Abstract
The hormonal environment experienced during prenatal development may affect adult phenotype and behaviour. Digit lengths may provide an estimate of steroid levels encountered during embryonic development in humans and other vertebrates. Finger patterns in humans have been shown to reveal sexual orientation or cooperative behaviour. We explored individual breeding behaviour in a monogamous seabird, the Balearic shearwater Puffinus mauretanicus and unexpectedly detected some cooperative breeders. Furthermore, we show evidence of correlation between digit lengths and cooperative breeding in this species. Additionally, we suggest that the first digit could be a possible indicator of prenatal steroid levels. These results are the starting point for further tests of the hypothesis that first digit length is an indicator of prenatal hormone levels in other vertebrate species. Moreover, these results may offer practical use in wild populations to study the implications of the changes in prenatal environment for adult social behaviour.
Keywords: behaviour, bird, cooperative breeding, digit ratios, digit lengths, finger patterns
1. Introduction
Prenatal androgenic steroids influence future adult phenotype and may have important consequences on future adult behaviour in vertebrates (Phoenix et al. 1959; Ehrhardt & Meyer-Bahlburg 1981; Dufty et al. 2002; Groothuis et al. 2005, Komdeur 2007). Thus, changes in prenatal androgen levels trigger effects in: sexual orientation (Williams et al. 2000); sex-role identity (Csathó et al. 2003) and cooperative behaviour (Millet & Dewitte 2006) in humans; sexual behaviour in rats (Rhees et al. 1997); and rates of aggression and mounting behaviour in hyenas (Dloniak et al. 2006). Sex steroids have a primary effect on sexual differentiation and activation of sexual and agonistic behaviour in birds (Adkins 1978); however, little is known about how variation in steroid levels in the prenatal period affects avian reproductive behaviour (Rhen & Crews 2002).
The second and fourth digit length ratio (2 D : 4 D) is sexually dimorphic in humans (Williams et al. 2000) and may provide an estimate of the steroid levels experienced during prenatal development (Manning et al. 1998). The link between digit development and prenatal exposure to steroids may be enforced by the simultaneous effect which some homeobox genes have on the development of both fingers and the urogenital system (Kondo et al. 1997; Manning et al. 1998). These genes are highly conserved across vertebrates and similar relationships between digit ratios and sex steroids have been documented in rodents (Brown et al. 2002), primates (Roney et al. 2004) and birds (Burley & Foster 2004; Romano et al. 2005; Saino et al. 2007). However, in birds, inconsistent patterns of variation appear (Romano et al. 2005; Garamszegi et al. 2007; Dreiss et al. 2008). Additionally, most studies analysing vertebrate digit patterns only involved 2 D : 4 D finger length ratios, but other digit lengths or ratios may be informative (McFadden & Shubel 2002), especially in (understudied) non-human species.
Most seabird species are monogamous, and cooperative breeding, when individuals additional to the male–female pair provide parental care, is rare (Stacey & Koenig 1990), occurring only in ground-nesting species (e.g. skuas: Hemmings 1994; gulls: Fitch & Shugart 1983). To our knowledge, cooperative breeding has never been documented in a burrow-nesting seabird species. In this study, we explored whether individual breeding behaviour (monogamy versus cooperative breeding) in the burrow-nesting Balearic shearwater Puffinus mauretanicus was linked with digit lengths or other morphometrics on this species. Determining whether breeding behaviour is reflected in digit lengths is of relevance to the debate about the effects of prenatal androgenic steroids on future adult behaviour, and may offer practical use in wild populations to study the implications in adult social behaviour.
