Abstract
The increase or decrease in yolk androgens over the laying sequence of a clutch in birds may mitigate or enhance, respectively, the disadvantage of the last-hatched chicks, providing a potentially adaptive tool to adjust brood size to food conditions. This variation may involve a genetic component on which Darwinian selection can act. We found that two lines of a wild bird species selected for bold and shy personalities show, respectively, increased and decreased androgen concentrations over the laying sequence. The line showing the increase laid earlier in the season, when food conditions are normally sufficient to raise the whole brood. The line showing the decrease laid later, when food is normally scarce, which may facilitate brood reduction. The results indicate a correlated response in maternal hormone transfer to genetic selection on personality, which relates to ecological conditions.
Keywords: maternal effects, testosterone, personality, laying date, hatching asynchrony
1. Introduction
Maternal effects influence offspring development with potentially adaptive consequences (Mousseau & Fox 1998). One example are avian yolk androgens of maternal origin, which affect hatching time, stimulate begging, growth and thereby survival in the offspring (Groothuis et al. 2005). Androgen concentrations vary between species, and among and within clutches of the same species (Groothuis et al. 2005; Gil et al. 2007; Martin & Schwabl 2008).
One adaptive hypothesis explaining such variation relates to within-clutch differences. In many species, yolk androgens increase over the laying sequence, but decrease in others, which may allow mothers to adjust brood size to food availability (Schwabl et al. 1997). Parents often start to incubate before clutch completion, first eggs receiving incubation before the last are laid, inducing earlier eggs to hatch sooner than later eggs (hatching asynchrony, HA). Consequently, last-hatched chicks experience a handicap in competition with older siblings. Thus, high androgen concentrations in later-laid eggs may enhance chick competitive ability, mitigating the disadvantages of HA when food is plentiful, while the opposite pattern exacerbates disadvantages of later hatching, facilitating brood reduction under poor conditions. These different patterns were revealed by between-species comparisons. Although theory would also predict systematical and heritable variations within species, this has been overlooked. One study on starlings (Eising et al. 2008) showed that androgen levels are characteristic of individual females, while another found an effect of selection on behavioural traits on yolk androgens (Gil & Faure 2007), suggesting such heritable variation.
We sampled great tits selected for distinct personalities (Drent et al. 2003) and differing in laying date. In the field, this would confront them with different food conditions. In natural populations, first clutches show less HA than late and second clutches, in which brood reduction is common (Barba et al. 1995). We hypothesized that birds from the early laying line would show increased androgens over the laying sequence. Birds of the late-laying line would follow the brood reduction strategy, by exhibiting the opposite pattern. The predominance of either of these strategies depending on laying date is suggested by a study in a similar songbird (Tobler et al. 2007).
Yolk androgens exert long-term effects on the behaviour of the offspring (Strasser & Schwabl 2004; Eising et al. 2006). Thus, intra-clutch variation could influence lifelong individual differences. Individual great tits differ consistently in exploration, aggression and responses to stressors referred to as syndromes, coping styles or personalities with fitness consequences (Dingemanse & Réale 2005; Groothuis & Carere 2005). Selection on exploration resulted in two lines of bold or shy explorers (Drent et al. 2003). Selection on risk taking resulted in lines similar to the original ones (van Oers et al. 2004a). A quantitative genetic analysis indicates a significant maternal effect (van Oers et al. 2004b). Although the artificial selection minimized it by standard procedures (cross fostering, hand rearing), an effect mediated by egg hormones could still have shaped the personalities. Indeed, testosterone injection in quail produced a phenotype resembling the bold personalities (Daisley et al. 2005, but see Tobler & Sandell 2007). Furthermore, a difference in yolk testosterone between lines of quail selected for social reinstatement has been reported (Gil & Faure 2007). Our lines may validate these findings in a functional context in a species in which the personalities and their ecological relevance are largely documented.
To study the effect of personalities on yolk hormones over the laying sequence and laying date, we collected eggs of both lines under identical conditions and analysed the concentration of testosterone (T), androstenedione (A4), 5α-dihydrotestosterone (DHT), 17β-oestradiol (E2) and corticosterone (B).
2. Material and methods
Details are reported in the electronic supplementary material. We collected 66 fresh laid eggs from first clutches of six shy and nine bold pairs, (Drent et al. 2003), for which we recorded: (i) onset of nest building, (ii) laying date (first egg), (iii) onset of incubation, (iv) clutch size, and (v) degree of HA. The exploration tests used for selection are described elsewhere (Drent et al. 2003).
