Abstract
Male blue monkeys (Cercopithecus mitis stuhlmanni) of Budongo Forest, Uganda, produce two acoustically distinct alarm calls: hacks to crowned eagles (Stephanoaetus coronatus) and pyows to leopards (Panthera pardus) and a range of other disturbances. In playback experiments, males responded to leopard growls exclusively with a series of pyows and to eagle shrieks predominantly with hacks. Responses to playbacks of these alarm call series matched the responses to the corresponding predators, suggesting that the calls conveyed something about the nature of the threat. When responding to a series of hacks, indicating an eagle, males responded predominately with hacks, but produced significantly more calls if their group members were close to the playback stimulus than far away, regardless of their own position. When responding to a series of pyows, indicating a range of disturbances, males responded with pyows, but call rates were independent of the distance of other group members. The results suggest that males took into account the degree of danger experienced by other group members.
Keywords: audience effect, vocal behaviour, predation, Cercopithecus, alarm call, referential
1. Introduction
A number of animal species possess predator-specific alarm calls, and often these signals are used by listeners to draw inferences about the nature of the event experienced by the caller (e.g. guenons: Arnold & Zuberbühler 2006; suricates: Manser et al. 2002; sciurids: Blumstein 1999; birds: Gil & Sealy 2004). In Seyfarth et al.'s (1980) classic study, vervet monkeys (Cercopithecus aethiops) produced several acoustically distinct predator-specific alarm calls to which nearby listeners responded adaptively. Although such findings have been taken to argue that animal signals can function in rudimentary referential ways (Zuberbühler 2003), a current theory suggests that alarm call behaviour is largely inflexible, and not the product of signallers intending to inform their nearby audience about the nature of the threat (Cheney & Seyfarth 1990; Tomasello et al. 2005).
However, there are some challenges to the notion of socially unaware primate alarm callers. In a recent field experiment, Wich & de Vries (2006) demonstrated that male Thomas langurs (Presbytis thomasi) continued alarm calling to a tiger model until all adult group members had produced at least one alarm call, suggesting that male callers attend to their audience while producing alarm calls. Whether such behaviour is more widely spread and perhaps a general feature of primate cognition is currently unknown (Zuberbühler 2008).
Here, we describe the alarm calling behaviour of adult male blue monkeys (Cercopithecus mitis stuhlmanni) of Budongo Forest, Uganda. The blue monkeys are relatively common in East and South African forests and groups typically consist of one adult male and several adult females and their offspring. After reaching adulthood, males typically leave their natal group to try and take over another group to maintain tenure for a number of years, and multi-male influxes have been observed (Cords 1988). Males thus have a strong biological interest to protect the group from predation, and to keep out rival males, during this reproductively limited time. The male blue monkeys vigorously produce loud alarm calls to predators, best described as ‘hacks’ (or ‘ka-trains’) and ‘pyows’. Crowned eagles (Stephanoaetus coronatus) pose a severe threat to the monkeys of Budongo Forest, but leopards (Panthera pardus) have become exceedingly rare and many monkeys may have no experience with this predator.
To investigate whether males took into account the degree of threat experienced by other group members, we played back a series of alarm calls recorded from males that responded to eagles and leopards. As an indicator of threat, we measured the distance of both the caller and the nearest female to the speaker during each trial.
2. Material and methods
(a) Data collection
Playbacks were conducted on unhabituated blue monkeys between March and December 2006 in a 16 km2 study area in the Budongo Forest Reserve (1°35′–1°55′ N, 31°18′–31°42′ E), surrounding the Budongo Conservation Field Station in western Uganda. The groups were recruited from formerly logged forest where the primate density was particularly high.
The blue monkeys were systematically searched throughout the study area by sight or by hearing female vocalizations. We identified at least 34 different groups within the study area. Although we were unable to map out the exact home ranges of all groups, it was possible to assign a core area to each group, at least 200 m apart from each other. To avoid multiple testing with the same stimulus, we drew an imaginary 200 m radius circle around the location of each experiment and excluded all groups within this area from further testing. The groups within 300 m of each other were not tested on the same day. None of the groups were habituated to the presence of humans. If the group produced alarm calls prior to a playback they were not tested.