2. Material and methods
The Balearic shearwater is a long-lived, critically endangered, species that breeds colonially in the Balearic Islands (Mediterranean Sea; Oro et al. 2004; Genovart et al. 2007). During 2001–2004, we examined individuals attending nests in two colonies. We only considered as breeders those individuals either incubating or attending their chick in the nest, so the possibility of confounding residents with neighbours is neglected. Several body measurements were taken from breeders: head plus bill length; minimum bill depth; tarsus length; first digit length; middle-toe length (third digit); and wing and tail length (see Genovart et al. 2003). All measures were taken with Vernier callipers (×0.02 mm) except for wing and tail length which were measured with a ruler (×0.5 mm). From most captured birds, a small blood sample (approx. 25 μl) was taken and stored in ethanol. DNA was extracted using the phenol–chloroform method, following digestion with proteinase K (Sambrook et al. 1989), and birds sexed using the polymerase chain reaction to amplify two CHD genes (Fridolfsson & Ellegren 1999). Those individuals from whom we had no blood sample were sexed by unequivocal scoring in the discriminant function specifically developed (Genovart et al. 2003). As this is an endangered species, visits were limited and we cannot guarantee the record of all breeding adults. Thus, as the most conservative assumption, we considered cooperative breeders as those individuals sharing nest attendance duties with a same-sex individual or with more than one individual, and the remainder were considered monogamous birds. Some individuals were recorded in different years but were included only once in morphometric analyses. Morphometric characters of monogamous and cooperative breeders were compared applying a general linear model with sex and breeding behaviour as factors and also testing for their interaction. We check for normality for all tested traits and for homoscedacity in the tests.
3. Results
During the four consecutive breeding seasons, a total of 206 breeders (111 males and 95 females) from 162 different nests were measured and sexed. We captured more than one male (7 cases) and more than one female (2 cases) attending the same egg or chick among the 67 monitored active nests, thus getting evidence of cooperative breeding in 13.5 per cent of the breeding attempts.
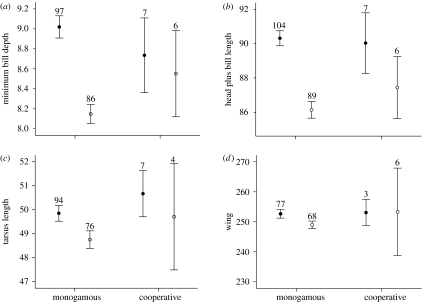
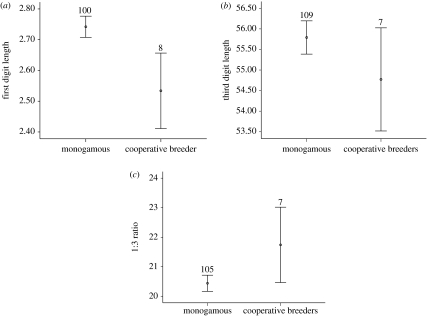
As in previous studies of this species (Genovart et al. 2003), minimum bill depth and head plus bill length were the most sexually dimorphic characters (F3,195=13.59, p<0.001; F3,205=27.37, p<0.001; figure 1). Tarsus length showed slight sexual dimorphism (F3,180=4.09, p<0.05; figure 1) but wing length did not show sexual dimorphism (F3,153=0.664, p>0.05; figure 1). Despite having a small sample, cooperative breeders seemed to show lower sexual dimorphism in characters (figure 1), especially in minimum bill depth, the most dimorphic character: this character was found to be different between sexes in monogamous birds, but not between cooperative breeders (F3,195=5.76, p<0.05). None of these morphometric characters differ significantly between cooperative breeders and monogamous birds (figure 1). When looking at digits, neither the first digit, the middle-toe, nor the 1 : 3 digit ratio showed sexual dimorphism (F3,104=0.036, p>0.05; F3,106=0.401, p>0.05; F3,100=0.108, p>0.05; figure 2). Third digit length did not differ between breeding groups (F3,106=0.967, p>0.05); however, despite having small sample sizes, cooperative breeders clearly differed from monogamous breeders in having shorter first digits (F3,104=7.72, p<0.01; figure 2) and greater 1/3 digit length ratios (F3,100=4.71, p<0.05; figure 2).
Figure 1.
