Abstract
Parasites seldom have predators but often fall victim to those of their hosts. How parasites respond to host predation can have important consequences for both hosts and parasites, though empirical investigations are rare. The exposure of wild juvenile salmon to sea lice (Lepeophtheirus salmonis) from salmon farms allowed us to study a novel ecological interaction: the response of sea lice to predation on their juvenile pink and chum salmon hosts by two salmonid predators—coho smolts and cut-throat trout. In approximately 70% of trials in which a predator consumed a parasitized prey, lice escaped predation by swimming or moving directly onto the predator. This trophic transmission is strongly male biased, probably because behaviour and morphology constrain female movement and transmission. These findings highlight the potential for sea lice to be transmitted up marine food webs in areas of intensive salmon aquaculture, with implications for louse population dynamics and predatory salmonid health.
Keywords: Pacific salmon, ectoparasite, host–parasite, Oncorhynchus kisutch, Oncorhynchus clarki
1. Introduction
Parasites are prisoners of their own habitat, and while few parasites have their own predators, they often fall victim to those of their hosts. Host predation can shape parasite life history by driving selection for (i) early maturity (Poulin 2007) ensuring that a parasite reproduces before its host is predated upon, (ii) manipulation of host behaviour to reduce the probability a host encounters a predator (Moore 2002) and (iii) parasitizing the predator itself, thus incorporating it into the parasite's life cycle (Parker et al. 2003). These adaptive responses can then have important consequences for hosts by influencing parasite virulence (Ebert & Herre 1996), behaviour (Moore 2002) and distribution (Poulin 2007).
Empirical investigations into how parasites respond to host predation and the consequences of these are rare (but see Ponton et al. 2006). This is probably in part due to the logistical constraints of manipulating host, parasite and predator under experimental conditions. Opportunities to study evolutionary processes are often created by anthropogenic change to ecological interactions (Palumbi 2001). The exposure of wild juvenile salmon to parasitic sea lice from salmon farms permits the study of a novel ecological interaction: that between parasitism and predation among salmonids and the sea louse (Lepeophtheirus salmonis), a marine ectoparasite with a direct life cycle (Pike & Wadsworth 1999).
Sea lice are ubiquitous on farmed and wild adult salmon throughout the Northern Hemisphere and have a life cycle consisting of non-infectious and infectious free-living stages, attached stages and motile stages (Johnson & Albright 1991). Transmission occurs primarily when the infective stage seeks out and attaches to a host fish, although motiles can move directly among hosts (Hull et al. 1998). In areas without salmon farms, sea lice are less than 5% prevalent on juvenile pink (Oncorhynchus gorbuscha) and chum (Oncorhynchus keta) salmon (Morton et al. 2004; Krkošek et al. 2007b), but in areas with salmon farms they can be orders of magnitude more abundant (Morton et al. 2004; Krkošek et al. 2006).
During early marine life, juvenile pink and chum salmon experience high rates of predation from other, larger salmonids, including coho (Oncorhynchus kisutch) salmon smolts and anadromous cut-throat trout (Oncorhynchus clarki), both of which are also sea louse hosts. Because infection with sea lice in unperturbed systems is rare until after three to four months of marine life (Krkošek et al. 2007b), when predator–prey interactions between salmonids have ceased (Groot & Margolis 1991), sea louse exposure to juvenile salmon caused by salmon farms allowed us to study the response of sea lice to host predation by juvenile coho salmon and cut-throat trout. We report the results of experiments, designed to investigate how louse infection affects predation risk, that reveal an extraordinary behaviour of a parasite. Sea lice respond to predation on their host by moving or swimming from prey to predator during predation.
2. Material and methods
We collected fishes used in the experiments from marine waters of the Broughton Archipelago, British Columbia, Canada, during a period of sea lice infestations of wild juvenile Pacific salmon (Krkošek et al. 2006, 2007a). We used beach seines, and hook and line to collect juvenile pink and chum salmon (mean±s.d.; 68.9 mm FL) and two of their primary marine predators, coho salmon smolts (120±11 mm FL; May 2005 and 2006) and cut-throat trout (226±29 mm FL; May 2006). All fishes were non-lethally examined for motile lice as described in Krkošek et al. (2005) and housed in floating pens for 24–48 hours prior to experimentation.
