Abstract
Are parasites always harmful to their hosts? By definition, indeed, but in a few cases and particular environments, hosts experience higher fitness in the presence than in the absence of their parasites. Symbiotic associations form a continuum of interactions, from deleterious to beneficial effects on hosts. In this paper, we investigate the outcome of parasite infection of Arabidopsis thaliana by its natural pathogen Hyaloperonospora arabidopsis. This system exhibits a wide range of parasite impact on host fitness with, surprisingly, deleterious effects on high fecundity hosts and, at the opposite extreme, seemingly beneficial effects on the least fecund one. This phenomenon might result from varying levels of tolerance among host lines and even overcompensation for parasite damage analogous to what can be observed in plant–herbivore systems.
Keywords: host–parasite interaction, symbiosis, tolerance, evolved dependence
1. Introduction
Parasitism and mutualism are the two extreme types of interactions between associated species. Both imply the exploitation of resources of one organism by its interacting partner but they fundamentally differ in that mutualistic associations benefit both of them, whereas one partner gains at the expense of the other in parasitic ones. This distinction is not always clear, however, and numerous cases where mutualistic organisms occasionally cheat on their partner have been reported. Examples include well-known mutualisms such as mycorrhyzae, yucca moths and cleaner fishes (Bronstein 2001). At the other extreme, rare reports exist of notorious parasites having a positive effect on their host in particular ecological contexts, when the negative impact of parasite exploitation is compensated by an indirect advantage of parasite presence, like protection against heavy metal toxicity (Thomas et al. 2000). Symbiotic associations thus form a continuum of interactions, ranging from deleterious to beneficial (Bronstein 1994) and the outcome of a given association may change depending on the individual partners or external factors. Elucidating the evolution of interspecies associations along this continuum will require identifying these factors and determining whether they are available to selection.
Many studies investigating the fitness consequences of parasitic infections have found variation in virulence, i.e. reduction in host fitness, among parasite genotypes (e.g. Peever et al. 2000; Schulenburg & Ewbank 2004) and/or variation in tolerance among hosts (e.g. Peever et al. 2000; Kover & Schaal 2002). Such studies, however, usually focused on parasites with a large, and thus easily assessed, deleterious impact. On the other hand, mild to asymptomatic parasites represent an opportunity to investigate variation in infection effects from negative to positive. The model plant Arabidopsis thaliana, provides such a case, as recent experiments with different natural pathogens have revealed occasional cases where infection increase host fitness, albeit not significantly (Salvaudon et al. 2005; Goss & Bergelson 2007).
Here, we report the results of an experiment designed specifically to investigate whether host or parasite genotypes, or both, determine the fitness variation and advantage in A. thaliana when infected with its natural oomycete pathogen Hyaloperonospora arabidopsis, using several plant lines and parasite genotypes of different origins. The deleterious impact of parasite infection varied dramatically among plant genotypes, despite their similar susceptibility profile, from clear negative impacts to benevolence, and we confirm that this parasite can even increase reproductive success of its host.
2. Material and methods
(a) Material
The oomycete H. arabidopsis is a natural pathogen of A. thaliana (Brassicaceae), its specific host. This parasite is biotrophic and produces asexual conidiophores on leaf surfaces a few days after infection, as well as sexual oospores that remain within leaves until host death. This pathogen is seldom lethal for its host, but nonetheless constitutes a selective pressure on A. thaliana, as more than 20 resistance genes targeted against H. arabidopsis have been reported (Slusarenko & Schlaich 2003). Six inbred lines of A. thaliana and seven parasite strains constituting a complete matrix of successful infections were selected from our collections. We used parasite strains of three different types of origin. Three strains, Emco, Emwa and Noco, were ‘laboratory strains’ provided by the Sainsbury Laboratory (John Innes Center, Norwich, UK) from isolates collected more than 10 years ago and subsequently maintained as asexual cultures on specific A. thaliana lines (Holub et al. 1994). ‘Orsay strains’, Ors3 and Ors5, were collected in spring 2004 from conidiospores on infected host plants from one population in Orsay, France. The ‘Fribourg strains’, Fri3 and Fri5, were obtained from oospores of two infected plants sampled in spring 2004 in a population in Fribourg, Switzerland. All strains were maintained on susceptible hosts for several asexual generations and thus are probably genetically homogeneous. The six A. thaliana inbred lines were issued from seeds collected in wild populations across Europe (Finland (Fin), England (Gb), Pyrenees (Pyr), Sweden (Sue) and Czech Republic (Tch)) or from the ecotype Tsu (Japan). Only the combination Fin/Emco unexpectedly failed to produce any successful infection.