Morphometric comparisons of (a) bill depth, (b) head plus bill depth, (c) tarsus length and (d) wing, between males (solid circles) and females (open circles) and between monogamous birds and cooperative breeders. Bars represent confidence intervals (95%) for each measure (in mm) and sample sizes are shown above bars.
Figure 2.
Confidence intervals (95%) for (a) first digit length, (b) third digit length and (c) 1 : 3 digit ratios, in monogamous and cooperative breeders. Sample sizes are shown above bars.
4. Discussion
Any character that provides an index of prenatal steroid levels could help us tease out the hormonal determinants of variation in social behaviour; for instance, cooperative breeding is one of the poorest known breeding systems in birds and examining the endocrine pathways could shed light on the occurrence of such behaviour. Several studies evidenced the link between hormonal environment experienced during prenatal development, digit lengths and adult behaviour in humans (Williams et al. 2000; Millet & Dewitte 2006). Interestingly, though our study lacks hormonal data, our results suggest that digit lengths may be related to breeding behaviour in birds, probably reflecting the hormonal environment experienced during their prenatal period. Our results also suggest that not only the 2 : 4 digit ratio but also other digit ratios or lengths may serve as indicators of hormone levels during early development. These results encourage further research, either on the hypothesis that first digit length is an indicator of prenatal hormone levels, as well as on the underlying endocrine processes related to certain social behaviour.
Previous studies have shown that variations in prenatal hormonal environment may play a role in body size differences between sexes on rats (Slob & Van Derr Werff Ten Bosch 1975; Cikos et al. 1992), and von Engelhardt et al. (2006) showed that variation in maternal testosterone in birds enhanced growth of daughters and reduced growth of sons. In agreement with both studies, we show that most dimorphic characters, such as minimum bill depth (Genovart et al. 2003), are much less dimorphic in cooperative breeders than in monogamous birds, and also indicate that individuals showing cooperative breeding could have been subject to different prenatal hormone environment than monogamous birds.
In conclusion, this study could be the starting point for further tests on the hypothesis that first digit length is an indicator of prenatal hormone levels in this and other vertebrate species. Moreover, our results suggest that analysis of androgen-sensitive digit lengths and their correlates in wild populations of birds may enable us to trace important prenatal influences on individual social behaviour.
Acknowledgments
The relevant legal and ethical approval was given for this research.
We are very grateful to V. Rodríguez and M. Bauzà who helped with the laboratory work, to D. Bowler, M. McMinn, R. Fernández and I. Afán for helping with the fieldwork and B. Morales for their logistic support. We warmly thank H. Drummond, R. Pradel, A. Martínez-Abraín, two anonymous referees and the corresponding editor for providing helpful comments on an earlier version of the manuscript. Permits were provided by the Consellería de Medio ambiente del Govern Balear. M.G. was funded by an I3P postdoctoral fellowship from the Spanish Ministry of Education and Science and M.L. by a predoctoral research fellowship of the Balearic Government. This work received partial financial support from the project 024B/2002 from the Spanish Ministry of the Environment.
Footnotes
This work is dedicated to Dr Xavier Ruiz, who died suddenly in April 2008.
