Abstract
Investment in one life-history stage can have delayed effects on subsequent life-history stages within a single reproductive bout. We experimentally heated tree swallow (Tachycineta bicolor) nests during incubation to test for effects on parental and nestling conditions. Females incubating in heated boxes maintained higher body condition and fed nestlings at higher rates. We cross-fostered nestlings and found that young nestlings (4–7 days old) incubated in heated nests had higher body condition and body mass, regardless of treatment status of their rearing parent. However, older nestlings which were fed by heated females maintained higher condition and body mass regardless of treatment status of their incubating parent. These results indicate that investment in one life-history stage can have multiple pathways of carry-over effects on future life-history stages.
Keywords: carry-over effects, environmental variation, life-history trade-offs
1. Introduction
Optimal energetic investment in each life-history stage is governed by trade-offs in allocation to other stages (Stearns 1992). Previous work on birds has shown that changes in energetic investment during one life-history stage can have carry-over effects on subsequent life-history stages within reproductive bouts (Heaney & Monaghan 1996; Reid et al. 2000a). Here, we experimentally modified the energetic costs of incubation to assess the consequences of such a manipulation on two stages within the same reproductive bout: incubation and nestling provisioning. We modified nest microclimate because investment in incubation and embryonic development is particularly sensitive to fluctuations in environmental conditions (Conway & Martin 2000; Cresswell et al. 2003; Olson et al. 2006). Our experiment sought to determine whether there were (i) effects on incubating females of heating nests during incubation that carry-over to nestling provisioning and (ii) effects of changes in the thermal environment during incubation on nestling body condition. Direct modification of thermal environment (Reid et al. 2000b; Engstrand & Bryant 2002; de Heij et al. 2006; Dobbs et al. 2006) may provide a clearer assessment of the short-term effects of modifying incubation demand than would clutch size manipulations (Reid et al. 2002). We studied the tree swallow, Tachycineta bicolor, a species in which only females incubate and males do not feed incubating females. We predicted that heating nests would reduce the energetic costs of incubation for females and produce the following effects: (i) a smaller decline in adult female body mass and (ii) an increased nestling feeding rate among females whose nests were heated (hereafter ‘heated females’).
To separate the effect of the heating treatment on eggs from the effects of being raised by a heated female, we cross-fostered nestlings between heated and non-heated (control) nests. We predicted that nestlings heated as embryos during incubation would have higher body condition (i.e. residual mass) than control nestlings due to greater retention of energy reserves, as previous work has suggested that suboptimal developmental conditions lead to reduced body condition (Larsen et al. 2003), though increased temperatures can lead to increased water loss and lower mass at hatching (Reid et al. 2000a).
2. Material and methods
We conducted our experiment from May to July 2006 in Amherst, MA. We paired the nests by clutch initiation date and clutch size, and assigned one nest of each pair to heated and non-heated (control) treatment groups. On the sixth day after clutch completion, we installed a Peltier thermoelectric heating device to each nest; heated boxes were attached to a battery, control nests to a sham battery (see the electronic supplementary material). On the fourth day following clutch completion, we measured adult female body mass, head-bill length and flattened ninth primary wing feather length. We recaptured all adult females on the third or fourth day of the nestling period to measure change in body mass.
We manipulated 30 boxes during incubation, but owing to nest failures, mass change was recorded on 25 birds (control 16 and heated 9), and cross-fostering was conducted between 18 nests (control 9 and heated 9). Imbalanced sample sizes arose due to destruction of nests from house wrens. A total of 29 nestlings were cross-fostered. On nestling day 1 (hatching 0), we measured the mass of each nestling. Nine pairs of nests with the same hatch date were subjected to a split-nest partial cross-fostering experiment of individually marked nestlings on nestling day 3. Nests did not differ in brood size prior to cross-fostering (control 3.2, heated 3.3, t17=0.55, p=0.55) and did not differ in average size rank hierarchy following brood manipulation (F1,57=1.06, p=0.31). Nestling body mass was measured on days 1, 4, 7, 10 and 13, head-bill and tarsus beginning on day 4 and wing length beginning on day 7. Growth rate of 13-day-old nestlings was compared by fitting logistic growth equations to each individual (details in the electronic supplementary material; Ardia 2006). For each nest, 50 min feeding observations were conducted on nestling days 8 and 11. Nests were paired such that cross-fostered nests were observed simultaneously to control for the effects of weather, time of day and aerial insect availability, the main food source. Additional information on feeding visits and insect availability is available in the electronic supplementary material.