In individual predation experiments, we paired size-matched pink salmon (one unparasitized and one parasitized with up to three motile lice) and left them undisturbed in a 10 l aquarium for approximately 15 min before releasing them into a 1.5×1.5×1 m ocean enclosure with a single unparasitized cut-throat trout (n=60). Trials ended once one prey had been consumed. We then coaxed the trout into a 10 l aquarium where its entire surface was visually assessed for motile sea lice, which we removed and identified to stage and sex according to Johnson & Albright (1991). To further estimate the rates of trophic transmission, we repeated this experiment with single parasitized juvenile pink salmon (n=30).
We also conducted group predation experiments in which we exposed approximately 200 parasitized juvenile pink or chum salmon (mean±95% bootstrap CI: 2±0.33 motile sea lice fish−1) to 40–50 coho salmon smolts (n=8; 0.49±0.1 motile sea lice fish−1) or 50–100 juvenile pink or chum salmon as a control (n=4; 0.57±0.04 motile sea lice fish−1). Juvenile pink and chum salmon occupy the same ecological niche (Groot & Margolis 1991) and were considered ecologically interchangeable in this study. We conducted the experiments in a 3×4×4 m marine net pen that was divided into half for approximately 2 hours to separate prey and predators while the fishes acclimatized to their new environment. The divider was removed and coho were allowed to feed on prey for 24–48 hours. We examined predators and prey for lice before and after each trial as described above.
Chi-squared tests were used to test for differences in the proportion of adult male and female sea lice trophically transmitted in individual predation experiments and generalized linear mixed-effects models (GLMM) to test for the differences in the number of male and female sea lice on predators before and after predation on parasitized prey in the group predation experiments. The GLMM used a Poisson distribution for the dependent variable (sea louse abundance) and included before versus after each trial as a fixed effect, the abundance of motile sea lice on prey before each trial as a covariate and replicate as a random effect. Differences in trophic transmission between sexes were evaluated by including a before versus after predation×sex interaction term. All analyses were performed in R v. 2.3.1.
3. Results
In individual predation experiments, trophic transmission occurred in 70% of trials (52 out of 74) when cut-throat trout consumed parasitized juvenile pink salmon. No sea lice were found on predators in trials when an unparasitized fish was consumed and the parasitized fish was not (n=16). This suggests that the trophic transmission of motile sea lice resulted solely from predation upon parasitized prey and not passively when prey and predator were in proximity. Adult male lice transferred 3.8 times more often than adult female lice (Χ2-test: Χ2=49.6, d.f.=1, p<0.0001; figure 1). In instances when it could be directly observed (n=17), sea lice responded to host predation by swimming from the host fishes to the predator's dorsal or lateral surface, or by moving directly from the captured prey onto the roof of the predator's mouth and then head.
Figure 1.
The proportion of the available adult male and female L. salmonis trophically transmitted from the infected juvenile pink salmon to cut-throat trout in individual predation experiments (n=74).
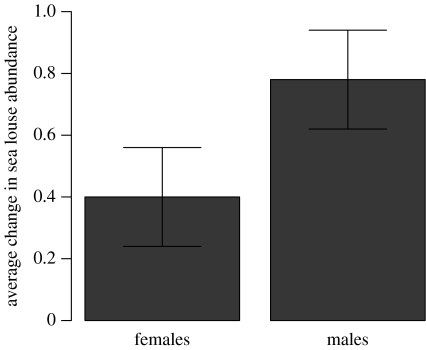
A similar pattern emerged in the group predation experiments. Predation upon the parasitized juvenile pink and chum salmon resulted in a significant increase in motile sea louse abundance on predators after 24–36 hour (Z=2.613, p<0.01), with males transferring significantly more often than females (before versus after predation×sex interaction, Z=2.006, p<0.05; figure 2). Motile sea louse abundance on control fish did not increase after 24–36 hour (Z=−0.9295, p>0.05).
