Abstract
Our ability to accurately forecast species' geographical responses to climate change requires knowledge of the proximate and ultimate drivers of their distribution. Here, we consider the ecophysiological and demographic determinants of the distribution of a partial migrant, the North American field sparrow, Spizella pusilla. From 1940 to 1963, the field sparrow extended its winter northern range margin 222 km polewards. Such expansion was coincident with not only a geographical expansion into suitable breeding habitats, but also a decrease in mean abundance across sites occupied during the winter surveys. Combined, these trends suggest that declining populations along the expansion front either stopped migrating or altered their autumn migration. The poleward expansion was not coincident with climatically induced decreases in peak metabolic energy demand, but it did track increases in ecosystem net primary productivity. After 1963, the species' lower lethal temperature prevented further poleward movement. These findings show how different ecophysiological constraints can interact to change migration and distribution in a demographically declining species.
Keywords: climate change, demographic decline, ecophysiological niche tracking, geographical range expansion, migration
1. Introduction
Species are colonizing new areas as a result of recent climate change (Parmesan 2006). The ability to accurately forecast distributional responses to ongoing changes in climate requires knowledge of the mechanisms that govern the species' observed and potential range limits (Kearney & Porter 2004; Bernardo & Spotila 2005; Hijmans & Graham 2006). Most studies assume that species' distributions are in equilibrium with the environment (Guisan & Thuiller 2005), and thus track climatically induced changes in the boundaries of the geographical realization of their potential niche (Jackson & Overpeck 2000). However, this assumption (i) ignores the possibility that species are still expanding out of refugia (Hewitt 2000; Svenning & Skov 2004), (ii) disregards behavioural (Kearney 2006) and evolutionary (Thomas et al. 2001; Smith & Betancourt 2006) changes enabling species to colonize new areas of niche space and (iii) overlooks the fact that multiple—often non-mutually exclusive and spatio-temporally variable—mechanisms can act to maintain distributional equilibrium (Brown et al. 1996). Here, we use an historical time-series analysis that combines weather, distribution, abundance and physiological data to characterize the ecophysiological and demographic determinants of geographical range in a migrating bird species.
2. Material and methods
(a) Study species
The field sparrow, Spizella pusilla (Emberizidae) is a partial migrant in eastern North America. Annual latitudinal migration occurs once in the early spring and again in the late summer or early autumn, but winter and summer distributions exhibit extensive geographical overlap, and the species is found year-round throughout much of the eastern USA (Carey et al. 1994). Habitats include grasslands and forest edges (Allaire & Fisher 1975), and densities appear to be highly dependent on the availability of suitable habitat (Fretwell 1970). Recent increases in urbanization and agriculture have probably altered many habitats that were suitable in the late nineteenth century (Carey et al. 1994), and the species has been declining since the mid-1960s (Sauer et al. 2007). During this period, the species' mean winter northern range margin was constrained energetically by an average winter temperature isocline of −7°C (Root 1988). Importantly, previous research considered only the location of the winter northern range boundary at a single point in time and did not investigate whether peak metabolic energy demand was coincident with lower lethal temperature or whether environmental energy supply, as measured by ecosystem net primary productivity (NPP), was a better predictor of the northern range margin. Owing to the availability of field surveys and physiological experiments conducted during the non-breeding season, we also focus on the winter distribution of the species, but in contrast to previous work we use a long time series to infer the mechanisms responsible for periods of range movement and stasis.
(b) Distribution and abundance
Time-series data on the winter distribution and abundance of the field sparrow were obtained from the Audubon Christmas Bird Count (CBC; Butcher 1990). We restricted our analysis to CBC data collected between 1940 and 1997 because these were the years with publicly available data that enabled us to estimate abundance. Data were further restricted to the 72 sites in suitable habitats (according to Carey et al. 1994) with 95% or more temporal coverage. The selected sites also fully encompassed the summer or breeding range of the species. Relative abundance, an index of population size, was estimated by dividing the log-transformed number of birds counted by survey effort (number of ‘party’ hours), 1000×log(x+1)/effort; this estimate assumed constant detection probability per unit effort. Mean relative abundance was calculated in two ways: aseasonal abundance was calculated by averaging estimates of relative abundance across all 72 selected sites (irrespective of occupancy), while seasonal abundance was calculated by averaging relative abundance across only those sites where the species was observed in a given year. We conducted several diagnostic analyses to ensure that our CBC results were not artefacts of survey design. These and other important features of our analysis, including published estimates of physiological parameters, weather data and the mapping of ecophysiological and geographical range limits, are presented in the electronic supplementary material.