We tested our hypotheses using stepwise forward linear regression and general linear models (see the electronic supplementary material for more information).
3. Results
Heated females gained body mass and body condition (residual body mass) between the incubation and the early nestling period, while control females did not (change in body mass (g); control females=−0.73±0.34, n=16; heated females=0.60±0.43, n=9; F1,21=8.17, p=0.002, partial R2=0.42; change in residual body mass; control: −0.62±1.37, n=16, heated 1.46±2.45, n=9; F1,21=7.91, p=0.01, partial R2=0.14). In models predicting change in body mass, initial body mass was retained as a covariate (β=−0.49, F1,24=11.56, p=0.002, partial R2=0.31); all other covariates were dropped from models.
Heated females made more feeding visits to offspring on nestling day 11 (feeding visits per hour; control 6.91±0.9; heated 9.36±1.1; F1,17=7.3, p=0.01, partial R2=0.21); there were no differences in male feeding rates (see the electronic supplementary material). Larger brood sizes marginally led to increased feeding visits (β=2.3, F1,17=3.43, p=0.08, partial R2=0.12); no other covariates entered the model. There was no difference in behaviour between treatment groups on nestling day 8.
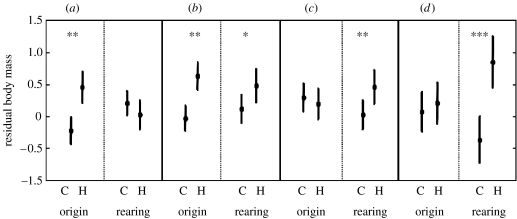
A mixed model analysis controlling for box of origin versus box of rearing revealed both effects of experimental heating on nestling condition (table 1). Early in the nestling period (nestling days 4 and 7), experimental heating had the strongest effect on nestling mass. Nestlings heated during incubation, regardless of box of rearing, were larger and had higher residual body mass and nestling mass on days 4 and 7 (table 1; figure 1). By contrast, 4-day-old nestlings raised by heated females were not larger in absolute mass or residual body mass, but 7-day-old nestlings show a trend towards being larger and having greater residual body mass if being fed by a heated female (table 1; figure 1). Both 10- and 13-day-old nestlings were larger and in better condition in the boxes of heated females regardless of whether nestlings experienced heated conditions as eggs (table 1; figure 1). In addition, growth rates of control nestlings fed by heated mothers were greater than control nestlings fed by unheated mothers (composite growth rate±s.e.; heated 0.34±0.14, control 0.05±0.13; mixed model F1,44=12.7, p<0.001).
Table 1.
Results of general linear mixed model showing factors affecting nestling residual body mass and body mass in tree swallows (Tachycineta bicolor) following experimental heating of nest boxes during the incubation period. (Nestlings were cross-fostered on day 3 of the nestling period. Origin refers to treatment (heated versus control) nestlings experienced as eggs; final refers to treatment that feeding females experienced during the nestling period. Effect size is given as partial η2. Numbers in bold indicate p<0.05; numbers in italics indicate p<0.10.)
