Abstract
Sexual conflict is ubiquitous across taxa. It often results in male harassment of females for mating opportunities that are costly for females, in some cases reducing reproductive success and increasing mortality. One strategy that females may employ to avoid sexual harassment is to segregate spatially from males. In fact, we do find sexual segregation in habitat use in species that have high levels of sexual conflict; however, the role of sexual harassment in driving such segregation remains poorly understood. Here, we demonstrate experimentally in a population of wild Trinidadian guppies Poecilia reticulata that male sexual harassment drives females into habitats that they otherwise do not prefer to occupy. In support of the social factors hypothesis for sexual segregation, which states that social factors such as harassment drive sexual segregation, this female behaviour leads to segregation of the sexes. In the presence of males, females actively select areas of high predation risk, but low male presence, and thus trade off increased predation risk against reduced sexual harassment.
Keywords: sexual segregation, sexual conflict, sexual harassment, Poecilia reticulata, predation risk, social factors hypothesis
1. Introduction
Often in nature the reproductive strategies of males and females are not aligned in that fitness gains for one sex may have negative fitness consequences for the other sex (Chapman et al. 2003). Divergence of interests in aspects related to reproduction is common among sexually reproducing species and results in conflict between the sexes (Hosken & Stockley 2005). As a result of sexual conflict, females often experience harassment from males, which can be costly to the point of reducing female condition and ultimately reproductive success (Chapman et al. 2003). In females, selection has resulted in a number of adaptations to reduce male harassment and associated costs. For example, female behavioural strategies have been documented to include associating with protective males (Silk 2007) and accepting suboptimal matings (Lee & Hays 2004). Another strategy that may be available to females is to segregate spatially from males. In fact, sexual harassment has been hypothesized to be one of the driving forces for sexual segregation under the social factors hypothesis (Bon & Campan 1996), but to our knowledge there is currently no experimental support for this hypothesis and the role of sexual harassment in driving sexual segregation remains unclear.
In this study, we examine male harassment of females as a factor promoting sexual segregation in habitat use through experimental manipulation of a wild population of Trinidadian guppies Poecilia reticulata. Levels of male harassment are high in this species (Magurran & Seghers 1994) and previous work has shown that in wild guppy populations inhabiting rivers with a high risk of predation by piscivorous fishes, the sexes segregate in the habitat such that areas of the river with the highest predation risk (deep water) have female-biased sex ratios and areas with the lowest predation risk (shallow water) are male biased (Croft et al. 2006). Guppies are highly sexually dimorphic with males being small and brightly coloured and females being large and cryptically coloured, making males more conspicuous to aquatic predators (Olendorf et al. 2006). Deep water in rivers with large piscivorous fishes is thus less optimal habitat for male than for female guppies due to this sexual dimorphism; however, females would still be at lower risk from aquatic predators in shallower water. We can thus ask the question: does the presence of males induce a female behavioural strategy that results in sexual segregation in habitat use, supporting the social factors hypothesis for sexual segregation? Here, we present the first empirical study to experimentally investigate the social factors hypothesis (sexual conflict) for sexual segregation in habitat use in a wild animal population by testing the hypothesis that females occupy areas of high predation risk to reduce encounters with males and thus trade off risk of predation against sexual harassment to mediate sexual conflict.
2. Material and methods
The study was carried out in May–June 2007 in the Turure River (10°40′20″ N, 61°09′60″ W) on the island of Trinidad. We manipulated the sex ratio that female guppies experienced in this high predation river by stocking enclosures in the river with either five large females and five males (mixed-sex experimental treatment, n=12) or five large females and five small females (same-sex control treatment, n=12). We chose a pool with a slanted bank where we could set up 100×140 cm bottomless enclosures constructed of plastic mesh (2×2 mm mesh size) buried into the substrate that allowed a throughflow of water (<1 m s−1) but constrained the fish within enclosures. This set-up left the fish exposed to naturally occurring predator cues and left the environment as natural as possible with the fish being able to forage on rocks before and during trials. Markers were placed on the bottom of each enclosure to mark four zones of increasing depth (total depth gradient: 0– 60 cm over a 1.4 m distance). Each zone had the dimension 35×100 cm in the horizontal plane.