(c) Statistical tests
We used linear models to establish whether our estimates of distribution, abundance and ecophysiological constraints were related to time. Based on these results, we then determined whether the northern range limit tracked climatically induced changes in NPP. This was accomplished by first calculating the temporal rate of change in NPP with respect to the movement of the northern range margin and then comparing this time-series rate to the magnitude of the latitudinal rate of change in mean NPP using a two-tailed t-test. Ecophysiological niche tracking was considered synonymous with a failure to reject the null hypothesis of identical slopes.
3. Results
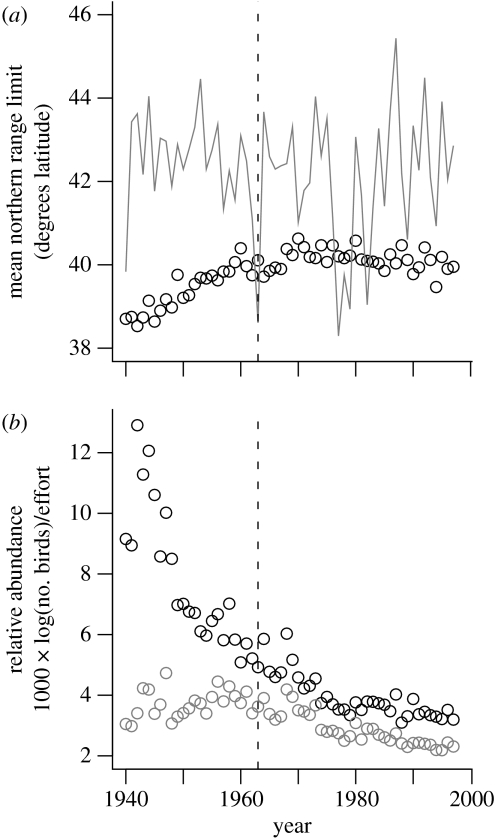
The mean winter northern range boundary of the field sparrow increased from 38.5° to 40.5° N latitude (Δ=222 km) between 1940 and 1963 (F1,22=121.8, p<0.001, slope=0.07° yr−1; figure 1a).
Figure 1.
Winter distribution and abundance of the field sparrow, 1940–1997. (a) Observed (black circles) and lower lethal (grey line) mean northern range limits. (b) Mean seasonal abundance (black circles) and mean aseasonal abundance (grey circles). The species stopped moving polewards in the year 1963 (vertical dashed lines). See the electronic supplementary material for details concerning trend calculations.
The poleward expansion was coincident with a small but non-significant increase in mean aseasonal abundance (F1,22=1.94, p=0.18; figure 1b) and a strong decrease in mean seasonal abundance (F1,22=61.54, p<0.001; figure 1b). Combined, the two abundance trends indicate that the species increasingly occupied new sites in the north while continuing to occupy the same sites in the south, but that abundance on a per occupied site basis decreased. Considering just the sites where the species was detected in 95% or more of the survey years, 1940–1963, mean seasonal abundance still declined significantly during the range expansion (F1,22=22.41, p<0.001).
The poleward range expansion was not coincident with winter warming because the species moved northwards during a period when the lower lethal temperature isocline fluctuated about a mean of 42.5° N latitude (F1,22=1.92, p=0.18; figure 1a) and peak metabolic energy demand did not change significantly through time (F1,22=1.16, p=0.29). Instead, the winter range expansion was coincident with climatically induced changes in NPP, which increased significantly from approximately 0.58 to 0.63 (Carbon) kg m−2 yr−1 (F1,22=9.87, p=0.004, slope=0.002 (Carbon) kg m−2 yr−2). Relating the estimated time-series change in NPP to the observed rate of latitudinal expansion, the field sparrow colonized areas where climatically induced increases in NPP approached 0.03 (Carbon) kg m−2 yr−1 deg−1 latitude of movement. This time-series rate of change was not significantly different from the magnitude of the latitudinal rate of change in NPP (t36=−14.97, p=0.50).
The field sparrow first contacted its mean lower lethal temperature isocline in the year 1963. From 1963 to 1997, both mean aseasonal and seasonal abundance decreased (aseasonal: F1,32=93.86, seasonal: F1,32=56.49, both p<0.001; figure 1b), and the species' mean winter northern range boundary fluctuated between 39.5° N (1994) and 40.6° N (1980) while maintaining an average position of 40.1° N latitude (F1,32=1.20, p=0.28; figure 1a). Meanwhile, the species' mean lower lethal temperature isocline fluctuated between 38.3° N (1977) and 45.4° N (1987) while maintaining an average position of 42.1° N latitude (F1,32=0.15, p=0.70; figure 1a).
4. Discussion
Our results suggest that climatically induced increases in NPP occurring in northeastern North America between 1940 and 1963 caused the field sparrow to alter or eliminate autumn migration in areas where historically the species never overwintered. This resulted in the winter northern range limit moving polewards, even when the species was declining throughout its winter range. In the year 1963, the field sparrow contacted its lower lethal temperature isocline and, at least until the year 1997, a lack of winter warming along the expansion front prevented further poleward movement.