| predictors | day 4 | day 7 | day 10 | day 13 | ||||
|---|---|---|---|---|---|---|---|---|
| residual body mass | mass | residual body mass | mass | residual body mass | mass | residual body mass | mass | |
| heating treatment at nest of origin | F=5.12 | F=5.23 | F=6.01 | F=5.23 | F=1.06 | F=0.74 | F=0.41 | F=0.00 |
| p=0.028 | p=0.0028 | p=0.018 | p=0.027 | p=0.31 | p=0.39 | p=0.52 | p=0.96 | |
| =0.33 | =0.53 | =0.29 | =0.25 | |||||
| heating treatment of nest of rearing | F=0.55 | F=0.62 | F=3.01 | F=2.77 | F=4.88 | F=4.77 | F=10.32 | F=5.16 |
| p=0.046 | p=0.43 | p=0.09 | p=0.10 | p=0.03 | p=0.04 | p=0.002 | p=0.03 | |
| =0.20 | =0.17 | =0.37 | =0.25 | |||||
| clutch size | F=0.81 | F=1.13 | F=1.11 | F=0.60 | F=1.48 | F=0.85 | F=0.58 | F=0.43 |
| p=0.38 | p=0.29 | p=0.30 | p=0.44 | p=0.23 | p=0.36 | p=0.45 | p=0.52 | |
| female age at origin | F=0.01 | F=0.39 | F=2.33 | F=0.50 | F=2.61 | F=1.99 | F=2.09 | F=2.71 |
| p=0.90 | p=0.53 | p=0.13 | p=0.48 | p=0.13 | p=0.17 | p=0.15 | p=0.11 | |
| female age at final box | F=0.31 | F=0.15 | F=2.01 | F=1.31 | F=0.67 | F=0.00 | F=0.28 | F=0.98 |
| p=0.58 | p=0.70 | p=0.16 | p=0.26 | p=0.41 | p=0.99 | p=0.60 | p=0.33 | |
| average ambient temperature during incubation | F=0.08 | F=0.23 | F=4.31 | F=2.07 | F=1.59 | F=1.03 | F=1.43 | F=2.00 |
| p=0.77 | p=0.63 | p=0.04 | p=0.16 | p=0.21 | p=0.32 | p=0.24 | p=0.16 | |
| =0.11 | ||||||||
| insect availability during incubation | F=2.88 | F=1.40 | F=1.88 | F=1.32 | F=6.48 | F=12.03 | F=0.55 | F=0.00 |
| p=0.09 | p=0.24 | p=0.18 | p=0.26 | p=0.01 | p=0.001 | p=0.47 | p=0.96 | |
| =0.17 | =0.18 | |||||||
| clutch initiation date | F=5.59 | F=0.42 | F=1.47 | F=0.19 | F=0.18 | F=0.17 | F=0.63 | F=0.28 |
| p=0.02 | p=0.02 | p=0.23 | p=0.67 | p=0.68 | p=0.68 | p=0.43 | p=0.60 | |
| =0.09 | =0.05 | |||||||
| nestling measure in previous time period | F=0.77 | F=1.11 | F=2.01 | F=1.73 | F=1.81 | F=1.93 | ||
| p=0.39 | p=0.30 | p=0.16 | p=0.20 | p=0.19 | p=0.17 | |||
| d.f. | 1,50 | 1,50 | 1,49 | 1,49 | 1,49 | 1,49 | 1,44 | 1,44 |
Figure 1.
Effect of experimental heating treatment on the least square mean residual body mass of nestling tree swallows (Tachycineta bicolor) following cross-fostering manipulation intended to separate effects of heating nest boxes during incubation. Residual body mass calculated as the residuals of a regression of head-bill length on body mass. Effect size (partial η2) is given for significant variables. Origin refers to treatment experienced during the incubation period. Rearing refers to the treatment experienced by rearing female. C, control; H, heated. *p<0.10, **p<0.05, ***p<0.01. (a) Day 4, (b) day 7, (c) day 10 and (d) day 13.
4. Discussion
We found carry-over life-history effects by experimentally manipulating the thermal environment of tree swallow nests during incubation. Nestlings that were incubated in heated nests were heavier and in better body condition early in the nestling period, regardless of whether their mother or foster mother's nest was heated during incubation. This change could be due to egg temperature (Hepp et al. 2006; Olson et al. 2006; Martin et al. 2007); a parallel study found that heated females raised on-bout and off-bout egg temperatures during and following heating (Ardia et al. in press). The finding reported here that chicks from heated nests were larger suggests a link between enhanced developmental conditions and nestling size at hatching, though differences at age four could have resulted from early rearing conditions.