(b) Methods
We inoculated all six host lines with the seven parasite strains and a water control, with five replicates for each of the 48 combinations. The plants were grown, inoculated and maintained under controlled conditions following the protocol described in Salvaudon et al. (2007), which reports on parasite transmission from this experiment. The life cycle of all plants was completed under greenhouse conditions (natural photoperiod: 23°C day–15°C night). Plants were watered ad libitum until they began fructification, and then only when pots were completely dry to hasten fruit production. Seeds were harvested progressively as they matured in order to collect all seeds before fruits opened. We estimated host fitness as the total weight of all seeds produced per plant, this parameter representing the lifetime investment in reproduction. Plants that died before flowering had a seed production of zero.
(c) Statistical analyses
All statistical analyses were performed with JMP software version 5.1.2 (SAS institute, Cary, NC). As the selected host and parasite genotypes did not represent a random sample of their respective populations but rather a subsample of intercompatible genotypes, we chose to treat them as fixed factors. Our purpose was not then to assess the existing variation for virulence in natural populations but to test whether host (or parasite) genotypes that have a similar susceptibility (or infectivity) profile can differ for virulence. The impact of inoculation treatment on host fitness was thus analysed with a nested ANOVA testing for the effect of host line, treatment type (water-inoculated controls or inoculation with laboratory, Fribourg or Orsay strains), parasite strain nested within the treatment type and interactions between host line and each of the two other factors.
We also tested whether virulence was linked to the intrinsic fecundity of host lines. By analogy with the type of analysis used to investigate costs of tolerance in plant–herbivore interactions, we investigated the relationship between the average fitness of attacked plants and the fitness in control plants of the same family (Strauss & Agrawal 1999). But instead of looking for a negative relationship that would indicate a cost, we tested whether the slope of this relationship was significantly different from one (Student's t-test), as this value would be expected if virulence was completely unrelated to the intrinsic fecundity of host lines. This relationship was tested at all the levels of parasite treatment (mean of all pooled strains, parasite strains averaged over origins or each parasite strain separately), followed by Bonferroni correction of the critical α-value to account for multiple testing. Three plants were excluded from the analyses as there were doubts about their possible contamination or errors during inoculation.
3. Results
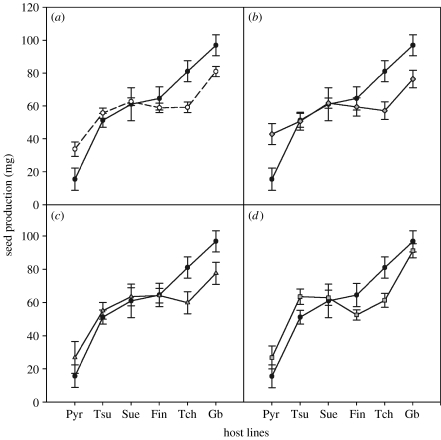
Of the 240 plants, six died before flowering. The weight of total seed production varied significantly among the six host lines (F5,189=31.007, p<0.0001) but not among the treatment types (controls, laboratory, Fribourg or Orsay) or parasite strains within the treatment types. However, there was a significant, albeit small, interaction between treatment type and host line (F15,189=1.733; p=0.0476), which was due to a difference in seed production between control and inoculated plants in some host lines: seed yield was lower in infected plants of Gb and Tch lines, unchanged in Fin, Sue and Tsu lines and higher in the Pyr line. This small interaction term was nonetheless robust to the type of analysis performed, and remained significant with a mixed model (Hocking formulation; Strauss & Agrawal 1999) incorporating only host lines as random factor, the type of treatment and their interaction (F15,213=1.758, p=0.0424). This interaction was due to significant differences between control and laboratory strains that were both positive (contrast on Pyr, T-ratio=2.56, p=0.011) and negative (Gb, T-ratio=−2.097, p=0.037; Tch, T-ratio=−2.51, p=0.025). Orsay and Fribourg strains showed little significance effect (figure 1). Furthermore, these changes in seed production covaried with the fecundity of control plants, as revealed by the relationship between the average fecundity of infected plants and controls of the corresponding line (table 1). This relationship was significantly lower than one both globally and for several parasite strains or origins taken separately. Negative impact of infection thus increased with host line fecundity.
