Abstract
Sexual selection by female choice can maintain male traits that are counter selected by natural selection. Alteration of the potential for sexual selection can thus lead to shifts in the expression of male traits. We investigated female mate choice for large male body size in a fish (Poecilia mexicana) that, besides surface streams, also inhabits two caves. All four populations investigated, exhibited an ancestral visual preference for large males. However, only one of the cave populations also expressed this female preference in darkness. Hence, the lack of expression of female preference in darkness in the other cave population leads to relaxation of sexual selection for large male body size. While P. mexicana populations with size-specific female mate choice are characterized by a pronounced male size variation, the absence of female choice in one cave coincides with the absence of large bodied males in that population. Our results suggest that population differences in the potential for sexual selection may affect male trait variation.
Keywords: non-visual mate choice, sensory shift, sexual selection, size variation, Poecilia mexicana (Poeciliidae)
1. Introduction
Female mate choice can be a strong selective force in the evolution and maintenance of male secondary sexual traits (Andersson 1994), balancing the costs such traits may confer in terms of natural selection (Zuk & Kolluru 1998). Male secondary traits can disappear in populations both if costs of trait expression increase (Zuk et al. 2006) or if the potential for sexual selection by female choice declines; for example, through changes in environmental conditions that impair the use of sensory systems (Seehausen et al. 1997; Fisher et al. 2006). A drastic example is the colonization of cave habitats by previously surface-dwelling diurnal animals. In the absence of light, all visual female preferences become obsolete, and accordingly male traits should vanish. Alternatively, male traits may remain under sexual selection if females evolve the capability of detecting male traits using another sensory system (‘sensory shift’, Plath et al. 2004).
We examined visual and non-visual female mate choice for large male body size in surface and cave populations of a small live-bearing fish (Poecilia mexicana). Female choice for large body size based on visual cues is widespread in poeciliid fishes (e.g. Reynolds & Gross 1992; Rosenthal & Evans 1998) and, in conjunction with natural counter-selection, seems to stabilize male size variation (Ryan et al. 1992). We compared female mating preferences and male size variation in two P. mexicana populations that independently colonized subterranean habitats (Tobler et al. submitted). Specifically, we tested whether the cave populations retained the ancestral poeciliid preference for large male size when exposed to light, and whether females evolved the ability to discriminate between different-sized males in darkness, indicating a sensory shift. Finally, we provide correlational evidence that non-visual female mate choice affects male size distributions in natural populations.
2. Material and methods
(a) Populations
Cave-dwelling P. mexicana were collected in the Cueva del Azufre (CDA) and Cueva Luna Azufre (CLA) in Tabasco, Mexico. Surface-dwelling fish were collected from the nearby El Azufre creek and from the nearest bigger river, the Río Oxolotan (for details see Tobler et al. (2006, 2008)). Gene flow among the populations investigated is low (Tobler et al. submitted). All test fish were descendants of wild-caught individuals and were reared in large, randomly outbred populations at the Universities of Hamburg and Oklahoma. Mate choice data for two surface populations and the CDA cave form were reanalysed from Plath et al. (2004).
(b) Female mate choice
Mate choice experiments followed (Plath et al. 2004). Individual focal females (31.8±1.6 mm standard length (SL), mean±s.e.) could choose between a large (33.0±0.2 mm) and a small stimulus male (23.7±0.4 mm) from the same population that were presented simultaneously on either side of a test tank. Males were confined to transparent Plexiglas cylinders in the visible light treatment or to wire mesh cylinders under infrared light to test for a derived non-visual preference. Unlike other cave fishes, cave-dwelling P. mexicana possess functional eyes, but none of the populations have IR-sensitive retinal photopigments (Körner et al. 2006). Association times during two subsequent 10 min observation periods (with interchanged stimuli) were used as a measure of preference. Owing to the absence of large males from the CLA laboratory stocks, CDA males were used as stimulus males in this case. To detect the female preferences for large male body size, association times near the two types of males were compared using paired t-tests. We also calculated the strength of preference (SOP=% timelargemale−% timesmallmale). Arcsine-(square-root)-transformed SOPs were used as dependent variable in an ANOVA with ‘treatment’ (light or dark) and ‘population’ as independent variables.
(c) Male size variation
To assess the size variation, we collected P. mexicana from the four natural habitats in spring 2007. Male and female SLs were measured using callipers to the closest millimetre. For data analysis, males and females were assigned to three size classes: SL<25 mm, 25–29 mm and >29 mm (see Morris et al. 1996). The relative frequencies of the different size classes were compared using chi-squared tests.