In addition, increased nest temperatures may have reduced depletion of female energy reserves, allowing females to spend more time incubating (Chaurand & Weimerskirch 1994; Bryan & Bryant 1999; Cresswell et al. 2004) and maintain egg temperatures nearer to their developmental optima, thus accelerating development (Hepp et al. 2006; Kim & Monaghan 2006; Martin et al. 2007). Incubating females probably modify investment based on energy reserves. When conditions allow, breeding females improve development conditions for offspring with the resultant effect of increasing nestling condition. Our results reveal that normal incubation conditions are suboptimal from the perspective of a developing embryo, but are likely optimal from the perspective of the trade-offs faced by an iteroparous intermittent incubator, such as the tree swallow.
By contrast, later in the nestling period, the strongest predictor of offspring mass and condition was whether the rearing female's nest had been heated. This effect is probably due to the behaviour of heated females, as heated females provisioned their nestlings at a greater rate than did control females. As nestlings age, the effects of incubation conditions are superceded by the conditions experienced during the nestling period and are now attributable to its effects on the rearing female. These results suggest that heating nest boxes served to maintain female condition during the incubation period, allowing them to alter the trade-off between self maintenance and parental investment later in the nestling period, which resulted in an improvement in nestling condition. Our results are conservative given that we heated boxes for slightly less than 50% of the incubation period. The degree of heating in our boxes reflects the natural range in internal box temperatures observed across the tree swallow range (D. R. Ardia 2006, unpublished data). Thus, differences in life histories among populations may be due, in part, to the energetic demands during incubation. For a widespread species that occurs across a broad latitudinal range of environmental conditions, our results suggest that warmer breeding areas may provide reduced energetic costs for females, thus allowing for reduced costs of breeding. The findings of this and our parallel study are important for our understanding of life-history evolution, as we have shown that females alter the trade-off between self-maintenance and parental investment during each stage and that trade-offs can have carry-over effects for future stages (Gorman & Nager 2004; Naguib & Gil 2005).
Acknowledgments
This work was conducted under the approval of Institutional Animal Care and Use Committees of the University of Massachusetts-Amherst and Amherst College.
We thank Ben Taft for field assistance and Renae Brodie, Sarah Partan, Beth Jakob, and five anonymous reviewers for reading a previous version of this manuscript. Funding for the research was provided by the Organismic and Evolutionary Biology Programme at the University of Massachusetts and the Faculty Research Award Programme as funded by the H. Axel Schupf '57 Fund for Intellectual Life at Amherst College.
Supplementary Material
Additional information on methods
References
- Ardia D.R. Geographic variation in the tradeoff between nestling growth rate and body condition in the tree swallow. Condor. 2006;108:601–611. doi:10.1650/0010-5422(2006)108[601:GVITTB]2.0.CO;2 [Google Scholar]
- Ardia, D. R., Perez, J. H., Chad, E. K., Voss, M. A. & Clotfelter, E. D. In press. Temperature and life history: experimental heating leads female tree swallows to modulate egg temperature and incubation behavior. J. Anim. Ecol [DOI] [PubMed]
- Bryan S.M, Bryant D.M. Heating nest-boxes reveals an energetic constraint on incubation behaviour in great tits, Parus major. Proc. R. Soc. B. 1999;266:157–162. doi:10.1098/rspb.1999.0616 [Google Scholar]
- Chaurand T, Weimerskirch H. Incubation routine, body-mass regulation and egg neglect in the blue petrel Halobaena caerulea. Ibis. 1994;136:285–290. doi:10.1111/j.1474-919X.1994.tb01097.x [Google Scholar]