A total of 240 fish (120 large females (29.9±0.2 mm), 60 males (21.2±0.2 mm) and 60 small females (22.1±0.2 mm)) were individually marked with implant elastomer (see Croft et al. 2003 for details) and tested in the enclosures in the mixed- or same-sex groups of 10 individuals across the study period. In the same-sex groups, small females were matched to the size of the males in the mixed-sex groups. After fish had been left to acclimatize in an enclosure for 90 min we consecutively recorded the behaviour and location of four focal individuals (two large females and two males in mixed-sex treatment and two large females and two small females in same-sex treatment) for a period of 10 min per individual in the 12 independent groups of fish per treatment (n=24 groups). Pilot trials demonstrated that 90 min was more than sufficient for the fish to resume normal behaviour (e.g. shoaling, foraging, male courtship displays). Location was noted as the focal individual's position in four zones and the mean values for individuals in a trial of the same test class (i.e. males, large females and small females) were used for further analysis to give one value for each class in each trial. All observations were carried out by a single observer and recorded onto a handheld computer using Fit System software (Held & Manser 2005). The 24 trials were balanced across four enclosures and across time of day so that an equal number of replicates for each treatment was carried out under a given set of conditions. We tested for differences in male and female space use, female space use in the presence and absence of males, and female movement on encountering a male using t-tests and a repeated-measures general linear model (for details see the electronic supplementary material).
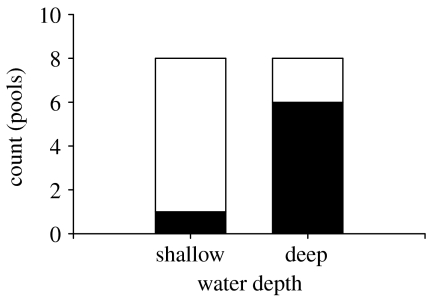
To examine how predation risk differed as a function of water depth for our study population and to confirm the generality of previous findings (Croft et al. 2006), we assessed predation risk to female guppies in shallow (23 cm) and deep (60 cm) areas in a sample of eight pools spanning the lower Turure River (including the pool where the study was carried out) using a standardized method (Croft et al. 2006). In each pool, we lowered single female guppies (28–31 mm in length) confined in a clear, colourless plastic container tethered to a monofilament line at the two depths to observe predator behaviour towards this prey stimulus. A single observer recorded all approaches (within one predator body length) and other behaviour of predatory fishes (Crenicichla sp. and Aequidens pulcher) from a distance of 3 m from the submerged container over a 10 min period. We tested for differences in predation risk in the two habitats with a Fisher's exact probability test by comparing the number of shallow and deep areas with predator visits.
3. Results
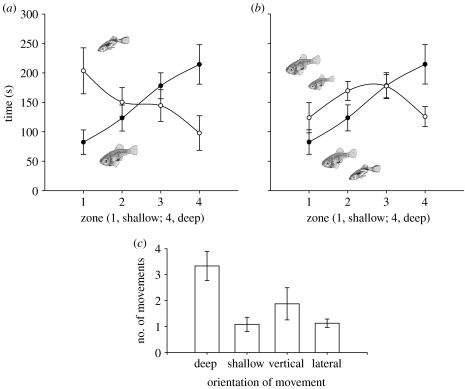
Males and females clearly used habitat differently in mixed-sex trials (t11=4.33, p=0.001, figure 1a) with males using the shallow zone more than females (t11=−4.19, corrected p=0.003) and females using the deep zone more than males (t11=3.27, corrected p=0.015). We found that this segregation was due to females altering space use in the presence of males (t22=2.37, p=0.0271, figure 1b), spending more time in deep water compared with the same-sex treatment. Moreover, our focal-individual follows showed that females preferentially sought out deeper water when encountering a male (F4,8=16.01, p=0.0007, figure 2). Finally, our assessment of predation risk in deep and shallow water confirmed that with increased use of deep water, females exposed themselves to a higher risk of predation by piscivorous fishes (Fisher's exact probability test, p=0.041, figure 2).