The procedures for extraction and radioimmunoassays of the hormones were as in Schwabl (1993). Data were analysed in MLwiN v. 1.10.0006 by hierarchical linear models (Rasbah et al. 2004).
3. Results
The analyses of T and its precursor A4 yielded similar results. Therefore, we report only T data. No significant results were found for DHT, E2 or B (see the electronic supplementary material for levels and intercorrelations).
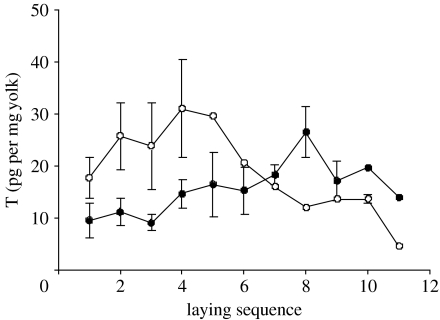
The lines differed in T concentrations across laying sequence (figure 1). In the bold line, T increased, while in the shy line, it decreased (line×sequence: Χ12=24.08, n=66, p<0.0001). The increase in T in the bold line was also significant (Χ12=19.182, n=45, p<0.0001). In the shy line, the best fit was for the model egg sequence+(egg sequence)2 (Χ12=13.821, n=21, p<0.0001), indicating that the levels first increased, then decreased with sequence.
Figure 1.
T yolk concentration in eggs across laying sequence of shy and bold great tits. Means±s.e.m. are shown. n shy eggs: 5, 4, 3, 3, 1, 1, 1, 1, 1, 2, 1; n bold eggs: 7, 5, 4, 6, 4, 4, 4, 3, 4, 1, 1. Filled circles, bold line; open circles, shy line.
To test whether differences over the sequence relate to laying date, we analysed the line effect in onset of laying and clutch size. Bold females laid earlier than shy females (F1,13=4.72, p=0.049, table 1; no effect of year or its interaction with line). A difference in laying date occurred in the same years in a larger sample of females (bold, n=19; shy, n=21, p<0.001). To test whether laying date was an important predictor of T, we ran the previous model with T as dependent variable and line, sequence, and their interaction as predictors, but now adding laying date and its interaction with line. The model did not improve (Χ22=1.799, n=66, p=0.417), while the interaction line×sequence was still significant (Χ12=8.812, p=0.0029), supporting line as the main explanatory factor. No line effect on egg mass, clutch size or its interaction with sequence was found.
Table 1.
Breeding parameters (mean±s.e.m.) in the selection lines.
| bold | shy | n | p | |
|---|---|---|---|---|
| clutch size | 8.3±0.8 | 7.0±1.6 | (9) (6) | n.s. |
| onset of nest buildinga | 19.9±6.4 | 37.2±3.2 | (8) (9) | 0.036 |
| laying datea | 27.0±5.5 | 42.7±2.9 | (9) (6) | 0.049 |
| onset of incubationa | 36.4±6.7 | 51.4±1.9 | (7) (5) | n.s. |
| hatching asynchronyb | 0.43±0.3 | 1.8±1.1 | (7) (5) | n.s. |
day 1, 1 April
days
We did not find an overall line effect, because in the model including line×sequence it disappeared when the interaction effect was removed (Χ12=2.245, p=0.134). This might be due to the different patterns in eggs 8–11. Average clutch size in the lines is 7–8. The analysis of T for the first eight eggs yielded an overall effect of line (Χ12=5.185, p=0.0227), with higher concentrations in the shy birds.
4. Discussion
The data support the hypothesis explaining variation in yolk androgens over the laying sequence: an increase from first- to later-laid eggs (bold line) would mitigate effects of HA under good food conditions, enabling parents to rear the full brood, while a decrease (shy line) would enhance them (brood reduction) under poor conditions (Schwabl et al. 1997). Until recently (Tobler et al. 2007), these strategies were described at the level of the species. We show that they occur within a species, in identical environmental conditions, have a genetic basis, and relate to laying date. In free-living great tits, late clutches show a larger degree of HA, face reduced food abundance and their late-hatching chicks have low survival (Barba et al. 1995). Indeed, the degree of HA tended to be larger in the shy than in the bold line. The results are consistent with the bold and shy phenotypes, being, respectively, risk taker and risk aversive in a life-history context (Wolf et al. 2007) the bold line taking the risk of rearing the full brood and the shy line avoiding rearing too many chicks.
