Abstract
Sexual selection and signal detection theories predict that females should be selective in their responses to mating signals in mate choice, while the response of males to signals in male competition should be less selective. The neural processes underlying this behavioural sex difference remain obscure. Differences in behavioural selectivity could result from differences in how sensitive sensory systems are to mating signals, distinct thresholds in motor areas regulating behaviour, or sex differences in selectivity at a gateway relaying sensory information to motor systems. We tested these hypotheses in frogs using the expression of egr-1 to quantify the neural responses of each sex to mating signals. We found that egr-1 expression in a midbrain auditory region was elevated in males in response to both conspecific and heterospecific calls, whereas in females, egr-1 induction occurred only in response to conspecific signals. This differential neural selectivity mirrored the sex differences in behavioural responsiveness to these stimuli. By contrast, egr-1 expression in lower brainstem auditory centres was not different in males and females. Our results support a model in which sex differences in behavioural selectivity arise from sex differences in the neural selectivity in midbrain areas relaying sensory information to the forebrain.
Keywords: immediate-early gene, Physalaemus pustulosus, túngara frogs, inferior colliculus, torus semicircularis
1. Introduction
Sexual selection theory predicts that females, due to their greater reproductive investment, should be more selective in mate choice than males (Darwin 1871; Trivers 1972). Signal detection theory makes a similar prediction about the differences in selectivity between the sexes in their responses to reproductive social signals due to costs of different types of errors (Green & Swets 1966; Wiley 2006). Females' considerable reproductive investment is usually wasted if they mate with heterospecifics, and conspecifics are usually abundant. Thus, there is a high cost to females for missed identification and little cost for missed opportunities. Males, however, infrequently encounter females and their investment in reproduction is usually much less than the females'. Thus, males bear a high cost if opportunities to compete for females are missed, but little cost for missed identification; males should therefore respond broadly to competitive signals, as, for example, when they vocally interact with other males. Although the predicted sexual differences in response to reproductive signals are well known, the neural bases of these differences remain obscure (Jacobs 1996).
To examine the neural basis of sex differences in behavioural selectivity, we measured neural responses to mating calls in túngara frogs, Physalaemus pustulosus. In most of the frog species, mating calls elicit mate choice via phonotaxis from females, and vocal responses from males. In P. pustulosus, the conspecific call elicits the normative reproductive behaviours from each sex (Ryan 1980; Ryan & Rand 1998). Females do not respond with phonotaxis to the call of Physalaemus petersi (Ryan & Rand 1995), while males escalate their vocalizations in response to the same call (Bernal et al. 2007). We ask, what are the neural mechanisms of this sex difference in stimulus selectivity?
We compared the neural responses of males and females using the expression of egr-1. The egr-1 levels increase in many neurons following depolarization, thereby marking neural activation (Clayton 2000). We measured egr-1 expression in the superior olivary nucleus, a lower brainstem auditory nucleus, and in four divisions of the midbrain torus semicircularis (the amphibian homologue of the mammalian inferior colliculus): laminar; principal; midline; and ventral. The principal and ventral regions are auditory areas with major inputs from the lower brainstem nuclei (Wilczynski & Endepols 2007), and the midline region is also acoustically responsive (Hoke et al. 2004). The laminar nucleus is a relay centre, connecting brainstem auditory nuclei with forebrain motor and limbic areas, thereby acting as an anatomical sensorimotor interface (Walkowiak & Luksch 1994). We asked whether the sex difference in the behavioural selectivity is matched by the differences in neural selectivity within the auditory system, and where that difference emerges.
2. Material and methods
Experimental procedures and analyses were similar to those previously described (Hoke et al. 2004, 2005, 2007; methods in the electronic supplementary material). Nine to eleven amplexed frogs of each sex were assigned to one of three stimulus groups: no acoustic stimulation (silence); natural call of P. petersi; or natural call of P. pustulosus. After tissue processing, the final sample sizes were as follows: female: silence n=9, heterospecific n=8, conspecific n=11; male: silence n=9, heterospecific n=9, conspecific n=5. The stimuli were broadcast for 30 min while we videotaped the locomotion under infrared illumination. Males did not vocalize.