Figure 1.
Spline curves (mean and s.e.) showing differences in (a) male (open circles) and large female (filled circles) space use during mixed-sex trials and (b) large female space use during same-sex trials (open circles) and mixed-sex trials (filled circles). (c) Female movement when encountering males (mean and s.e., vertical and lateral: change in position in water column, but not in depth of water column; deep and shallow: change in depth of water column), showing female preference for movement into deep water.
Figure 2.
Proportion of pools where female test guppies were approached by predators (black boxes, predator approach; white boxes, no predator approach) in shallow and deep water illustrating higher predation risk in deep water.
4. Discussion
We show that although females in rivers that have a high risk of predation by aquatic predators should avoid deep water areas of pools, they only do so when males are not present. This behaviour indicates that in the presence of males, females use deep water, a habitat with a higher risk of predation, as a refuge from male harassment. As such, this is the first time that support for the social factors hypothesis for sexual segregation has been demonstrated experimentally.
Pinpointing the forces driving sexual segregation has been under investigation for sometime (Ruckstuhl & Neuhaus 2005). Currently, the three prevailing hypotheses for the occurrence of sexual segregation, which have largely been applied to ungulates, are the predation-risk, forage-selection and activity-budget hypotheses (Ruckstuhl & Neuhaus 2002). Examples include differences in susceptibility to predation that, in species where females are the more vulnerable sex, keeps them from using otherwise high-quality habitats (Jakimchuk et al. 1987), gender differences in habitat use stemming from sex differences in the ability to digest and absorb nutrients from the dominant vegetation (Beier 1987) and activity synchrony of same-sex individuals leading to segregation of the sexes within the same habitat (Conradt 1998). The social factors hypothesis seems to have been overlooked in much of the current literature (although see Perez-Barberia et al. 2005) even though there has previously been observational evidence that provides support for this hypothesis (for a summary see Croft et al. 2006).
The type of female control over levels of exposure to male harassment demonstrated here is particularly interesting since it is a behavioural strategy that is only available when there are habitat conditions that predominantly exclude males. This is emphasized in the Trinidadian guppy by the fact that we do not see sexual segregation in habitat use in river populations where large predators are absent in deeper waters (low-risk rivers; Croft et al. 2006). Female ability to actively segregate from males will reduce levels of male contact and harassment. The influence of this segregation on sex ratios experienced by both males and females will have implications for other behaviours, including female mate-choice and male mating strategies (e.g. Owens & Thompson 1994) and female–female interactions (e.g. Weckerly et al. 2001). These effects will be reflected in patterns of sexual selection and other evolutionary processes and represent exciting avenues of research that have relevance across taxa and disciplines.
Acknowledgments
All work was carried out in accordance with the ethical guidelines of the Fisheries Division of the Ministry of Agriculture, Land and Marine Resources, of Trinidad and Tobago.
We thank M. Edenbrow for assistance and discussion in the field. We would like to thank J. Kelley, J. Krause and R. Thorpe for their comments on an earlier version of this manuscript and I. Ramnarine for support in Trinidad. The study was funded by a National Environmental Research Council UK grant (NE/E001181/1) to D.P.C.