Figure 1.
Seed production, in milligrams (±s.e.), averaged over control- and parasite-inoculated plants among host lines, ordered by seed production in the absence of pathogen. (a) Controls versus all parasite strains, (b) controls versus laboratory strains, (c) controls versus Fribourg strains and (d) controls versus Orsay strains. Filled circle, controls; open circle, all parasite-inoculated plants; diamonds, plants inoculated with laboratory strains (Emco, Emwa and Noco); triangles, Fribourg strains (Fri3 and Fri5); squares, Orsay strains (Ors3 and Ors5).
Table 1.
Slope of the relationship between average seed production of inoculated and control plants of the same host line. The Student t-test (5 d.f.) tests whether the slope equals one. Since we test the same hypothesis multiple times we mark in italics only those tests significant after Bonferroni correction of the critical α-value.
| slope coefficient | Student t | p-value | |
|---|---|---|---|
| mean of all inoculated plants | 0.511 | 5.242 | 0.0033 |
| mean by origin | |||
| Laboratory | 0.364 | 7.074 | 0.0009 |
| Fribourg | 0.571 | 4.074 | 0.0096 |
| Orsay | 0.671 | 2.022 | 0.0991 |
| mean by strain | |||
| Emco | 0.226 | 5.308 | 0.0032 |
| Emwa | 0.197 | 5.395 | 0.0030 |
| Noco | 0.669 | 4.057 | 0.0098 |
| Fri3 | 0.488 | 5.312 | 0.0032 |
| Fri5 | 0.655 | 2.571 | 0.0500 |
| Ors3 | 0.587 | 2.832 | 0.0366 |
| Ors5 | 0.755 | 1.175 | 0.2928 |
4. Discussion
We found that the negative impact of parasite infection on Arabidopsis hosts varied depending on host genotype and, to a lesser extent, on parasite genotype, even though genotypes with similar susceptibility profiles were selected. Furthermore, the impact of H. arabidopsis was linked to the intrinsic host fecundity, i.e. host fitness without parasite infection. Indeed, host fitness was more reduced in most fecund hosts, whereas intermediate seed producers suffered no significant deleterious effect and the poorest one even benefited from infection. Our parasite thus acted like Robin Hood, stealing fitness from the wealthiest hosts but benefiting the poorest one, though parasites are not expected to act so chivalrously! The intensity of this pattern varied among parasite strains, being the most striking for laboratory strains. These strains have been maintained through repeated asexual cycles under laboratory conditions, which might have released them from constraints on their virulence (Ebert 1998) and led to an amplified response of infected hosts compared with wild strains.