Figure 2.
The average change in male and female L. salmonis abundance (±1 s.e.) on coho salmon smolts after predation upon groups of parasitized pink and chum salmon (n=8).
4. Discussion
Our results demonstrate that sea lice can escape predation on their hosts by moving from prey to predator. This trophic transmission was strongly male biased, suggesting that females do not exhibit this behaviour frequently. Male-biased dispersal in free-living species is correlated with polygynous mating systems, where male reproductive success is limited by mating opportunities and female reproductive success is limited by investment in offspring (Clobert et al. 2001). Sea lice are polygynous and males provide no investment in offspring beyond sperm, whereas females invest both time and energy into producing offspring. As a result, male fitness is dependent on the total number of successful matings, whereas female fitness is dependent on the available energy reserves and nutrients for egg production. This has probably selected for sex-specific behavioural strategies where females remain on a host to sequester resources and males move among hosts to increase the mate encounters. Experimental work supports these predictions (Hull et al. 1998). Morphological differences between sexes parallel the differences in dispersal: females are larger and have expanded genital segments (Johnson & Albright 1991). These differences in behaviour and morphology may restrict motile female movement among hosts, thereby constraining their ability to escape predation on their hosts.
There are few empirical examples of parasites escaping host predation. Ponton et al. (2006) demonstrated that parasitic gordian worms escape host predation by wriggling out of their host's predator's mouth, gills or nose approximately 23% of the time their cricket host is predated upon. Ticks and fleas are also commonly observed leaving a dying host, presumably increasing the probability of ending up on a scavenger consuming their dying host. What is so extraordinary about the trophic transmission of sea lice is the frequency at which it occurs (e.g. approx. 70% of predation events in individual trials) and the fact that sea lice end up on a suitable host. Whether or not trophic transmission of sea lice is accompanied by an increase in fitness is contingent upon mating opportunities on their new host. These may be limited by increased male–male mate competition as a result of male-biased sea louse populations on the predatory salmonids with consequences for sea louse population dynamics.
The trophic transmission of sea lice may also affect the health of predatory salmonid populations sympatric with infested juvenile pink and chum salmon. Sea louse pathogenicity is intensity dependent (Pike & Wadsworth 1999) and because coho smolts and cut-throat trout are orders of magnitude larger than juvenile pink and chums during early marine residence (Groot & Margolis 1991), they are likely to be less negatively affected by the transmission of sea lice via free-living infectious stages from wild and farmed hosts than are their smaller prey. However, predation on infested juvenile pink and chum may result in the accumulation of motile lice, the stage most pathogenic to hosts (Pike & Wadsworth 1999), in numbers sufficient to compromise the health of predatory salmonid populations. These results therefore also have a conservation message: the addition of salmon farms to coastal waters where wild salmonids rear and interact may have important indirect health consequences for larger predatory salmonid hosts than just the direct transmission of parasite larvae. However, if lice make prey easier for predators to capture, then the increased exposure to motile lice may be compensated for by an increased availability of prey. The capture and handling of prey by predators was similar in our small enclosures, larger net pens, and in annual field surveys in the region. These similarities in predator–prey behaviour across scales of observation suggest the trophic transmission of sea lice is prevalent among wild juvenile salmonids in areas of intensive salmon aquaculture.
Acknowledgments
This research was approved by Simon Fraser University's Animal Care Committee.
We thank Sara Henderson, Douglas Braun, Paul Mages, Caitlin Currey, Dane Stabel and Helen Ford for their hard work in the field. This work was supported by the Watershed Watch Salmon Society, the Sierra Club of British Columbia, the National Geographic Society, the David Suzuki Foundation, the Canadian Sablefish Association, the Pacific Salmon Forum, an NSERC Canada Industrial Postgraduate scholarship, an NSERC Canada Graduate Scholarship, NSERC Canada grant A6869 and individual donors: D. Bradshaw, B. and J. Hager, R. North and B. Wheeler.