The correlated response to selection in androgen levels suggests a genetic component for this trait. Consequently, Darwinian selection could act on hormone levels, supporting evolutionary explanations of hormone-mediated maternal effects. Although genetic variation in yolk hormones has previously been found in lines of domesticated species (Hayward et al. 2005; Gil & Faure 2007), this is the first report showing variation of within-clutch concentrations in association with laying date and personality.
The possibility that differences in attractiveness between males of the lines affected the results (Gil et al. 1999) is unlikely, since mate preference tests did not give an indication of females preferring males in relation to line (Groothuis & Carere 2005). Further, it is unlikely that attractiveness explains differences within clutch patterns.
Maternal androgens can affect various behaviours into adulthood, including those that form part of the tits' personality (see §1). Given that the majority of the birds for selection came from the first eight eggs, the typical clutch size in the lines, we analysed T for only these first eight eggs. An overall line difference emerged, suggesting that the bold offspring would develop under lower androgen exposure than the shy offspring, contrary to expectations from effects of T injections (Groothuis et al. 2005, but see Tobler & Sandell 2007). However, this is consistent with findings in lines of mice similar to those of the great tit (Groothuis & Carere 2005). SAL mice (proactive, bold) experience lower embryonic T exposure than LAL (reactive, shy) mice (de Ruiter et al. 1992).
A potential problem is that the selection lines are not replicated. However, data from unselected birds collected from the field and data from another selection experiment provide validation (van Oers et al. 2004a,b; Groothuis & Carere 2005; see the electronic supplementary material).
In conclusion, we found a correlated response between selection on personality and yolk androgen concentrations over the laying sequence, in conjunction with co-selection for laying date, implying that the mechanisms underlying maternal effects could be heritable and may allow rapid responses to natural selection on reproductive strategies. Different personalities could result from differential exposure to hormones rather than the selection for behaviour traits and the selection may have acted on a maternal effect. Experimental evidence is necessary to confirm these interpretations.
Acknowledgments
The Animal Experiment Committee of the University of Groningen (DEC 2124) approved this project.
We thank Sjoerd Veenstra and Roelie Wiegman for animal caretaking, Nikolaus von Engelhardt for statistical advice and Gregory Ball, Serge Daan, Jaap Koolhaas, Ben Sheldon, Judy Stamps and Marcel Visser for comments. C.C and T.G.G. were funded by NWO (805-33-324p). H.S. was supported by NSF (IBN-9604370) and NIH (MH-4987).
Supplementary Material
Full methods, additional results and further discussion
References
- Barba E, Gil Delgado J.A, Monros J.S. The costs of being late: consequences of delaying great tit first clutches. J. Anim. Ecol. 1995;64:642–651. doi:10.2307/5806 [Google Scholar]
- Daisley J.N, Bromundt V, Möstl E, Kotrschal K. Enhanced yolk testosterone influences behavioral phenotype independent of sex in Japanese quail chicks. Horm. Behav. 2005;47:185–194. doi: 10.1016/j.yhbeh.2004.09.006. doi:10.1016/j.yhbeh.2004.09.006 [DOI] [PubMed] [Google Scholar]
- de Ruiter A.J.H, Koolhaas J.M, Keijser J.N, van Oortmerssen G.A. Differential testosterone secretory capacity of the testes of aggressive and non aggressive mice during ontogeny. Aggr. Behav. 1992;18:149–157. doi:10.1002/1098-2337(1992)18:2<149::AID-AB2480180209>3.0.CO;2-J [Google Scholar]
- Dingemanse N.J, Réale D. Natural selection and animal personalities. Behaviour. 2005;142:1165–1190. doi:10.1163/156853905774539445 [Google Scholar]