3. Results
(a) Female mate choice
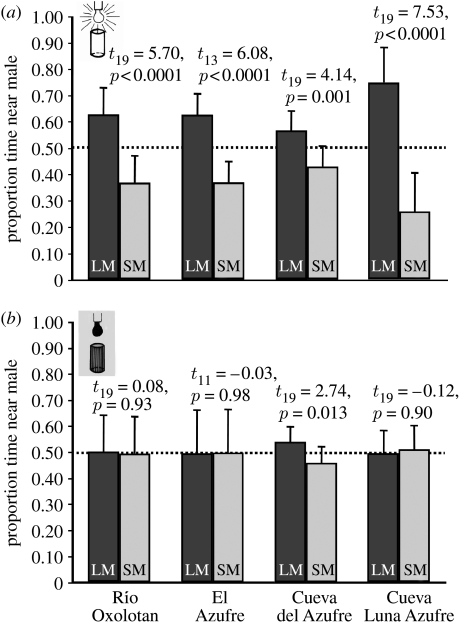
Females from all populations showed a highly significant visual preference for the larger of the two stimulus males (figure 1a). In the absence of visible light, only the CDA females showed a significant preference (figure 1b). Comparing the strength of preference across treatments and populations revealed a significant interaction effect (F3,138=6.72, p<0.001) indicating that populations responded differently in the two experimental treatments. Both the factor population (F3,138=3.35, p=0.021) and treatment (F1,138=49.37, p<0.001) were also significant.
Figure 1.
The mean (±s.d.) time P. mexicana females spent associating with a large (black bars, LM) and a small stimulus male (grey bars, SM). Males were presented in (a) transparent Plexiglas cylinders under visible light or (b) wire mesh cylinders under infrared conditions. Association times were compared using paired t-tests.
(b) Male size variation
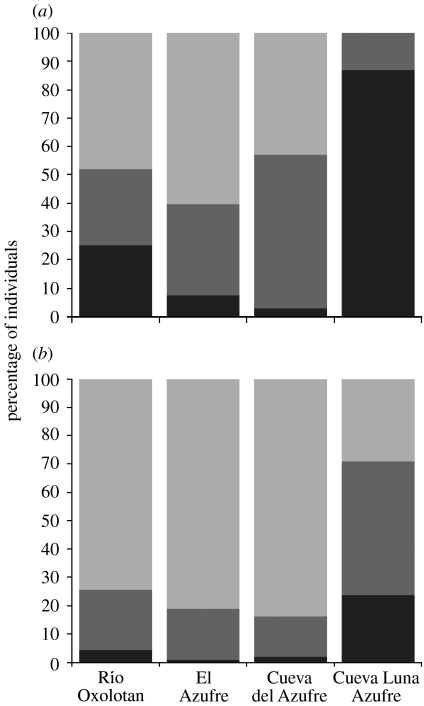
Male and female size distributions differed significantly among populations (figure 2). Large males were absent in the CLA population (n=38, mean=21 mm (range: 18–29 mm)), but common in the others (Río Oxolotan: n=131, mean=31 mm (19–56 mm); El Azufre: n=53, mean=30 mm (24–39 mm); CDA: n=203, mean=29 mm (21–45 mm)). Although large females were present in the CLA (figure 2b), females were, on average, smaller (n=38, mean=29 mm (20–49 mm)) than those from other populations (Río Oxolotan: n=263, mean=37 mm (22–62 mm); El Azufre: n=117, mean=35 mm (23–55 mm); CDA: n=303, mean=35 mm (20–52 mm)).
Figure 2.
(a) Male (Χ2=178.4, p<0.001) and (b) female (Χ2=79.7, p<0.001) size distributions differed significantly among populations. The bars depict the relative frequency of small (<25 mm SL; black), medium (25–29 mm; grey) and large individuals (>29 mm; light grey) in the different populations.
4. Discussion
Female P. mexicana from all populations exhibited a visual preference for large males, suggesting the persistence of the ancestral character state in both cave populations even though visual preferences are not currently under selection. However, the potential for sexual selection through female mate choice appears to differ among the two cave populations. CDA females also discriminated among different-sized males in darkness, indicating a sensory shift, whereby both mechano-sensory and chemical cues may play a role (Plath et al. 2007a). By contrast, CLA females, just like surface populations, did not exhibit a preference in darkness. Conceivably, CLA females might not have responded to non-visual cues emanating from CDA males; however, females readily responded in visual choice tests, and tests with CLA males were not feasible due to the absence of large males in this population.