Because the different host responses did not covary with parasite asexual spore production (Salvaudon et al. 2007) they cannot be explained by different levels of resistance that reduces parasite infectivity or growth. But they could reflect variation in tolerance that reduces the negative effect of infection on host fitness (Strauss & Agrawal 1999). Indeed, genetic variation for tolerance against herbivores (e.g. Weinig et al. 2003) or other pathogens (Kover & Schaal 2002) has previously been demonstrated in Arabidopsis. A cost of this tolerance might also explain why the deleterious effects of H. arabidopsis infection were linked to host fitness (Strauss & Agrawal 1999). Our chivalrous parasite would thus be an ordinary parasite attacking hosts more or less adequately ensured against it.
Another, though not exclusive, hypothesis is that the water stress imposed on plants at the end of their cycle influenced infected and uninfected plants differently. Indeed, responses against pathogens and abiotic stresses can involve shared metabolic pathways; for instance, the jasmonate phytohormone mediates responses against both desiccation and pathogen attack (Wasternack & Parthier 1997). The differential fitness loss observed could then also reflect a variation in their induction of long-term protection against various stresses by an early pathogen aggression: infected plants from more induced lines would cope better with a late stress, thus reducing their difference in fitness with uninfected controls.
The more striking aspect of our results, which cannot be explained by tolerance alone (either to stress or parasites), is the fact that the less fecund host line benefited from the infection. Here again it is doubtful that H. arabidopsis behaved generously by providing some advantage. However, such an increase in host fitness has sometimes been reported for plants damaged by herbivores and a similar phenomenon could possibly have occurred with our parasite. This increase in plant reproduction following damage, or ‘overcompensation’, has been explained as an adaptation of plants to recurrent and predictable herbivory, with plants delaying inflorescence development until grazers have passed. The corollary is that such plants then become dependent on herbivores to trigger the initiation of reproduction and perform less well in their absence. Overcompensation is thus an artefact of the plants' evolved dependence on their herbivores (Agrawal 2000; de Mazancourt et al. 2005): their being unprepared to live without them. This phenomenon is less documented for parasites (but see Pannebakker et al. 2007), but there is no reason why a predictable attack by parasites would not select for the same kind of dependence. We do not exclude that other factors could also be invoked (like other aspects of the experimental conditions for which this line was maladapted, or even a real, yet undetermined, advantage given by the parasite). However, our hypothesis provides testable predictions. We expect that the gradient observed among the host lines tested reflects a gradient in parasite pressure in their population of origin, with low tolerance in populations with low parasite pressure and high tolerance even associated with evolved dependence in those undergoing regular pathogen epidemics. In any case, the dramatic variation in parasite impact we observed among host lines in our experiment points towards an important role in host–parasite coevolution played by host variation in compensating for infection.
Acknowledgements
We thank G. Félix and L. Saunois for their technical assistance in the greenhouse, and two anonymous reviewers for their constructive comments on an earlier version of the manuscript.
References
- Agrawal A.A. Overcompensation of plants in response to herbivory and the by-product benefits of mutualism. Trends Plant Sci. 2000;5:309–313. doi: 10.1016/s1360-1385(00)01679-4. doi:10.1016/S1360-1385(00)01679-4 [DOI] [PubMed] [Google Scholar]