References
- Clobert J, Danchin E, Dhondt A.A, Nichols J.D. Oxford University Press; Oxford, UK: 2001. Dispersal. [Google Scholar]
- Ebert D, Herre E.A. The evolution of parasitic diseases. Parasitol. Today. 1996;12:96–101. doi: 10.1016/0169-4758(96)80668-5. doi:10.1016/0169-4758(96)80668-5 [DOI] [PubMed] [Google Scholar]
- Groot C, Margolis L. UBC Press; Vancouver, BC: 1991. Pacific salmon life histories. [Google Scholar]
- Hull M.Q, Pike A.W, Mordue A.J, Rae G.H. Patterns of pair formation and mating in an ectoparasitic caligid copepod Lepeophtheirus salmonis (Krøyer 1837): implications for its sensory and mating biology. Phil. Trans. R. Soc. B. 1998;353:753–764. doi:10.1098/rstb.1998.0241 [Google Scholar]
- Johnson S.C, Albright L.J. The developmental stages of Lepeophtheirus–Salmonis (Kroyer, 1837) (Copepoda, Caligidae) Can. J. Zool. 1991;69:929–950. [Google Scholar]
- Krkošek M, Morton A, Volpe J.P. Nonlethal assessment of juvenile pink and chum salmon for parasitic sea lice infections and fish health. Trans. Am. Fish. Soc. 2005;134:711–716. doi:10.1577/T04-133.1 [Google Scholar]
- Krkošek M, Lewis M.A, Morton A, Frazer L.N, Volpe J.P. Epizootics of wild fish induced by farm fish. Proc. Natl Acad. Sci. USA. 2006;103:15 506–15 510. doi: 10.1073/pnas.0603525103. doi:10.1073/pnas.0603525103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krkošek M, Ford J.S, Morton A, Myers R.A, Lewis M.A. Declining wild salmon populations in relation to parasites from farmed salmon. Science. 2007a;318:1711–1713. doi: 10.1126/science.1148744. doi:10.1126/science.1148744 [DOI] [PubMed] [Google Scholar]
- Krkošek M, Gottesfeld A, Proctor B, Rolston D, Carr-Harris C, Lewis M.A. Effects of host migration, diversity, and aquaculture on disease threats to wild fish populations. Proc. R. Soc. B. 2007b;274:3141–3149. doi: 10.1098/rspb.2007.1122. doi:10.1098/rspb.2007.1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. Oxford University Press; Oxford, UK: 2002. Parasites and the behaviour of animals. [Google Scholar]
- Morton A, Routledge R, Peet C, Ladwig A. Sea lice (Lepeophtheirus salmonis) infection rates on juvenile pink (Oncorhynchus gorbuscha) and chum (Oncorhynchus keta) salmon in the nearshore marine environment of British Columbia, Canada. Can. J. Fish. Aquat. Sci. 2004;61:147–157. doi:10.1139/f04-016 [Google Scholar]
- Palumbi S.R. Humans as the world's greatest evolutionary force. Science. 2001;293:1786–1790. doi: 10.1126/science.293.5536.1786. doi:10.1126/science.293.5536.1786 [DOI] [PubMed] [Google Scholar]
- Parker G.A, Chubb J.C, Ball M.A, Roberts G.N. Evolution of complex life cycles in helminth parasites. Nature. 2003;425:480–484. doi: 10.1038/nature02012. doi:10.1038/nature02012 [DOI] [PubMed] [Google Scholar]
- Pike A.W, Wadsworth S.L. Sea lice on salmonids: their biology and control. Adv. Parasitol. 1999;44:233–337. doi: 10.1016/s0065-308x(08)60233-x. doi:10.1016/S0065-308X(08)60233-X [DOI] [PubMed] [Google Scholar]
- Ponton F, Lebarbenchon C, Lefevre T, Biron D.G, Duneau D, Hughes D.P, Thomas F. Parasite survives predation on its host. Nature. 2006;440:756. doi: 10.1038/440756a. doi:10.1038/440756a [DOI] [PubMed] [Google Scholar]
- Poulin R. Princeton University Press; Princeton, NJ: 2007. Evolutionary ecology of parasites. [Google Scholar]