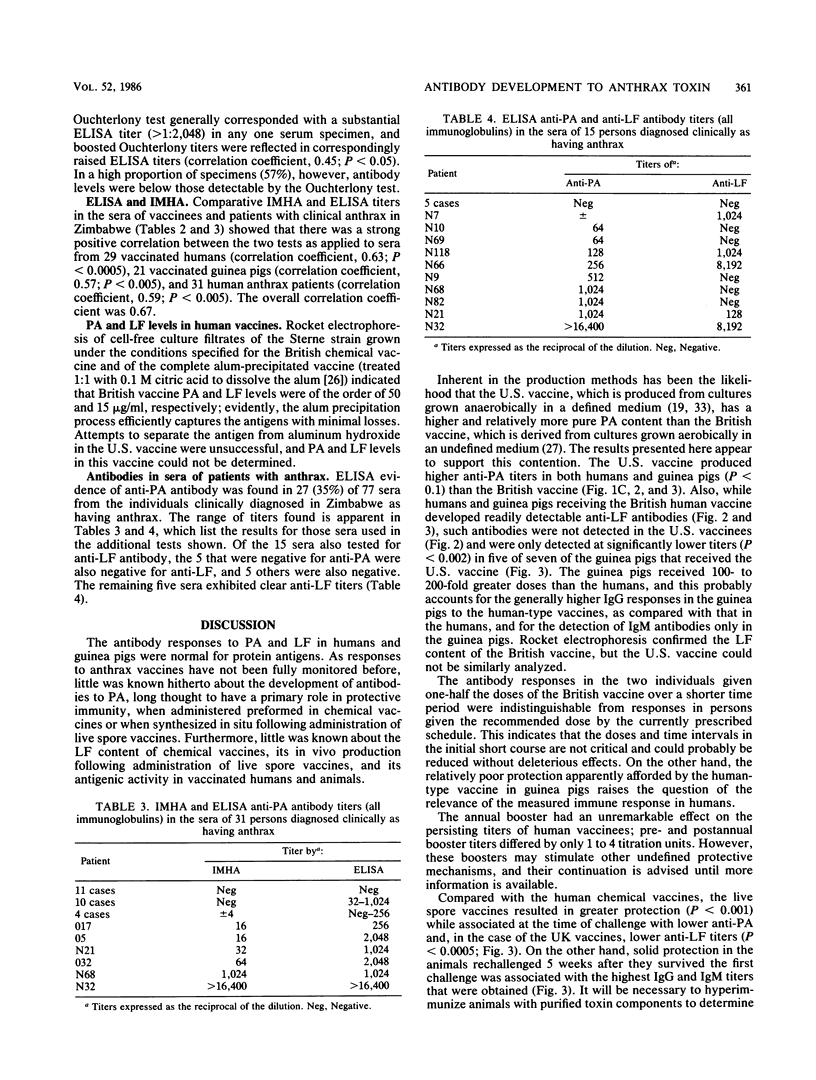
A competitive inhibition enzyme-linked immunosorbent assay (ELISA) was developed to detect antibodies in serum to the protective antigen (PA) and lethal factor (LF) components of anthrax toxin. Current human vaccination schedules with an acellular vaccine induce predictable and lasting antibody titers to PA and, when present in the vaccine, to LF. Live spore vaccine administered to guinea pigs in a single dose conferred significantly better protection than the human vaccines (P less than 0.001), although they elicited significantly lower (P less than 0.0005) anti-PA and anti-LF titers at time of challenge with virulent Bacillus anthracis. Substantial anti-PA and anti-LF titers may not, therefore, indicate solid protective immunity against anthrax infection. The ELISA system was also shown to be capable of detecting anti-PA and anti-LF antibodies in the sera of individuals with histories of clinical anthrax. The advantage of ELISA over the Ouchterlony gel diffusion test and indirect microhemagglutination assay are demonstrated. There was a highly significant degree of correlation between ELISA and the indirect microhemagglutination assay (P less than 0.0005); but ELISA was markedly superior in terms of reproducibility, reliability, specificity, and simplicity in performance and stability of the bound antigen.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELTON F. C., DARLOW H. M., HENDERSON D. W. The use of anthrax antigen to immunise man and monkey. Lancet. 1956 Sep 8;271(6941):476–479. doi: 10.1016/s0140-6736(56)91968-7. [DOI] [PubMed] [Google Scholar]
- BELTON F. C., HENDERSON D. W. A method for assaying anthrax immunising antigen and antibody. Br J Exp Pathol. 1956 Apr;37(2):156–160. [PMC free article] [PubMed] [Google Scholar]
- BELTON F. C., STRANGE R. E. Studies on a protective antigen produced in vitro from Bacillus anthracis: medium and methods of production. Br J Exp Pathol. 1954 Apr;35(2):144–152. [PMC free article] [PubMed] [Google Scholar]
- Brachman P. S., Gold H., Plotkin S. A., Fekety F. R., Werrin M., Ingraham N. R. Field Evaluation of a Human Anthrax Vaccine. Am J Public Health Nations Health. 1962 Apr;52(4):632–645. doi: 10.2105/ajph.52.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Feeley J. C., Hayes P. S., Brachman P. S. Anthrax indirect microhemagglutination test. J Immunol. 1971 Dec;107(6):1631–1636. [PubMed] [Google Scholar]
- Davies J. C. A major epidemic of anthrax in Zimbabwe. Cent Afr J Med. 1982;28(12):291–298. [PubMed] [Google Scholar]
- Ezzell J. W., Ivins B. E., Leppla S. H. Immunoelectrophoretic analysis, toxicity, and kinetics of in vitro production of the protective antigen and lethal factor components of Bacillus anthracis toxin. Infect Immun. 1984 Sep;45(3):761–767. doi: 10.1128/iai.45.3.761-767.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish D. C., Mahlandt B. G., Dobbs J. P., Lincoln R. E. Purification and properties of in vitro-produced anthrax toxin components. J Bacteriol. 1968 Mar;95(3):907–918. doi: 10.1128/jb.95.3.907-918.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton P., Carman J. A., Melling J. Anthrax: the disease in relation to vaccines. Vaccine. 1984 Jun;2(2):125–132. doi: 10.1016/0264-410x(84)90003-3. [DOI] [PubMed] [Google Scholar]
