Abstract
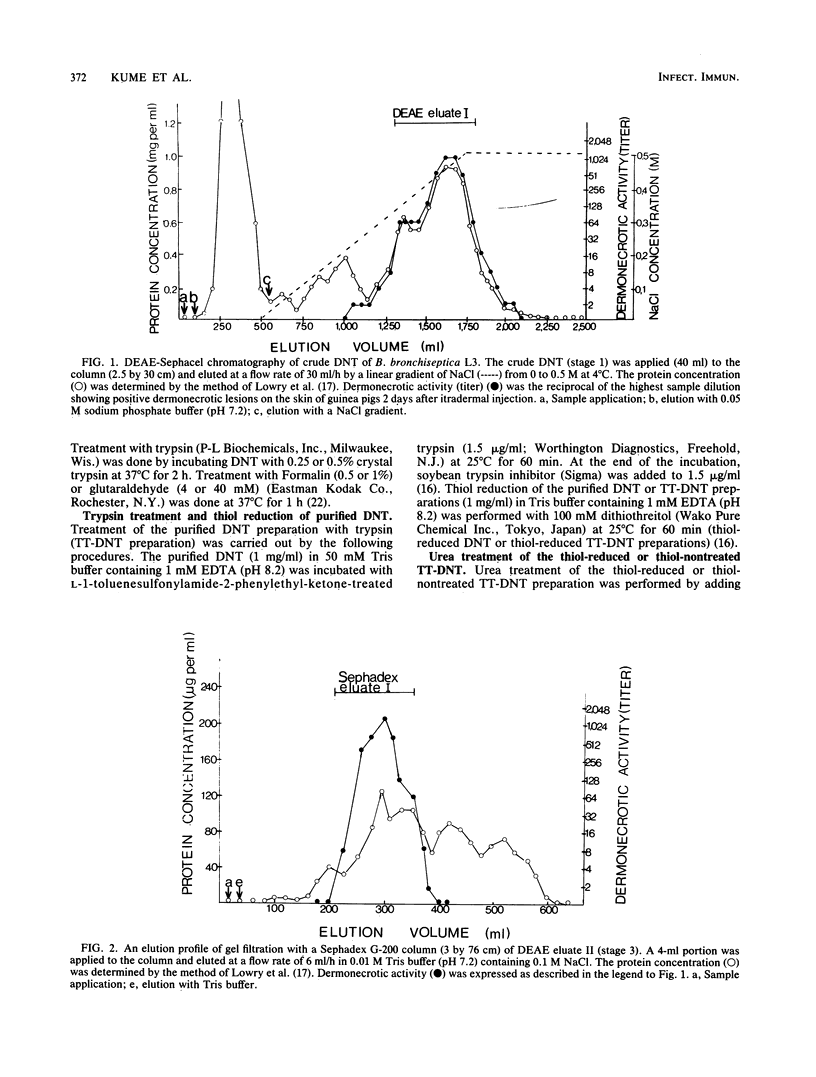
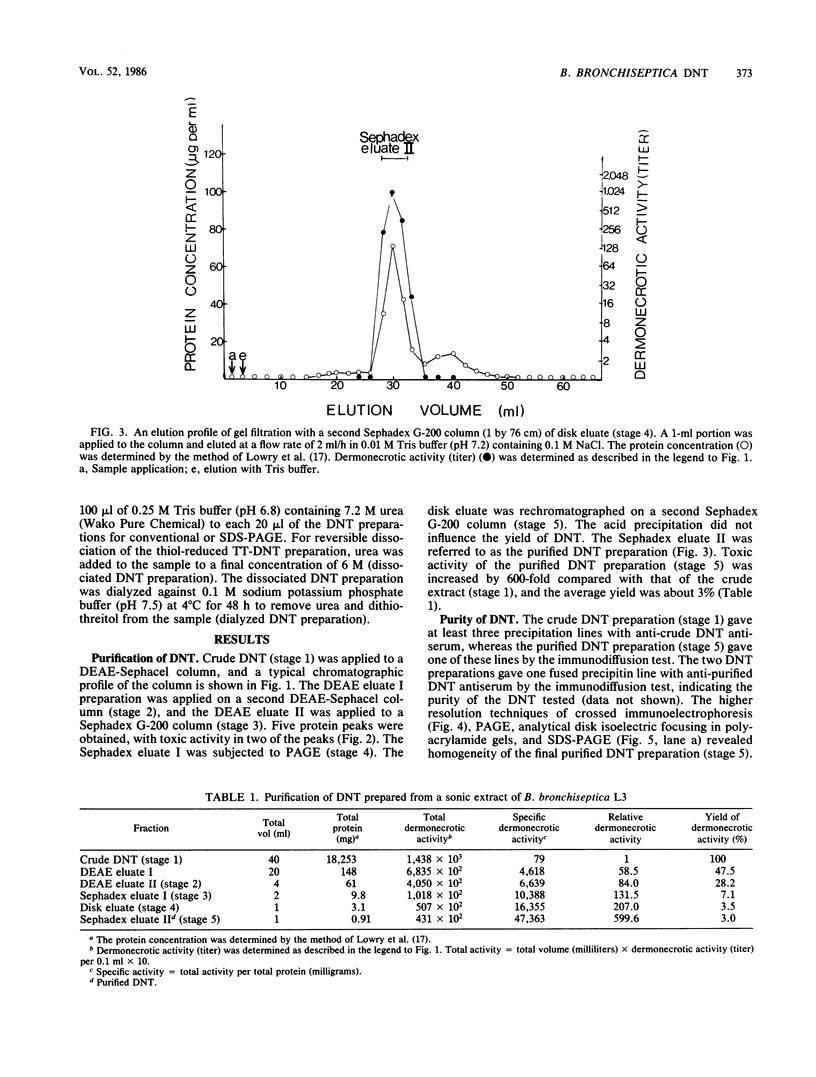
A toxin with dermonecrotic activity (DNT) was purified from sonic extracts of Bordetella bronchiseptica L3 of pig origin at phase I by chromatographic and electrophoretic methods. The purification procedure was one developed for obtaining the Pasteurella multocida DNT from sonic extracts with some modifications. Dermonecrotizing activity of B. bronchiseptica-purified DNT was increased by 600-fold compared with that of the crude extract, and the average yield was about 3%. The toxin was homogeneous, as determined by Ouchterlony double immunodiffusion, crossed immunoelectrophoresis, and disk isoelectric focusing in polyacrylamide gels. The toxin gave a single band on polyacrylamide disk gel electrophoresis (PAGE) and sodium dodecyl sulfate-SDS PAGE. The molecular weight of the toxin was ca. 190,000 +/- 5,000, as determined by SDS-PAGE. The isoelectric point of the toxin was ca. 6.5 to 6.6. The minimal necrotizing dose of the toxin for guinea pigs was about 2 ng of protein per 0.1 ml, the 50% lethal dose per mouse was about 0.3 micrograms, and the minimal cytotoxic dose for embryonic bovine lung cells was about 2 ng/ml. The toxin was heat labile and sensitive to inactivation by trypsin, Formalin, and glutaraldehyde. The mildly trypsinized B. bronchiseptica DNT preparation dissociated into two polypeptide chains, with molecular weights of ca. 75,000 +/- 4,000 (fragment 1) and ca. 118,000 +/- 5,000 (fragment 2), after treatment with dithiothreitol-SDS or urea. Upon removal of dithiothreitol and urea from the dissociated DNT preparation, the fragments reassociated, and the DNT that was formed was indistinguishable from the native toxin.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANERJEA A., MUNOZ J. Antigens of Bordetella pertussis. II. Purification of heat-labile toxin. J Bacteriol. 1962 Aug;84:269–274. doi: 10.1128/jb.84.2.269-274.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemis D. A., Plotkin B. J. Hemagglutination by Bordetella bronchiseptica. J Clin Microbiol. 1982 Jun;15(6):1120–1127. doi: 10.1128/jcm.15.6.1120-1127.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brassinne M., Dewaele A., Gouffaux M. Intranasal infection with Bordetella bronchiseptica in gnotobiotic piglets. Res Vet Sci. 1976 Mar;20(2):162–166. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Duncan J. R., Ross R. F., Switzer W. P., Ramsey F. K. Pathology of experimental Bordetella bronchiseptica infection in swine: atrophic rhinitis. Am J Vet Res. 1966 Mar;27(117):457–466. [PubMed] [Google Scholar]