References
- Adkins E.K. Sex steroids and the differentiation of avian reproductive behavior. Am. Zool. 1978;18:501–509. [Google Scholar]
- Brown W.M, Fin C.J, Breedlove S.M. Sexual dimorphism in digit-length ratios of laboratory mice. Anat. Rec. 2002;267:231–234. doi: 10.1002/ar.10108. doi:10.1002/ar.10108 [DOI] [PubMed] [Google Scholar]
- Burley N.T, Foster V.S. Digit ratio varies with sex, egg order and strength of mate preference in zebra finches. Proc. R. Soc. B. 2004;271:239–244. doi: 10.1098/rspb.2003.2562. doi:10.1098/rspb.2003.2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikos S, Kuchar S, Koppel J. The effect of administration of estradiol and testosterone on body growth of young male rats. Physiol. Res. 1992;41:387–392. [PubMed] [Google Scholar]
- Csathó A, Osváth A, Bicsák E, Karádi K, Manning J, Kállai J. Sex role identity related to the ratio of second to fourth digit length in women. Biol. Psychol. 2003;62:147–156. doi: 10.1016/s0301-0511(02)00127-8. doi:10.1016/S0301-0511(02)00127-8 [DOI] [PubMed] [Google Scholar]
- Dloniak S.M, French J.A, Holekamp K.E. Rank-related maternal effects of androgens on behavior in wild spotted hyaenas. Nature. 2006;440:1190–1193. doi: 10.1038/nature04540. doi:10.1038/nature04540 [DOI] [PubMed] [Google Scholar]
- Dreiss A.N, Navarro C, de Lope F, Møller A.P. Digit ratios, secondary sexual characters and condition in barn swallows Hirundo rustica. Behav. Ecol. 2008;19:16–21. doi:10.1093/beheco/arm095 [Google Scholar]
- Dufty A.M, Clobert J, Møller A.P. Hormones, development plasticity and adaptation. Trends Ecol. Evol. 2002;17:190–196. doi:10.1016/S0169-5347(02)02498-9 [Google Scholar]
- Ehrhardt A.A, Meyer-Bahlburg H.F.L. Effects of pre-natal sex hormones on gender-related behaviour. Science. 1981;211:1312–1318. doi: 10.1126/science.7209510. doi:10.1126/science.7209510 [DOI] [PubMed] [Google Scholar]
- Fitch M.A, Shugart G.W. Comparative biology and behaviour of monogamous pairs and one male–two female trios of herring gulls. Behav. Ecol. Sociobiol. 1983;14:1–7. doi:10.1007/BF00366649 [Google Scholar]
- Fridolfsson A.-K, Ellegren H. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 1999;30:116–121. doi:10.2307/3677252 [Google Scholar]
- Garamszegi L.Z, Hegy G, Szollosi E, Rosivall B, Torok J, Eens M, Moller A.P. Phenotypic correlates of digit ratio in a wild bird: implications for the study of maternal effects. Anim. Behav. 2007;74:641–647. doi:10.1016/j.anbehav.2006.11.023 [Google Scholar]
- Genovart M, McMinn M, Bowler D.A. Discriminant function for predicting sex in the Balearic shearwater. Waterbirds. 2003;26:72–76. doi:10.1675/1524-4695(2003)026[0072:ADFFPS]2.0.CO;2 [Google Scholar]
- Genovart M, Oro D, Juste J, Bertorelle G. What genetics tell us about the conservation of the critically endangered Balearic shearwater? Biol. Conserv. 2007;137:283–293. doi:10.1016/j.biocon.2007.02.016 [Google Scholar]
- Groothuis T.G, Muller W, von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 2005;29:239–352. doi: 10.1016/j.neubiorev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Hemmings A.D. Cooperative breeding in the skuas (Stercorariidae): history, distribution and incidence. J. R. Soc. New Zeal. 1994;24:245–260. [Google Scholar]
- Komdeur J. Constraints on evolutionary shifts in cooperative breeding. Behav. Process. 2007;76:75–77. doi: 10.1016/j.beproc.2007.01.012. doi:10.1016/j.beproc.2007.01.012 [DOI] [PubMed] [Google Scholar]
- Kondo T, Zakany J, Innis J.W, Duboule D. Of fingers, toes and penises. Nature. 1997;390:29. doi: 10.1038/36234. doi:10.1038/36234 [DOI] [PubMed] [Google Scholar]