We estimated egr-1 expression based on radioactive in situ hybridization, sampling throughout the superior olivary nucleus and four divisions of the torus (figure 1). We processed photomicrographs to calculate the fraction of the area covered by cells that contained silver grains, our measure of egr-1 expression. We used ANCOVA to test for sex differences in mean egr-1 measures in each of the five brain regions. The main effects were sex and stimulus, time in motion and overall brain activation were the covariates, and sex by stimulus was the interaction term.
Figure 1.
Photomicrographs of typical egr-1 expression in each brain region and background egr-1 expression alongside tissue. (a) Photomicrographs indicating locations of brain regions included in this analysis. Ltor, laminar nucleus, torus; Mtor, midline region, torus; Ptor, principal nucleus, torus; Vtor, ventral region, torus; SO, superior olivary nucleus. (b) High-magnification images of silver grain densities in the auditory regions and in a nearby blank area of the slide (typical background silver grain density) from a female in the P. pustulosus treatment condition. (i) Laminar nucleus, (ii) principal nucleus, (iii) midline region, (iv) ventral region, (v) superior olivary nucleus, and (vi) background.
3. Results
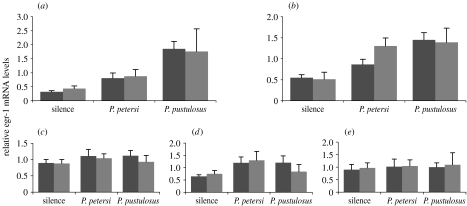
There were no sex differences in egr-1 levels in the superior olivary nucleus (table 1; figure 2). Both sexes showed graded egr-1 responses that varied with stimulus condition in a similar manner, with less elevation in response to heterospecific P. petersi calls than to conspecific calls. Thus, sex differences in the behavioural selectivity do not arise from differences through the lower brainstem.
Table 1.
Effects of sex, stimulus and locomotion on egr-1 levels in the auditory system using ANCOVA. Italics indicate statistical significance at p=0.05 level.
| brain region | stimulus | sex | time in motion | sex×stimulus | ||||
|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | |
| superior olive | 18.612 | <0.001 | 0.253 | 0.617 | 0.561 | 0.458 | 0.154 | 0.857 |
| laminar | 33.907 | <0.001 | 0.178 | 0.675 | 3.724 | 0.060 | 7.926 | 0.001 |
| midline | 3.075 | 0.056 | 0.474 | 0.495 | 6.405 | 0.015 | 0.772 | 0.469 |
| principal | 0.886 | 0.420 | 0.008 | 0.928 | 7.956 | 0.007 | 1.158 | 0.324 |
| ventral | 0.465 | 0.631 | 0.630 | 0.432 | 4.842 | 0.033 | 1.195 | 0.313 |
Figure 2.
Sex differences in selectivity of egr-1 responses occur in the laminar nucleus but not in the lower auditory centres. Bar heights depict mean (±s.e.) egr-1 levels in each acoustic condition, with females in black and males in grey. Stimulation induces egr-1 expression similarly in males and females in (a) the superior olivary nucleus (interaction, F2,42=0.154; p=0.857). These acoustic stimuli induce egr-1 expression in (b) the laminar nucleus of the torus semicircularis differently in males and females (F2,43=7.926; p=0.001). The egr-1 levels in (c–e) the principal, midline and ventral toral regions do not vary consistently with stimulus or sex.
By contrast, egr-1 levels in the laminar nucleus of the torus varied not only with stimulus but also had a significant sex by stimulus interaction (table 1; figure 2). The pattern of activation in the laminar nucleus matched the behavioural selectivity: in females, the egr-1 induction following heterospecific P. petersi calls was not significantly different from controls (p=0.112, pairwise comparisons of estimated marginal means), whereas the difference between conspecific and heterospecific was statistically significant (p=0.029). In males, egr-1 induction in response to heterospecific calls was higher than controls (p<0.001) and was similar to the response to conspecific signals (p=0.94). Other divisions of the torus did not vary consistently based on the stimulus or sex (figure 2).