- Eliás B., Krüger M. Epizootologische Untersuchungen der Rhinitis atrophicans des Schweines. III. Untersuchungen zu den Eigenschaften des Bordetella bronchiseptica-Exotoxins. Zentralbl Veterinarmed B. 1983 Jun;30(5):333–340. [PubMed] [Google Scholar]
- Eliás B., Krüger M., Rátz F. Epizootiologische Untersuchungen der Rhinitis atrophicans des Schweines. II. Biologische Eigenschaften der von Schweinen isolierten Bordetella bronchiseptica-Stämme. Zentralbl Veterinarmed B. 1982 Sep;29(8):619–635. [PubMed] [Google Scholar]
- Hanada M., Shimoda K., Tomita S., Nakase Y., Nishiyama Y. Production of lesions similar to naturally occurring swine atrophic rhinitis by cell-free sonicated extract of Bordetella bronchiseptica. Nihon Juigaku Zasshi. 1979 Feb;41(1):1–8. doi: 10.1292/jvms1939.41.1. [DOI] [PubMed] [Google Scholar]
- Iida T., Okonogi T. Lienotoxicity of Bordetella pertussis in mice. J Med Microbiol. 1971 Feb;4(1):51–61. doi: 10.1099/00222615-4-1-51. [DOI] [PubMed] [Google Scholar]
- Kume K., Nakai T. Dissociation of Pasteurella multocida dermonecrotic toxin into three polypeptide fragments. Nihon Juigaku Zasshi. 1985 Oct;47(5):829–833. doi: 10.1292/jvms1939.47.829. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maeda M., Shimizu T. Nasal infection of Alcaligenes bronchisepticus (Bordetella bronchiseptica) and lesions in newborn rabbits. Natl Inst Anim Health Q (Tokyo) 1975 Spring;15(1):29–37. [PubMed] [Google Scholar]
- Miniats O. P., Johnson J. A. Experimental atrophic rhinitis in gnotobiotic pigs. Can J Comp Med. 1980 Oct;44(4):358–365. [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M., Shimizu T., Motoi Y. Pathology of experimental atrophic rhinitis in swine infected with Alcaligenes bronchisepticus or Pasteurella multocida. Natl Inst Anim Health Q (Tokyo) 1974 Summer;14(2):61–71. [PubMed] [Google Scholar]
- Nakai T., Sawata A., Kume K. Intracellular locations of dermonecrotic toxins in Pasteurella multocida and in Bordetella bronchiseptica. Am J Vet Res. 1985 Apr;46(4):870–874. [PubMed] [Google Scholar]
- Nakai T., Sawata A., Tsuji M., Kume K. Characterization of dermonecrotic toxin produced by serotype D strains of Pasteurella multocida. Am J Vet Res. 1984 Nov;45(11):2410–2413. [PubMed] [Google Scholar]