These findings illustrate how different ecophysiological mechanisms can act at different times to maintain distributional equilibrium (Brown et al. 1996). Correlative distribution models are potentially able to accurately predict past and future distributions of such species because there is a spatio-temporal correspondence between the observed and ecophysiologically determined potential range margins (Guisan & Thuiller 2005). We stress ‘potentially’ because all empirical models projected to novel environments assume that the correct mechanism is being modelled using unbiased data and that the spatio-temporal correspondence between the response variable and the predictors remains constant (Austin 2002; Kadmon et al. 2003; Mustin et al. 2007), assumptions that can be violated if populations undergo demographic collapse or if other distributional constraints such as biotic interactions or dispersal limitations change significantly through time (Davis et al. 1998; Crozier & Dwyer 2006).
The winter survey areas included in the present analysis also encompassed the breeding range of the field sparrow. Nevertheless, a comprehensive analysis would include information on the breeding biology of the species because migrants do not necessarily track the same ecophysiological constraints across seasons (Nakazawa et al. 2004), and because winter distributional dynamics can be influenced by a multitude of intrinsic and extrinsic constraints operating in the breeding range (Sherry & Holmes 1995; Gordo et al. 2007). We were unable to match our winter range expansion analysis with a corresponding summer analysis because the North American Breeding Bird Survey started only in the mid-1960s (Sauer et al. 2007). Furthermore, we did not have access to ringing records for purposes of determining whether the expanding northern populations consisted of migratory or resident birds. Hence, our analysis was unable to distinguish among three possible autumn migratory changes capable of generating the observed patterns: (i) loss of migratory behaviour in expanding northern populations, (ii) shortening of autumn migration distance out of a geographically stable summer distribution, or (iii) maintenance of autumn migration distance out of a summer distribution that was also moving polewards.
Despite some uncertainty in the underlying autumn migratory response, our historical time-series analysis for the field sparrow provides a rare and important case study describing the demographic and ecophysiological mechanisms enabling species' distributions to either move or remain stationary in a changing climate. Four types of information are required to work towards applying this framework more broadly: (i) time-series data on species' distribution and abundance, (ii) estimates of species' physiological limits, (iii) knowledge of the climatic variables that interact with the physiological limits to determine both the observed and potential distribution of the organism, and (iv) spatio-temporal weather data for mapping observed and potential changes in distribution. Effective biodiversity conservation in the face of continued climate change requires increased efforts at obtaining and integrating across these disparate types of data, and ultimately selecting the appropriate spatio-temporal scales to ask questions related to the potential effects of climate change on species' distributions.
Acknowledgments
This work was supported by the National Science Foundation grant DEB-0407971 and graduate fellowship to W.B.M. We thank Catherine Graham, Walt Koenig, Eileen Lacey, Gary Langham, Craig Moritz, Juan Parra, Ken Wachter and Dave Wake for providing their helpful comments on the manuscript.
Footnotes
One contribution of 12 to a Special Feature on ‘Global change and biodiversity: future challenges’.
Supplementary Material
Additional material and methods
References
- Allaire P.N, Fisher C.D. Feeding ecology of three sympatric sparrows in eastern Texas. Auk. 1975;92:260–269. [Google Scholar]
- Austin M.P. Spatial prediction of species distribution: an interface between ecological theory and statistical modelling. Ecol. Model. 2002;157:101–118. doi:10.1016/S0304-3800(02)00205-3 [Google Scholar]
- Bernardo J, Spotila J.R. Physiological constraints on organismal response to global warming: mechanistic insights from clinally varying populations and implications for assessing endangerment. Biol. Lett. 2005;2:135–139. doi: 10.1098/rsbl.2005.0417. doi:10.1098/rsbl.2005.0417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.H, Stevens G.C, Kaufman D.M. The geographic range: size, shape, boundaries, and internal structure. Annu. Rev. Ecol. Syst. 1996;27:597–623. doi:10.1146/annurev.ecolsys.27.1.597 [Google Scholar]
- Butcher, G. S. 1990 Audubon Christmas Bird Counts. U.S. Fish and Wildlife Service biological report 90(1), pp. 5–13. ftp://ftp.nmt.edu/pub/people/john/cbc (March 2006).
- Carey, M., Burans, D. E. & Nelson, D. A. 1994 Field sparrow. In The birds of North America, no. 103 (eds A. Poole & F. Gill), pp. 1–20. Philadelphia, PA: The Birds of North America, Inc.