4. Discussion
We conclude that sex differences in stimulus selectivity at the behavioural level are not the result of a global sex difference in the auditory system because activation at lower stages of auditory processing is similar in males and females. Rather, our results are consistent with the emergence of a ‘gatekeeper’ nucleus that differentially relays sensory stimulation to motor centres (figure 1 in the electronic supplementary material). Our results implicate the laminar nucleus of the torus semicircularis as that gatekeeper controlling behavioural selectivity. Differential activation of the laminar nucleus matches sex differences in behavioural selectivity, with males and females showing different activation to heterospecific cues. Other midbrain divisions, such as the principal or ventral torus, do not exhibit sex differences, although inconsistent egr-1 induction in response to sound may obscure our ability to observe this. The stimulus representation in the superior olivary nucleus is thus transformed into sex-specific activation patterns in the laminar nucleus that match the patterns of behavioural responses.
The laminar nucleus is an important anatomical sensorimotor interface for acoustic communication, and its neurons have complex stimulus-response properties that match the behavioural preferences for calls (Walkowiak & Luksch 1994; Endepols & Walkowiak 1999; Wilczynski & Endepols 2007). As such, its anatomy and physiology are consistent with a strategic role in controlling the natural responses to social signals. Additionally, the laminar nucleus concentrates androgens and oestradiol (Kelley 1980), and has hormonally modulated egr-1 induction (Lynch & Wilczynski 2008), suggesting a potential mechanism for generating or modulating these sex differences. The identification of the laminar nucleus as a gatekeeper highlights the general criteria for identifying a sex-specific sensorimotor relay: complex sensory processing with neural responses linked to behaviourally relevant stimulus features, anatomical and functional links to motor control regions that mediate reproductive behaviours, sensitivity to sex steroid hormones, and, as found here, the key element of sex difference in the functional activation to natural signals.
Our results suggest how sex differences in stimulus selectivity could emerge. They do not delimit the extent of each sex's selectivity because we did not test a wide range of stimuli. They also do not address why the sexes differ in the behavioural responses they typically exhibit in reproductive social contexts, that is, phonotaxis in females and evoked calling in males. Recent work in mice demonstrated that effector circuits for both male and female sexual behaviours are present in each sex (Kimchi et al. 2007). In fact, when lacking the necessary substrate for calling (puddles of water), male túngara frogs perform phonotaxis with similar selectivity for mating signals as females (Bernal 2007), indicating that effector circuits for female-like behaviours are present in male frogs, and that the broad selectivity at midbrain levels may be narrowed at later processing stages depending on the response selected. In females, however, the narrow selectivity in the midbrain occurs prior to the effector circuits controlling phonotaxis. These observations suggest that in addition to the difference in selectivity of the midbrain gatekeeper, the relationship of this gatekeeper to the forebrain effector centres differs in males and females. Kimchi et al. (2007) suggested that an olfactory ‘sensory switch’ related to pheromones functions differently in male and female mice. This is concordant with our hypothesis that an auditory gatekeeper nucleus in the midbrain related to advertisement calls differentially regulates access to forebrain motor centres in males and females. Future analysis will be required to determine how the sex-typical sensory selectivity of this midbrain gateway is propagated through forebrain and motor networks to enact the sex differences in behaviour.
In conclusion, we have demonstrated that sex differences in behavioural selectivity for acoustic signals are mirrored by sex differences in sensory responses in a specific midbrain nucleus relaying auditory information to the forebrain, but not by activation patterns at the earlier stages of auditory processing. Our results support a model in which the sex differences in behavioural selectivity to social signals that are predicted by sexual selection theory and signal detection models are established by differential responsiveness at a gatekeeper relaying sensory information to motor centres.
Acknowledgments
All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin and by the Autoridad Nacional del Ambiente del Republica de Panama´.
This work was funded by the National Science Foundation, National Institutes of Health and American Association of University Women. We would like to thank Ryan Taylor for support in Panama.
Supplementary Material
This text contains additional details on methods as well as a figure depicting competing hypotheses
References
- Bernal, X. E. 2007 The role of sex on behavioral responses to mating signals: studies of phonotaxis and evoked calling in male and female túngara frogs. PhD dissertation, University of Texas, Austin.