- Nakai T., Sawata A., Tsuji M., Samejima Y., Kume K. Purification of dermonecrotic toxin from a sonic extract of Pasteurella multocida SP-72 serotype D. Infect Immun. 1984 Nov;46(2):429–434. doi: 10.1128/iai.46.2.429-434.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase Y., Takatsu K., Tateishi M., Sekiya K., Kasuga T. Heat-labile toxin of Bordetella pertussis purified by preparative acrylamide gel electrophoresis. Jpn J Microbiol. 1969 Dec;13(4):359–366. doi: 10.1111/j.1348-0421.1969.tb00479.x. [DOI] [PubMed] [Google Scholar]
- ONOUE K., KITAGAWA M., YAMAMURA Y. CHEMICAL STUDIES ON CELLULAR COMPONENTS OF BORDETELLA PERTUSSIS. III. ISOLATION OF HIGHLY POTENT TOXIN FROM BORDETELLA PERTUSSIS. J Bacteriol. 1963 Oct;86:648–655. doi: 10.1128/jb.86.4.648-655.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen K. B., Elling F. The pathogenesis of atrophic rhinitis in pigs induced by toxigenic Pasteurella multocida. J Comp Pathol. 1984 Apr;94(2):203–214. doi: 10.1016/0021-9975(84)90041-0. [DOI] [PubMed] [Google Scholar]
- Ross R., Munoz J., Cameron C. Histamine-sensitizing factor, mouse-protective antigens, and other antigens of some members of the genus Bordetella. J Bacteriol. 1969 Jul;99(1):57–64. doi: 10.1128/jb.99.1.57-64.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter J. M., Luther P. D. Cell culture assay for toxigenic Pasteurella multocida from atrophic rhinitis of pigs. Vet Rec. 1984 Apr 21;114(16):393–396. doi: 10.1136/vr.114.16.393. [DOI] [PubMed] [Google Scholar]
- Sawata A., Kume K. Nasal turbinate atrophy in young mice inoculated with Bordetella bronchiseptica of pig origin. Am J Vet Res. 1982 Oct;43(10):1845–1847. [PubMed] [Google Scholar]
- Sekiya K., Munoz J. J., Nakase Y. Effect of dermonecrotic toxin of Bordetella pertussis on the spleen of CFW and C57BL/10ScN mice. Microbiol Immunol. 1982;26(10):971–977. doi: 10.1111/j.1348-0421.1982.tb00244.x. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Nakagawa M., Shibata S., Suzuki K. Atrophic rhinitis produced by intranasal inoculation of Bordetella bronchiseptica in hysterectomy produced colostrum-deprived pigs. Cornell Vet. 1971 Oct;61(4):696–705. [PubMed] [Google Scholar]
- Tettamanti G., Pigman W. Purification and characterization of bovine and ovine submaxillary mucins. Arch Biochem Biophys. 1968 Mar 20;124(1):41–50. doi: 10.1016/0003-9861(68)90301-9. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Yokomizo Y., Shimizu T. Adherence of Bordetella bronchiseptica to swine nasal epithelial cells and its possible role in virulence. Res Vet Sci. 1979 Jul;27(1):15–21. [PubMed] [Google Scholar]