- Crozier L, Dwyer G. Combining population dynamic and ecophysiological models to predict climate-induced insect range shifts. Am. Nat. 2006;167:853–866. doi: 10.1086/504848. doi:10.1086/504848 [DOI] [PubMed] [Google Scholar]
- Davis A.J, Jenkinson L.S, Lawton J.H, Shorrocks B, Wood S. Making mistakes when predicting shifts in species range in response to global warming. Nature. 1998;391:783–786. doi: 10.1038/35842. doi:10.1038/35842 [DOI] [PubMed] [Google Scholar]
- Fretwell S. Breeding success in a local population of field sparrows. Acta Biotheor. 1970;19:45–52. doi:10.1007/BF01601955 [Google Scholar]
- Gordo O, Sanz J.J, Lobo J.M. Environmental and geographical constraints on common swift and barn swallow spring arrival patterns throughout the Iberian Peninsula. J. Biogeogr. 2007;34:1065–1076. doi:10.1111/j.1365-2699.2006.01679.x [Google Scholar]
- Guisan A, Thuiller W. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 2005;8:993–1009. doi: 10.1111/j.1461-0248.2005.00792.x. doi:10.1111/j.1461-0248.2005.00792.x [DOI] [PubMed] [Google Scholar]
- Hewitt G.M. The genetic legacy of the Quaternary ice ages. Nature. 2000;405:907–913. doi: 10.1038/35016000. doi:10.1038/35016000 [DOI] [PubMed] [Google Scholar]
- Hijmans R.J, Graham C.H. The ability of climate envelope models to predict the effect of climate change on species distributions. Glob. Change Biol. 2006;12:2272–2281. doi:10.1111/j.1365-2486.2006.01256.x [Google Scholar]
- Jackson S.T, Overpeck J.T. Responses of plant populations and communities to environmental changes of the Late Quaternary. Paleobiology. 2000;26:194–220. doi:10.1666/0094-8373(2000)26[194:ROPPAC]2.0.CO;2 [Google Scholar]
- Kadmon R, Farber O, Danin A. A systematic analysis of factors affecting the performance of climatic envelope models. Ecol. Appl. 2003;13:853–867. doi:10.1890/1051-0761(2003)013[0853:ASAOFA]2.0.CO;2 [Google Scholar]
- Kearney M. Habitat, environment and niche: what are we modelling? Oikos. 2006;115:186–191. [Google Scholar]
- Kearney M, Porter W.P. Mapping the fundamental niche: physiology, climate, and the distribution of a nocturnal lizard. Ecology. 2004;85:3119–3131. doi:10.1890/03-0820 [Google Scholar]
- Mustin K, Sutherland W.J, Gill J.A. The complexity of predicting climate-induced ecological impacts. Clim. Res. 2007;35:165–175. doi:10.3354/cr00723 [Google Scholar]
- Nakazawa Y, Peterson A.T, Martínez-Meyer E, Navarro-Sigüenza A.G. Seasonal niches of nearctic–neotropical migratory birds: implications for the evolution of migration. Auk. 2004;121:610–618. doi:10.1642/0004-8038(2004)121[0610:SNONMB]2.0.CO;2 [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006;37:637–669. doi:10.1146/annurev.ecolsys.37.091305.110100 [Google Scholar]
- Root T. Energy constraints on avian distributions and abundances. Ecology. 1988;69:330–339. doi:10.2307/1940431 [Google Scholar]
- Sauer, J. R., Hines, J. E. & Fallon, J. 2007 The North American Breeding Bird Survey, Results and Analysis 1966–2005, version 6.2.2006. USGS Patuxent Wildlife Research Center, Laurel, MD. http://www.mbr-pwrc.usgs.gov/bbs (March 2008).
- Sherry T.W, Holmes R.T. Summer versus winter limitation of populations: conceptual issues and evidence. In: Martin T, Finch D, editors. Ecology and management of neotropical migratory birds: a synthesis and review of the critical issues. Oxford University Press; New York, NY: 1995. pp. 85–120. [Google Scholar]
- Smith F.A, Betancourt J.L. Predicting woodrat (Neotoma) responses to anthropogenic warming from studies of the palaeomidden record. J. Biogeogr. 2006;33:2061–2076. doi:10.1111/j.1365-2699.2006.01631.x [Google Scholar]
- Svenning J.-C, Skov F. Limited filling of the potential range in European tree species. Ecol. Lett. 2004;7:565–573. doi:10.1111/j.1461-0248.2004.00614.x [Google Scholar]
- Thomas C.D, Bodsworth E.J, Wilson R.J, Simmons A.D, Davies Z.G, Musche M, Conradt L. Ecological and evolutionary processes at expanding range margins. Nature. 2001;411:577–581. doi: 10.1038/35079066. doi:10.1038/35079066 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional material and methods