- Drent P.J, van Oers K, van Noordwijk A.J. Realised heritability of personalities in the great tit (Parus major) Proc. R. Soc. B. 2003;270:45–51. doi: 10.1098/rspb.2002.2168. doi:10.1098/rspb.2002.2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eising C.M, Müller W, Groothuis T.G.G. Avian mothers create different phenotypes by hormone deposition in their eggs. Biol. Lett. 2006;2:20–22. doi: 10.1098/rsbl.2005.0391. doi:10.1098/rsbl.2005.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eising C.M, Pavlova D, Groothuis T.G.G, Eens M, Pinxten R. Maternal yolk androgens in European starlings: social environment or individual traits of the mother? Behaviour. 2008;145:51–72. doi:10.1163/156853908782687232 [Google Scholar]
- Gil D, Faure G.M. Correlated response in yolk testosterone levels following divergent genetic selection for social behaviour in quail. J. Exp. Zool. A. 2007;307:91–94. doi: 10.1002/jez.a.340. [DOI] [PubMed] [Google Scholar]
- Gil D, Graves J.A, Hazon N, Wells A. Male attractiveness and differential testosterone investment in zebra finch eggs. Science. 1999;286:126–128. doi: 10.1126/science.286.5437.126. doi:10.1126/science.286.5437.126 [DOI] [PubMed] [Google Scholar]
- Gil D, Biard C, Lacroix A, Spottiswood C.N, Saino N, Puerta M, Moller A.P. Evolution of yolk androgens in birds: development, coloniality, and sexual dichromatism. Am. Nat. 2007;169:802–819. doi: 10.1086/516652. doi:10.1086/516652 [DOI] [PubMed] [Google Scholar]
- Groothuis T.G.G, Carere C. Avian personalities: characterization and epigenesis. Neurosci. Biobehav. Rev. 2005;29:137–150. doi: 10.1016/j.neubiorev.2004.06.010. doi:10.1016/j.neubiorev.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Groothuis T.G.G, Müller W, von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. doi:10.1016/j.neubiorev.2004.12.002 [DOI] [PubMed] [Google Scholar]
- Hayward L.S, Satterlee D.G, Wingfield J.C. Japanese quail selected for high plasma corticosterone response deposit high levels of corticosterone in their eggs. Physiol. Biochem. Zool. 2005;78:1026–1031. doi: 10.1086/432854. doi:10.1086/432854 [DOI] [PubMed] [Google Scholar]
- Martin T.E, Schwabl H. Variation in maternal effects and embryonic development rates among passerine species. Phil. Trans. R. Soc. B. 2008;363:1663–1674. doi: 10.1098/rstb.2007.0009. doi:10.1098/rstb.2007.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau T.A, Fox C.W, editors. Maternal effects. Oxford University Press; New York, NY: 1998. [Google Scholar]
- Rasbah, J., Steele, F., Browne, W. & Prosser, B. 2004 A user's guide toMLwiN v. 2.0. London, UK: Institute of Education.
- Schwabl H. Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA. 1993;90:11 446–11 450. doi: 10.1073/pnas.90.24.11446. doi:10.1073/pnas.90.24.11446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabl H, Mock D.W, Gieg J.A. A hormonal mechanism for parental favouritism. Nature. 1997;386:231. doi:10.1038/386231a0 [Google Scholar]
- Strasser R, Schwabl H. Yolk testosterone organises behavior and male plumage coloration in house sparrows. Behav. Ecol. Sociobiol. 2004;56:491–497. doi:10.1007/s00265-004-0810-9 [Google Scholar]
- Tobler M, Sandell M. Yolk testosterone modulates persistence of neophobic responses in adult zebra finches. Horm. Behav. 2007;52:640–645. doi: 10.1016/j.yhbeh.2007.07.016. doi:10.1016/j.yhbeh.2007.07.016 [DOI] [PubMed] [Google Scholar]
- Tobler M, Granbom M, Sandell M. Maternal androgens in the pied flycatcher: timing of breeding and within female consistency. Oecologia. 2007;151:730–740. doi: 10.1007/s00442-006-0610-1. doi:10.1007/s00442-006-0610-1 [DOI] [PubMed] [Google Scholar]
- van Oers K, Drent P.J, de Goede P, van Noordwijk A.J. Realized heritability and repeatability of risk-taking behaviour in relation to avian personalities. Proc. R. Soc. B. 2004a;271:65–73. doi: 10.1098/rspb.2003.2518. doi:10.1098/rspb.2003.2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oers K, Drent P.J, de Jong G, van Noordwijk A.J. Additive and nonadditive genetic variation in avian personality traits. Heredity. 2004b;93:496–503. doi: 10.1038/sj.hdy.6800530. doi:10.1038/sj.hdy.6800530 [DOI] [PubMed] [Google Scholar]
- Wolf M, van Doorn G.S, Leimar O, Weissing F.J. Life-history trade-offs favour the evolution of animal personalities. Nature. 2007;447:581–584. doi: 10.1038/nature05835. doi:10.1038/nature05835 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Full methods, additional results and further discussion