Once a group was located, the experimenter and the field assistant positioned themselves close by, but out of sight. The playback equipment, an Audix powered speaker connected to a portable CD player, was positioned 0–2 m from the ground and directed towards the monkeys. The field assistant then moved to a location where it was possible to observe the monkeys' behaviour through an opening in the canopy. All playback stimuli were preceded by 5 min of silence, allowing the experimenter to move away from the equipment and observe the behaviour prior to playback and ensure that monkeys were unaware of the experimenter, the field assistant and the equipment. Recording of calls continued for at least 5 min after a stimulus was played, or 5 min after the focal male had stopped giving alarm calls. Trials were excluded from the analyses if the monkeys detected the experimenter, the field assistant or the equipment at any time before the end of the trial.
Predator playback stimuli were 15 s natural calling series, either purchased from the National Sound Archive, London, UK (leopard growls, n=3) or recorded by K.Z. in the Taï National Park, Ivory Coast (eagle calls, n=6, which are identical to those heard in the study area). The pyow and hack series were natural series recorded from the blue monkeys at the field site (hacks, n=4, 23–37 calls in 14–15 s series; pyows, n=4, 4 calls in 18–30 s series).
Vocalizations were recorded using a Sony DAT TCD-D7 professional walkman (sampling rate 44.1 kHz) and a Sennheiser K6/ME66 directional microphone. The following contextual information was recorded: (i) the distance between the speaker and the male, (ii) the distance between the speaker and the closest female, (iii) the density of local vegetation (‘open’: few trees, vegetation only dense at ground level; ‘medium’: average number of trees at all levels of growth; ‘dense’: high numbers of trees at all levels), (iv) illumination (‘dark’: sky overcast, no shadows or twilight; ‘light’: shadows visible, sunlight and few clouds), and (v) the behaviour before playback (eating, travelling, resting and grooming). Males often foraged at some distance from the rest of the group so that the caller's own distance to the speaker could be different from those of the rest of the group members. Response rates were calculated using data collected by S.P., A.S.B. and J.B. Four additional trials, collected with identical methodology by A.M.S., were included for the analyses of call characteristics to increase sample size.
(b) Analyses
All recordings were transferred to an Acer Aspire 3690 laptop at 44.1 kHz using Cool Edit 2000. We described the overall calling response by measuring (i) the number and type of male calls given in response to the stimulus, (ii) the total duration of the call series, (iii) the latency to male calling, (iv) the call rate over the whole series, (v) the proportion of pyows in the whole series and the first 10 calls, and (vi) the number of female calls given before the male started calling.
3. Results
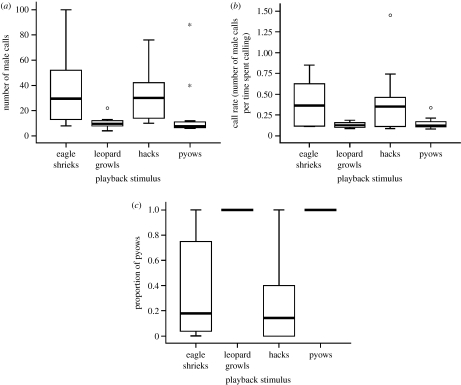
Males produced alarm calls in 7 (26%) out of 27 valid ‘leopard-growl’ trials and 9 (24%) out of 37 valid ‘eagle-shriek’ trials. Males produced significantly more calls and call rates were significantly higher, in response to eagle shrieks than to leopard growls, while the overall calling duration and latency to call did not differ between predators (figure 1; table 1). Males produced alarm calls in 12 (33%) out of 36 valid ‘pyow-series’ trials and in 14 (41%) out of 34 valid ‘hack-series’ trials. Considering only the males that responded with calls, the males produced significantly more calls in response to the hack than the pyow series, while call rates, overall calling duration and latency to call did not differ between the series types (figure 1; table 1). Vegetation density, illumination and prior behaviour did not affect the responses.
Figure 1.
Context-specific differences in calling behaviour in male blue monkeys (a) overall calling efforts, (b) relative overall call rates and (c) proportion of pyows in the calling bout.
Table 1.