- Bronstein J.L. Conditional outcomes in mutualistic interactions. Trends Ecol. Evol. 1994;9:214–217. doi: 10.1016/0169-5347(94)90246-1. doi:10.1016/0169-5347(94)90246-1 [DOI] [PubMed] [Google Scholar]
- Bronstein J.L. The exploitation of mutualisms. Ecol. Lett. 2001;4:277–287. doi:10.1046/j.1461-0248.2001.00218.x [Google Scholar]
- de Mazancourt C, Loreau M, Dieckmann U. Understanding mutualism when there is adaptation to the partner. J. Ecol. 2005;93:305–314. doi:10.1111/j.0022-0477.2004.00952.x [Google Scholar]
- Ebert D. Experimental evolution of parasites. Science. 1998;282:1432–1435. doi: 10.1126/science.282.5393.1432. doi:10.1126/science.282.5393.1432 [DOI] [PubMed] [Google Scholar]
- Goss E.M, Bergelson J. Fitness consequences of infection of Arabidopsis thaliana with its natural bacterial pathogen Pseudomonas viridiflava. Oecologia. 2007;152:71–81. doi: 10.1007/s00442-006-0631-9. doi:10.1007/s00442-006-0631-9 [DOI] [PubMed] [Google Scholar]
- Holub E.B, Beynon J.L, Crute I.R. Phenotypic and genotypic characterization of interactions between isolates of Peronospora parasitica and accessions of Arabidopsis thaliana. Mol. Plant Microbe Interact. 1994;7:223–239. [Google Scholar]
- Kover P.X, Schaal B.A. Genetic variation for resistance and tolerance among Arabidopsis thaliana accessions. Proc. Natl Acad. Sci. USA. 2002;99:11 270–11 274. doi: 10.1073/pnas.102288999. doi:10.1073/pnas.102288999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannebakker B.A, Loppin B, Elemans C.P.H, Humblot L, Vavre F. Parasitic inhibition of cell death facilitates symbiosis. Proc. Natl Acad. Sci. USA. 2007;104:213–215. doi: 10.1073/pnas.0607845104. doi:10.1073/pnas.0607845104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peever T.L, Liu Y.C, Cortesi P, Milgroom M.G. Variation in tolerance and virulence in the chestnut blight fungus–hypovirus interaction. Appl. Environ. Microb. 2000;66:4863–4869. doi: 10.1128/aem.66.11.4863-4869.2000. doi:10.1128/AEM.66.11.4863-4869.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvaudon L, Héraudet V, Shykoff J.A. Parasite–host fitness trade-offs change with parasite identity: Genotype-specific interactions in a plant–pathogen system. Evolution. 2005;59:2518–2524. doi:10.1554/05-299.1 [PubMed] [Google Scholar]
- Salvaudon L, Héraudet V, Shykoff J.A. Genotype-specific interactions and the trade-off between host and parasite fitness. BMC Evol. Biol. 2007;7:189. doi: 10.1186/1471-2148-7-189. doi:10.1186/1471-2148-7-189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulenburg H, Ewbank J.J. Diversity and specificity in the interaction between Caenorhabditis elegans and the pathogen Serratia marcescens. BMC Evol. Biol. 2004;4:49. doi: 10.1186/1471-2148-4-49. doi:10.1186/1471-2148-4-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarenko A.J, Schlaich N.L. Downy mildew of Arabidopsis thaliana caused by Hyaloperonospora parasitica (formerly Peronospora parasitica) Mol. Plant Pathol. 2003;4:159–170. doi: 10.1046/j.1364-3703.2003.00166.x. doi:10.1046/j.1364-3703.2003.00166.x [DOI] [PubMed] [Google Scholar]
- Strauss S.Y, Agrawal A.A. The ecology and evolution of plant tolerance to herbivory. Trends Ecol. Evol. 1999;14:179–185. doi: 10.1016/s0169-5347(98)01576-6. doi:10.1016/S0169-5347(98)01576-6 [DOI] [PubMed] [Google Scholar]
- Thomas F, Poulin R, Guegan J.F, Michalakis Y, Renaud F. Are there pros as well as cons to being parasitized? Parasitol. Today. 2000;16:533–536. doi: 10.1016/s0169-4758(00)01790-7. doi:10.1016/S0169-4758(00)01790-7 [DOI] [PubMed] [Google Scholar]
- Wasternack C, Parthier B. Jasmonate signalled plant gene expression. Trends Plant Sci. 1997;2:302–307. doi:10.1016/S1360-1385(97)89952-9 [Google Scholar]
- Weinig C, Stinchcombe J.R, Schimitt J. Evolutionary genetics of resistance and tolerance to natural herbivory in Arabidopsis thaliana. Evolution. 2003;57:1270–1280. doi: 10.1111/j.0014-3820.2003.tb00335.x. doi:10.1554/02-469 [DOI] [PubMed] [Google Scholar]