- Conway C.J, Martin T.E. Effects of ambient temperatures on avian incubation behavior. Behav. Ecol. 2000;11:178–188. doi:10.1093/beheco/11.2.178 [Google Scholar]
- Cresswell W, Holt S, Reid J.M, Whitfield D.P, Mellanby R.J. Do energetic demands constrain incubation scheduling in a biparental species? Behav. Ecol. 2003;14:97–102. doi:10.1093/beheco/14.1.97 [Google Scholar]
- Cresswell W, Holt S, Reid J.M, Whitfield D.P, Mellanby R.J, Norton D, Waldron S. The energetic costs of egg heating constrain incubation attendance but do not determine daily energy expenditure in the pectoral sandpiper. Behav. Ecol. 2004;15:498–507. doi:10.1093/beheco/arh042 [Google Scholar]
- de Heij M.E, van den Hout P.J, Tinbergen J.M. Fitness cost of incubation in great tits (Parus major) is related to clutch size. Proc. R. Soc. B. 2006;273:2353–2361. doi: 10.1098/rspb.2006.3584. doi:10.1098/rspb.2006.3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs R.C, Styrsky J.D, Thompson C.F. Clutch size and the costs of incubation in the house wren. Behav. Ecol. 2006;17:849–856. doi:10.1093/beheco/arl019 [Google Scholar]
- Engstrand S.M, Bryant D.M. A trade-off between clutch size and incubation efficiency in the barn swallow Hirundo rustica. Funct. Ecol. 2002;16:782–791. doi:10.1046/j.1365-2435.2002.00681.x [Google Scholar]
- Gorman H.E, Nager R.G. Prenatal developmental conditions have long-term effects on offspring fecundity. Proc. R. Soc. B. 2004;271:1923–1928. doi: 10.1098/rspb.2004.2799. doi:10.1098/rspb.2004.2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney V, Monaghan P. Optimal allocation of effort between reproductive phases: the trade-off between incubation costs and subsequent brood rearing capacity. Proc. R. Soc. B. 1996;263:1719–1724. doi:10.1098/rspb.1996.0251 [Google Scholar]
- Hepp G.R, Kennamer R.A, Johnson M.H. Maternal effects in wood ducks: incubation temperature influences incubation period and neonate phenotype. Funct. Ecol. 2006;20:307–314. doi:10.1111/j.1365-2435.2006.01108.x [Google Scholar]
- Kim S.Y, Monaghan P. Effects of early incubation constancy on embryonic development: an experimental study in the herring gull Larus argentatus. J. Therm. Biol. 2006;31:416–421. doi:10.1016/j.jtherbio.2006.02.002 [Google Scholar]
- Larsen V.A, Lislevand T, Byrkjedal I. Is clutch size limited by incubation ability in northern lapwings. J. Anim. Ecol. 2003;71:784–792. doi:10.1046/j.1365-2656.2003.00751.x [Google Scholar]
- Martin T.E, Auer S.K, Bassar R.D, Niklison A.M, Lloyd P. Geographic variation in avian incubation periods and parental influences on embryonic temperature. Evolution. 2007;61:2558–2569. doi: 10.1111/j.1558-5646.2007.00204.x. doi:10.1111/j.1558-5646.2007.00204.x [DOI] [PubMed] [Google Scholar]
- Naguib M, Gil D. Transgenerational effects on body size caused by early developmental stress in zebra finches. Biol. Lett. 2005;1:95–97. doi: 10.1098/rsbl.2004.0277. doi:10.1098/rsbl.2004.0277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson C.R, Vleck C.M, Vleck D. Periodic cooling of bird eggs reduces embryonic growth efficiency. Physiol. Biochem. Zool. 2006;79:927–936. doi: 10.1086/506003. doi:10.1086/506003 [DOI] [PubMed] [Google Scholar]
- Reid J.M, Monaghan P, Ruxton G.D. Resource allocation between reproductive phases: the importance of thermal conditions in determining the cost of incubation. Proc. R. Soc. B. 2000a;267:37–41. doi: 10.1098/rspb.2000.0963. doi:10.1098/rspb.2000.0963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid J.M, Monaghan P, Ruxton G.D. The consequences of clutch size for incubation conditions and hatching success in starlings. Funct. Ecol. 2000b;14:560–565. doi:10.1046/j.1365-2435.2000.t01-1-00446.x [Google Scholar]
- Reid J.M, Monaghan P, Nager R.G. Incubation and the costs of reproduction. In: Deeming D.C, editor. Avian incubation: behaviour, environment and evolution. Oxford University Press; Oxford, UK: 2002. pp. 314–325. [Google Scholar]
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information on methods