- Manning J.T, Scott D, Wilson J, Lewis-Jones D.I. The ratio of 2nd and 4th digit length: a predictor of sperm numbers and concentration of testosterone, leutinizing hormone and oestrogen. Hum. Reprod. 1998;1311:3000–3004. doi: 10.1093/humrep/13.11.3000. doi:10.1093/humrep/13.11.3000 [DOI] [PubMed] [Google Scholar]
- McFadden D, Shubel E. Relative lengths of fingers and toes in human males and females. Horm. Behav. 2002;42:492–500. doi: 10.1006/hbeh.2002.1833. doi:10.1006/hbeh.2002.1833 [DOI] [PubMed] [Google Scholar]
- Millet K, Dewitte S. Second to fourth digit ratio and cooperative behaviour. Biol. Psychol. 2006;71:111–115. doi: 10.1016/j.biopsycho.2005.06.001. doi:10.1016/j.biopsycho.2005.06.001 [DOI] [PubMed] [Google Scholar]
- Oro D, Aguilar J.S, Igual J.M, Louzao M. Modelling demography and extinction risk in the endangered Balearic shearwater. Biol. Conserv. 2004;116:93–102. doi:10.1016/S0006-3207(03)00180-0 [Google Scholar]
- Phoenix C.H, Goy R.W, Gerall A.A, Young W.C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Rhees R.W, Kirk B.A, Sephton S, Lephart E.D. Effects of prenatal testosterone on sexual behavior. Reproductive morphology and LH secretion in the female rat. Dev. Neurosci. 1997;19:430–437. doi: 10.1159/000111240. doi:10.1159/000111240 [DOI] [PubMed] [Google Scholar]
- Rhen T, Crews D. Variation in reproductive behaviour within a sex, neural systems and endocrine activation. J. Neuroendocrinol. 2002;14:517–531. doi: 10.1046/j.1365-2826.2002.00820.x. doi:10.1046/j.1365-2826.2002.00820.x [DOI] [PubMed] [Google Scholar]
- Romano M, Rubolini D, Martinelli R, Alquati A.B, Saino N. Experimental manipulation of yolk testosterone affects digit lengths ratios in the ring-necked pheasant (Phasianus colchicus) Horm. Behav. 2005;48:342–346. doi: 10.1016/j.yhbeh.2005.03.007. doi:10.1016/j.yhbeh.2005.03.007 [DOI] [PubMed] [Google Scholar]
- Roney J.R, Whitham J.C, Leoni M, Bellem A, Wielebnowski N, Maestripieri D. Relative digit lengths and testosterone levels in Guinea baboons. Horm. Behav. 2004;45:285–290. doi: 10.1016/j.yhbeh.2003.12.008. doi:10.1016/j.yhbeh.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Saino N, Rubolioni D, Romano N, Boncoraglio G. Increased egg estradiol concentration feminizes digit ratios of male pheasants (Phasianus colchicus) Naturwissenschaften. 2007;94:207–212. doi: 10.1007/s00114-006-0188-9. doi:10.1007/s00114-006-0188-9 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch E.F, Maniatis T. Cold Spring Harbor Laboratory Press; New York, NY: 1989. Molecular cloning: a laboratory manual. [Google Scholar]
- Slob A.K, Van Derr Werff Ten Bosch J.J. Sex differences in body growth in the rat. Physiol. Behav. 1975;14:353–361. doi: 10.1016/0031-9384(75)90044-x. doi:10.1016/0031-9384(75)90044-X [DOI] [PubMed] [Google Scholar]
- Stacey P.B, Koenig D. Cambridge University Press; Cambridge, UK: 1990. Cooperative breeding in birds. Long-term studies of ecology and behaviour. [Google Scholar]
- von Engelhardt N, Carere C, Dijkstra C, Groothuis T.G.G. Sex-specific effects of yolk testosterone on survival, begging and growth of zebra finches. Proc. R. Soc. B. 2006;273:65–70. doi: 10.1098/rspb.2005.3274. doi:10.1098/rspb.2005.3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T.J, Pepitone E.M, Christensen S.E, Cooke B.M, Huberman A.D, Breedlove N.J, Breedlove T.J, Jordan C.L, Breedlove S.M. Finger-length ratios and sexual orientation. Nature. 2000;404:455–456. doi: 10.1038/35006555. doi:10.1038/35006555 [DOI] [PubMed] [Google Scholar]