Supplementary Material
References
- Beier P. Sex differences in quality of white-tailed deer diets. J. Mammal. 1987;68:323–329. doi:10.2307/1381471 [Google Scholar]
- Bon R, Campan R. Unexplained sexual segregation in polygamous ungulates: a defense of an ontogenetic approach. Behav. Process. 1996;38:131–154. doi: 10.1016/s0376-6357(96)00029-0. doi:10.1016/S0376-6357(96)00029-0 [DOI] [PubMed] [Google Scholar]
- Chapman T, Arnqvist G, Bangham J, Rowe L. Sexual conflict. Trends Ecol. Evol. 2003;18:41–47. doi:10.1016/S0169-5347(02)00004-6 [Google Scholar]
- Conradt L. Could asynchrony in activity between the sexes cause intersexual social segregation in ruminants? Proc. R. Soc. B. 1998;265:1359–1363. doi: 10.1098/rspb.1998.0442. doi:10.1098/rspb.1998.0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft D.P, Albanese B, Arrowsmith B.J, Botham M, Webster M, Krause J. Sex biased movement in the guppy (Poecilia reticulata) Oecologia. 2003;137:62–68. doi: 10.1007/s00442-003-1268-6. doi:10.1007/s00442-003-1268-6 [DOI] [PubMed] [Google Scholar]
- Croft D.P, Morrell L.J, Wade A.S, Piyapong C, Ioannou C.C, Dyer J.R.G, Chapman B.B, Yan W, Krause J. Predation risk as a driving force for sexual segregation: a cross-population comparison. Am. Nat. 2006;167:867–878. doi: 10.1086/504853. doi:10.1086/504853 [DOI] [PubMed] [Google Scholar]
- Held J, Manser T. A PDA-based system for online recording and analysis of concurrent events in complex behavioral processes. Behav. Res. Methods. 2005;37:155–164. doi: 10.3758/bf03206410. [DOI] [PubMed] [Google Scholar]
- Hosken D.J, Stockley P. Sexual conflict. Curr. Biol. 2005;15:R535–R536. doi: 10.1016/j.cub.2005.07.014. doi:10.1016/j.cub.2005.07.014 [DOI] [PubMed] [Google Scholar]
- Jakimchuk R.D, Ferguson S.H, Sopuck L.G. Differential habitat use and sexual segregation in the Central Arctic caribou herd. Can. J. Zool. Rev. Can. Zool. 1987;65:534–541. doi:10.1139/z87-083 [Google Scholar]
- Lee P.L.M, Hays G.C. Polyandry in a marine turtle: females make the best of a bad job. Proc. Natl Acad. Sci. USA. 2004;101:6530–6535. doi: 10.1073/pnas.0307982101. doi:10.1073/pnas.0307982101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magurran A.E, Seghers B.H. Sexual conflict as a consequence of ecology: evidence from guppy, Poecilia reticulata, populations in Trinidad. Proc. R. Soc. B. 1994;255:31–36. doi:10.1098/rspb.1994.0005 [Google Scholar]
- Olendorf R, Rodd F.H, Punzalan D, Houde A.E, Hurt C, Reznick D.N, Hughes K.A. Frequency-dependent survival in natural guppy populations. Nature. 2006;441:633–636. doi: 10.1038/nature04646. doi:10.1038/nature04646 [DOI] [PubMed] [Google Scholar]
- Owens I.P.F, Thompson D.B.A. Sex differences, sex ratios and sex roles. Proc. R. Soc. B. 1994;258:93–99. doi: 10.1098/rspb.1994.0148. doi:10.1098/rspb.1994.0148 [DOI] [PubMed] [Google Scholar]
- Perez-Barberia F.J, Robertson E, Gordon I.J. Are social factors sufficient to explain sexual segregation in ungulates? Anim. Behav. 2005;69:827–834. doi:10.1016/j.anbehav.2004.06.011 [Google Scholar]
- Ruckstuhl K.E, Neuhaus P. Sexual segregation in ungulates: a comparative test of three hypotheses. Biol. Rev. 2002;77:77–96. doi: 10.1017/s1464793101005814. [DOI] [PubMed] [Google Scholar]
- Ruckstuhl K.E, Neuhaus P. Cambridge University Press; Cambridge, UK: 2005. Sexual segregation in vertebrates: ecology of the two sexes. [Google Scholar]
- Silk J.B. Social components of fitness in primate groups. Science. 2007;317:1347–1351. doi: 10.1126/science.1140734. doi:10.1126/science.1140734 [DOI] [PubMed] [Google Scholar]
- Weckerly F.W, Ricca M.A, Meyer K.P. Sexual segregation in Roosevelt elk: cropping rates and aggression in mixed-sex groups. J. Mammal. 2001;82:825–835. doi:10.1644/1545-1542(2001)082<0825:SSIREC>2.0.CO;2 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.