Contrasts of general response characteristics to different playback stimuli. (Asterisks denote significant results from Mann–Whitney U-tests.)
| measure | result | stimuli | |
|---|---|---|---|
| eagle (n=10)a versus leopard (n=10)a | pyow (n=12) versus hack (n=14) | ||
| number of calls | U | 11 | 27.5 |
| Z | −2.958 | −2.914 | |
| pexact | 0.002** | 0.003** | |
| duration of male call series (s) | U | 29 | 60 |
| Z | −1.587 | −1.234 | |
| pexact | 0.123 | 0.231 | |
| call rate (number of male calls per call bout length) | U | 23 | 46 |
| Z | −2.041 | −1.955 | |
| pexact | 0.043* | 0.053 | |
| latency to the first male call (s) | U | 21 | 53 |
| Z | −1.443 | −1.594 | |
| pexact | 0.167 | 0.118 | |
| proportion of pyows in the entire call sequence | U | 10 | 12 |
| Z | −3.414 | −4.038 | |
| pexact | 0.001*** | <0.001*** | |
| proportion of pyows in the first 10 calls | U | 10 | 12 |
| Z | −3.472 | −4.172 | |
| pexact | 0.001*** | <0.001*** | |
Sample sizes of eagle and leopard responses were increased to 10 using additional recordings collected by A.M.S. under identical methodology.
All the male vocal responses to playbacks of leopard growls consisted of only the pyow series (10 out of 10). Eagle shrieks elicited only the hack series in 6 out of the 10 males, and two males started with the hack series and later switched to pyows. Two further males produced pyows from the start and never any hacks. A significantly higher proportion of pyows was given in response to leopard growls than to eagle shrieks, both for the entire response and during the first 10 calls only (table 1; figure 1c). To the pyow series, all calling males gave pyows, never hacks (12 out of 12). To the hack series, 12 out of 14 responding males gave hacks, although two later switched to pyows. Two further males produced pyows from the start, never any hacks. A significantly higher proportion of pyows was given in response to the pyow than the hack series for the entire call, and during the first 10 calls only (table 1; figure 1c).
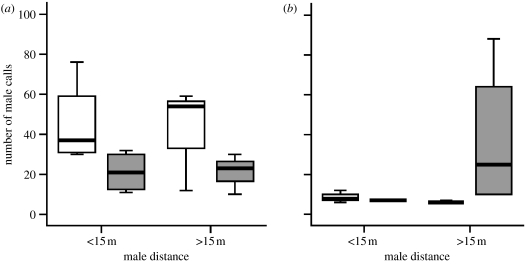
The analysis of covariance showed that the males' distance to the speaker did not affect their own call rates, both overall (F(1,19)=0.793, p=0.384) and within the stimulus types (F(1,19)=1.432, p=0.246). By contrast, although the females' distance did not influence male call rates overall (F(1,19)=0.088, p=0.770), there was a significant effect within the stimulus types (F1,19=6.351, p=0.021). Post hoc tests indicated that this effect was driven by response differences to the hack series, with males giving significantly more calls when females were close to the speaker (Mann–Whitney U-tests, medianclose=42.0 calls, medianfar=23.0; U=7, Z=-2.241, n1=n2=7, pexact=0.023; figure 2a). By contrast, male call rates in response to playbacks of the pyow series were not significantly affected by the females' distance (Mann–Whitney U-tests, medianclose=4.67 calls, medianfar=8.33; U=7, Z=−1.790, N1=N2=6, pexact=0.087; figure 2b).
Figure 2.
Male calling effort as a function of females' distance to the speaker responses to (a) the hack series and (b) the pyow series. White boxes represent cases when the females were close (less than 15 m) and grey boxes when they were far from the stimulus (greater than 15 m).
4. Discussion
Predator-specific alarm calling appears to be a general feature of primate behaviour, and, in several species, there is empirical evidence that calls are meaningful to nearby listeners (Zuberbühler 2003). In the blue monkeys, male calling patterns differed depending on the type of danger encountered by the caller. Males responded to leopards with a homogeneous series of pyows, whereas responses to eagles usually consisted of hack sequences. Leopards are probably no longer relevant as predators in our study area, although there have been some isolated reports of sightings. When a series of pyows or hacks were played back, the observed response patterns closely resembled those given to the corresponding predators, confirming that the calls conveyed relevant external information to the monkeys. In response to the eagle calls and the hack series, males often approached the speaker, while females usually moved down in the trees, whereas in response to the leopard growls and the pyow series no consistent patterns were noted. Under natural conditions, hacks are reliably given to eagles, while pyows are given to a range of disturbances, sometimes even in response to chimpanzees and humans, and regularly during conflicts with neighbouring groups, suggesting that listeners must rely on additional cues before being able to select an appropriate response.