Despite their spatial proximity, both caves were independently colonized by P. mexicana; the two populations represent morphologically and genetically distinct lineages (Tobler et al. 2008, submitted). The inability of the CLA population to evolve non-visual mate choice could be a consequence of lower evolutionary potential due to small population size (see Willi et al. 2006) or a more recent colonization as compared to the CDA population.
Female mate choice for large-sized males is thought to maintain male size variation in natural populations (Ryan et al. 1992). While large males are favoured due to sexual selection, they face a cost by natural selection as they take longer to mature (Morris & Ryan 1990), are more conspicuous in ornamentation and behavioural displays (Ptacek & Travis 1996) and, thus, are preferred targets of predators (Trexler et al. 1994; Johansson et al. 2004; Tobler et al. 2007). Lack of non-visual mate choice was accompanied by the absence of large-sized males in the CLA population. Although our sample size is limited due to the small population size in this cave, more than 80% of males were smaller than 25 mm. The relaxation of sexual selection thus could have directly favoured the early maturity, resulting in the loss of large-sized males; although, unlike in the CDA (Tobler et al. 2007), predators seem to be absent in this cave (M. Tobler et al. 2006, 2007, unpublished data).
The low average body size in CLA females also suggests a possible role of natural selection on body size reduction. For example, low energy availability could select for slow growth rates and smaller size at maturity (Reznick 1990; Arendt & Reznick 2005). But, although the CLA is indeed a resource-poor habitat (Tobler in press), energy limitation is unlikely to solely account for the loss of large-sized males. P. mexicana from the El Azufre and CDA population exhibit equally low body conditions (Tobler in press), probably owing to energetically costly adaptations necessary to cope with the toxic hydrogen sulphide present in the latter two habitats (Plath et al. 2007b); still large males are frequent in these populations.
Acknowledgments
The experiments reported in this paper comply with the current legislation of the European Union and the USA.
The Mexican Government kindly issued permits to collect fish (DGOPA02232.230706-1079). Financial support came from the Swiss National Science Foundation (PBZHA-121016 to M.T.) and the DFG (Pl 470/1-1, 2 to M.P.). This study used resources of the University of Oklahoma Aquatic Research Facility.
References
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Arendt J.D, Reznick D.N. Evolution of juvenile growth rates in female guppies (Poecilia reticulata): predator regime or resource level? Proc. R. Soc. B. 2005;272:333–337. doi: 10.1098/rspb.2004.2899. doi:10.1098/rspb.2004.2899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher H.S, Wong B.B.M, Rosenthal G.G. Alteration of the chemical environment disrupts communication in freshwater fish. Proc. R. Soc. B. 2006;273:1187–1193. doi: 10.1098/rspb.2005.3406. doi:10.1098/rspb.2005.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson J, Turesson H, Persson A. Active selection for large guppies, Poecilia reticulata, by the pike cichlid, Crenicichla saxatilis. Oikos. 2004;105:595–605. doi:10.1111/j.0030-1299.2004.12938.x [Google Scholar]
- Körner K.E, Schlupp I, Plath M, Loew E.R. Spectral sensitivity of mollies: comparing surface- and cave-dwelling Atlantic mollies, Poecilia mexicana. J. Fish Biol. 2006;69:54–65. doi:10.1111/j.1095-8649.2006.01056.x [Google Scholar]
- Morris M.R, Ryan M.J. Age at sexual maturity of male Xiphophorus nigrensis in nature. Copeia. 1990;1990:747–751. doi:10.2307/1446440 [Google Scholar]
- Morris M.R, Wagner W.E, Ryan M.J. A negative correlation between trait and mate preference in Xiphophorus pygmaeus. Anim. Behav. 1996;52:1193–1203. doi:10.1006/anbe.1996.0267 [Google Scholar]