- Johnson-Winegar A. Comparison of enzyme-linked immunosorbent and indirect hemagglutination assays for determining anthrax antibodies. J Clin Microbiol. 1984 Sep;20(3):357–361. doi: 10.1128/jcm.20.3.357-361.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S. H. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc Natl Acad Sci U S A. 1982 May;79(10):3162–3166. doi: 10.1073/pnas.79.10.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S. H. Bacillus anthracis calmodulin-dependent adenylate cyclase: chemical and enzymatic properties and interactions with eucaryotic cells. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:189–198. [PubMed] [Google Scholar]
- Mikesell P., Ivins B. E., Ristroph J. D., Dreier T. M. Evidence for plasmid-mediated toxin production in Bacillus anthracis. Infect Immun. 1983 Jan;39(1):371–376. doi: 10.1128/iai.39.1.371-376.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J., Friedlander A., Dreier T., Ezzell J., Leppla S. Effects of anthrax toxin components on human neutrophils. Infect Immun. 1985 Jan;47(1):306–310. doi: 10.1128/iai.47.1.306-310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUZISS M., MANNING L. C., LYNCH J. W., BARCLAYE, ABELOW I., WRIGHT G. G. Large-scale production of protective antigen of Bacillus anthracis in anaerobic cultures. Appl Microbiol. 1963 Jul;11:330–334. doi: 10.1128/am.11.4.330-334.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUZISS M., WRIGHT G. G. Studies on immunity in anthrax. X. Gel-adsorbed protective antigen for immunization of man. J Bacteriol. 1963 Jan;85:230–236. doi: 10.1128/jb.85.1.230-236.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAY J. G., Jr, KADULL P. J. AGAR-GEL PRECIPITIN TECHNIQUE IN ANTHRAX ANTIBODY DETERMINATIONS. Appl Microbiol. 1964 Jul;12:349–354. doi: 10.1128/am.12.4.349-354.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRANGE R. E., BELTON F. C. Studies on a protective antigen produced in vitro from Bacillus anthracis: purification and chemistry of the antigen. Br J Exp Pathol. 1954 Apr;35(2):153–165. [PMC free article] [PubMed] [Google Scholar]
- THORNE C. B., BELTON F. C. An agar-diffusion method for titrating Bacillus anthracis immunizing antigen and its application to a study of antigen production. J Gen Microbiol. 1957 Oct;17(2):505–516. doi: 10.1099/00221287-17-2-505. [DOI] [PubMed] [Google Scholar]
- Turner M. Anthrax in humans in Zimbabwe. Cent Afr J Med. 1980 Jul;26(7):160–161. [PubMed] [Google Scholar]
- Uchida I., Sekizaki T., Hashimoto K., Terakado N. Association of the encapsulation of Bacillus anthracis with a 60 megadalton plasmid. J Gen Microbiol. 1985 Feb;131(2):363–367. doi: 10.1099/00221287-131-2-363. [DOI] [PubMed] [Google Scholar]
- WRIGHT G. G., PUZISS M., NEELY W. B. Studies on immunity in anthrax. IX. Effect of variations in cultural conditions on elaboration of protective antigen by strains of Bacillus anthracis. J Bacteriol. 1962 Mar;83:515–522. doi: 10.1128/jb.83.3.515-522.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie M. H., Ward M. K. Characterization of anthrax toxin. Fed Proc. 1967 Sep;26(5):1527–1531. [PubMed] [Google Scholar]
- Williams J. E., Arntzen L., Robinson D. M., Cavanaugh D. C., Isaäcson M. Comparison of passive haemagglutination and enzyme-linked immunosorbent assay for serodiagnosis of plague. Bull World Health Organ. 1982;60(5):777–781. [PMC free article] [PubMed] [Google Scholar]
- Williams J. E., Gentry M. K., Braden C. A., Leister F., Yolken R. H. Use of an enzyme-linked immunosorbent assay to measure antigenaemia during acute plague. Bull World Health Organ. 1984;62(3):463–466. [PMC free article] [PubMed] [Google Scholar]