An important finding in our study was that males responded differently to the hack series, depending on the distance of their females and offspring to the stimulus, and regardless of their own. We checked whether there were differences in the quality or quantity of female alarm calls, depending on their distance to the hack series but did not find anything. No effect of the number of female alarm calls was found, and we did not note any differences in the acoustic structure of these calls.
We concluded that, in the presence of a crowned eagle, the male blue monkeys were sensitive to the differences in the threat experienced by other group members, which affected their calling behaviour. In many species of primates, adult males play a special role during predation defence and are highly motivated to protect individuals that are crucial for their reproductive success (e.g. van Schaik & van Noordwijk 1989; Zuberbühler et al. 1999). Our results suggest that this important evolutionary pressure has had an impact on their vocal behaviour and underlying cognitive skills.
Acknowledgments
This research and its ethical implications was approved by the Uganda National Council for Science and Technology and the University of St Andrews.
We thank the National Forest Authority, Ugandan Wildlife Authority, Uganda National Council for Science and Technology and the President's Office for permission to conduct research in the Budongo Forest Reserve, and especially Alfred Afeku for helping with data collection. We are thankful to E. Bowman and S. Townsend for their statistical advice. This study was funded by the EU FP6 Pathfinder initiative ‘What it means to be human’ and the Leverhulme Trust. The Budongo Conservation Field Station receives core funding from the Royal Zoological Society of Scotland.
References
- Arnold K, Zuberbühler K. Semantic combinations in primate calls. Nature. 2006;441:303. doi: 10.1038/441303a. doi:10.1038/441303a [DOI] [PubMed] [Google Scholar]
- Blumstein D.T. Alarm calling in 3 species of marmot. Behaviour. 1999;136:731–757. doi:10.1163/156853999501540 [Google Scholar]
- Cheney D.L, Seyfarth R.M. Attending to behaviour versus attending to knowledge: examining monkey's attribution of mental states. Anim. Behav. 1990;40:742–753. doi:10.1016/S0003-3472(05)80703-1 [Google Scholar]
- Cords M. Mating systems of forest guenons: a preliminary review. In: Gautier-Hion A, Bourliere F, Gautier J.-P, Kingdon J, editors. A primate radiation: evolutionary biology of the African guenons. Cambridge University Press; Cambridge, UK: 1988. pp. 323–339. [Google Scholar]
- Gil S.A, Sealy S.G. Functional reference in an alarm signal given during nest defence: seet calls of yellow warblers denote brood-parasitic brown-headed cowbirds. Behav. Ecol. Sociobiol. 2004;56:71–80. doi:10.1007/s00265-003-0736-7 [Google Scholar]
- Manser M.B, Seyfarth R.M, Cheney D.L. Suricate alarm calls signal predator class and urgency. Trends. Cogn. Sci. 2002;6:55–57. doi: 10.1016/s1364-6613(00)01840-4. doi:10.1016/S1364-6613(00)01840-4 [DOI] [PubMed] [Google Scholar]
- Seyfarth R.M, Cheney D.L, Marler P. Vervet monkey alarm calls: semantic communication in a free-ranging primate. Anim. Behav. 1980;28:1070–1094. doi:10.1016/S0003-3472(80)80097-2 [Google Scholar]
- Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: the origins of cultural cognition. Behav. Brain. Sci. 2005;28:675–691. doi: 10.1017/S0140525X05000129. doi:10.1017/S0140525X05000129 [DOI] [PubMed] [Google Scholar]
- van Schaik C.P, van Noordwijk M.A. The special role of male cebus monkeys in predation avoidance and its effect on group composition. Behav. Ecol. Sociobiol. 1989;24:265–276. doi:10.1007/BF00290902 [Google Scholar]
- Wich S.A, de Vries H. Male monkeys remember which group members have given alarm calls. Proc. R. Soc. B. 2006;273:735–740. doi: 10.1098/rspb.2005.3320. doi:10.1098/rspb.2005.3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuberbühler K. Referential signalling in non-human primates: cognitive precursors and limitations for the evolution of language. Adv. Study Behav. 2003;33:265–307. doi:10.1016/S0065-3454(03)33006-2 [Google Scholar]
- Zuberbühler K. Audience effects. Curr. Biol. 2008;18:189–190. doi: 10.1016/j.cub.2007.12.041. doi:10.1016/j.cub.2007.12.041 [DOI] [PubMed] [Google Scholar]
- Zuberbühler K, Jenny D, Bshary R. The predator deterrence function of primate alarm calls. Ethology. 1999;105:477–490. [Google Scholar]