- Bernal X.E, Rand A.S, Ryan M.J. Sexual differences in receiver permissiveness to advertisement calls in túngara frogs, Physalaemus pustulosus. Anim. Behav. 2007;73:955–964. doi:10.1016/j.anbehav.2006.10.018 [Google Scholar]
- Clayton D.F. The genomic action potential. Neurobiol. Learn. Mem. 2000;74:185–216. doi: 10.1006/nlme.2000.3967. doi:10.1006/nlme.2000.3967 [DOI] [PubMed] [Google Scholar]
- Darwin C. Murray; London, UK: 1871. The descent of man and selection in relation to sex. [Google Scholar]
- Endepols H, Walkowiak W. Influence of descending forebrain projections on processing of acoustic signals and audiomotor integration in the anuran midbrain. Eur. J. Morphol. 1999;37:182–184. doi: 10.1076/ejom.37.2.182.4753. doi:10.1076/ejom.37.2.182.4753 [DOI] [PubMed] [Google Scholar]
- Green D, Swets J. Wiley; New York, NY: 1966. Signal detection theory and psychophysics. [Google Scholar]
- Hoke K.L, Burmeister S.S, Fernald R.D, Rand A.S, Ryan M.J, Wilczynski W. Functional mapping of the auditory midbrain during mate call reception. J. Neurosci. 2004;24:11 264–11 272. doi: 10.1523/JNEUROSCI.2079-04.2004. doi:10.1523/JNEUROSCI.2079-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke K.L, Ryan M.J, Wilczynski W. Social cues shift functional connectivity in the hypothalamus. Proc. Natl Acad. Sci. USA. 2005;102:10 712–10 717. doi: 10.1073/pnas.0502361102. doi:10.1073/pnas.0502361102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoke K.L, Ryan M.J, Wilczynski W. Integration of sensory and motor processing underlying social behaviour in túngara frogs. Proc. R. Soc. B. 2007;274:641–649. doi: 10.1098/rspb.2006.0038. doi:10.1098/rspb.2006.0038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs L. Sexual selection and the brain. Trends Ecol. Evol. 1996;11:82–86. doi: 10.1016/0169-5347(96)81048-2. doi:10.1016/0169-5347(96)81048-2 [DOI] [PubMed] [Google Scholar]
- Kelley D.B. Auditory and vocal nuclei in the frog brain concentrate sex hormones. Science. 1980;207:553–555. doi: 10.1126/science.7352269. doi:10.1126/science.7352269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448:1009–1014. doi: 10.1038/nature06089. doi:10.1038/nature06089 [DOI] [PubMed] [Google Scholar]
- Lynch K.S, Wilczynski W. Reproductive hormones modify reception of species-typical communication signals in a female anuran. Brain Behav. Evol. 2008;71:143–150. doi: 10.1159/000111460. doi:10.1159/000111460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M.J. Female mate choice in a Neotropical frog. Science. 1980;209:523–525. doi: 10.1126/science.209.4455.523. doi:10.1126/science.209.4455.523 [DOI] [PubMed] [Google Scholar]
- Ryan M.J, Rand A.S. Female responses to ancestral advertisement calls in the túngara frog. Science. 1995;269:390–392. doi: 10.1126/science.269.5222.390. doi:10.1126/science.269.5222.390 [DOI] [PubMed] [Google Scholar]
- Ryan M.J, Rand A.S. Evoked vocal response in male túngara frogs: pre-existing biases in male responses? Anim. Behav. 1998;56:1509–1516. doi: 10.1006/anbe.1998.0928. doi:10.1006/anbe.1998.0928 [DOI] [PubMed] [Google Scholar]
- Trivers R. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and descent of man. Aldine; Chicago, IL: 1972. pp. 136–179. [Google Scholar]
- Walkowiak W, Luksch H. Sensory motor interfacing in acoustic behavior of anurans. Am. Zool. 1994;34:685–695. doi:10.1093/icb/34.6.685 [Google Scholar]
- Wilczynski, W. & Endepols, H. 2007 Central auditory pathways in anuran amphibians: the anatomical basis of hearing and sound communication. In Hearing and sound communication in amphibians, vol. 28 (eds A. N. Popper, A. S. Feng & P. N. Narins). Springer Handbook of Auditory Research, pp. 221–249. Berlin, Germany: Springer.
- Wiley R.H. Signal detection and animal communication. Adv. Stud. Behav. 2006;36:217–247. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This text contains additional details on methods as well as a figure depicting competing hypotheses