- Plath M, Parzefall J, Körner K, Schlupp I. Sexual selection in darkness? Female mating preferences in surface- and cave-dwelling Atlantic mollies, Poecilia mexicana (Poeciliidae, Teleostei) Behav. Ecol. Sociobiol. 2004;55:596–601. doi:10.1007/s00265-003-0750-9 [Google Scholar]
- Plath M, Schlupp I, Parzefall J, Riesch R. Female preference for large body size in the cave molly, Poecilia mexicana (Poeciliidae, Teleostei): influence of species-and sex-specific cues. Behaviour. 2007a;144:1147–1160. [Google Scholar]
- Plath M, Tobler M, Riesch R, Garcia de Leon F.J, Giere O, Schlupp I. Survival in an extreme habitat: the role of behaviour and energy limitation. Naturwissenschaften. 2007b;94:991–996. doi: 10.1007/s00114-007-0279-2. doi:10.1007/s00114-007-0279-2 [DOI] [PubMed] [Google Scholar]
- Ptacek M.B, Travis J. Inter-population variation in male mating behaviours in the sailfin molly, Poecilia latipinna. Anim. Behav. 1996;52:59–71. doi:10.1006/anbe.1996.0152 [Google Scholar]
- Reynolds J.D, Gross M.R. Female mate preference enhances offspring growth and reproduction in a fish, Poecilia reticulata. Proc. R. Soc. B. 1992;250:57–62. doi:10.1098/rspb.1992.0130 [Google Scholar]
- Reznick D.N. Plasticity in age and size at maturity in male guppies (Poecilia reticulata): an experimental evaluation of alternative models of development. J. Evol. Biol. 1990;3:185–203. doi:10.1046/j.1420-9101.1990.3030185.x [Google Scholar]
- Rosenthal G.G, Evans C.S. Female preferences for swords in Xiphophorus helleri reflects a bias for large apparent size. Proc. Natl Acad. Sci. USA. 1998;95:4431–4436. doi: 10.1073/pnas.95.8.4431. doi:10.1073/pnas.95.8.4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M.J, Pease C, Morris M.R. A genetic polymorphism in the swordtail Xiphophorus nigrensis: testing the prediction of equal fitnesses. Am. Nat. 1992;139:21–32. doi:10.1086/285311 [Google Scholar]
- Seehausen O, Van Alphen J.J.M, Witte F. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science. 1997;277:1808–1811. doi:10.1126/science.277.5333.1808 [Google Scholar]
- Tobler, M. In press. Divergence in trophic ecology characterises colonisation of extreme habitats. Biol. J. Linn. Soc.
- Tobler M, Schlupp I, Heubel K, Riesch R, Garcia de Leon F.J, Giere O, Plath M. Life on the edge: hydrogen sulfide and the fish communities of a Mexican cave and surrounding waters. Extremophiles. 2006;10:577–585. doi: 10.1007/s00792-006-0531-2. doi:10.1007/s00792-006-0531-2 [DOI] [PubMed] [Google Scholar]
- Tobler M, Schlupp I, Plath M. Predation of a cave fish (Poecilia mexicana, Poeciliidae) by a giant water-bug (Belostoma, Belostomatidae) in a Mexican sulfur cave. Ecol. Entomol. 2007;32:492–495. doi:10.1111/j.1365-2311.2007.00892.x [Google Scholar]
- Tobler M, Riesch R, Garcia de Leon F.J, Schlupp I, Plath M. A new and morphologically distinct cavernicolous population of Poecilia mexicana (Poeciliidae, Teleostei) Environ. Biol. Fish. 2008;82:101–108. doi:10.1007/s10641-007-9258-x [Google Scholar]
- Tobler, M., DeWitt, T. J., Schlupp, I., Garcia de Leon, F. J., Herrmann, R., Feulner, P., Tiedemann, R. & Plath, M. Submitted. Phenotypic and genetic divergence across two abiotic environmental gradients in Poecilia mexicana [DOI] [PubMed]
- Trexler J, Tempe R, Travis J. Size-selective predation of sailfin mollies by two species of heron. Oikos. 1994;69:250–259. doi:10.2307/3546145 [Google Scholar]
- Willi Y, Van Buskirk J, Hoffmann A. Limits to the adaptive potential of small populations. Annu. Rev. Ecol. Evol. Syst. 2006;37:433–458. doi:10.1146/annurev.ecolsys.37.091305.110145 [Google Scholar]
- Zuk M, Kolluru G.R. Exploitation of sexual signals by predators and parasitoids. Quart. Rev. Biol. 1998;73:415–438. doi:10.1086/420412 [Google Scholar]
- Zuk M, Rotenberry J.T, Tinghitella R.M. Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol. Lett. 2006;2:521–524. doi: 10.1098/rsbl.2006.0539. doi:10.1098/rsbl.2006.0539 [DOI] [PMC free article] [PubMed] [Google Scholar]