Abstract
This paper discusses biotic interactions in agroecosystems and how they may be manipulated to support crop productivity and environmental health by provision of ecosystem services such as weed, pest and disease management, nutrient cycling and biodiversity conservation. Important elements for understanding biotic interactions include consideration of the effects of diversity, species composition and food web structure on ecosystem processes; the impacts of timing, frequency and intensity of disturbance; and the importance of multitrophic interactions. All of these elements need to be considered at multiple scales that depend in part on the range of the movement of the organisms involved. These issues are first discussed in general, followed by an examination of the application of these concepts in agricultural management. The potential for a greater use of ecological management approaches is high; however, owing to the nature of complex interactions in ecosystems, there is some inherent unpredictability about responses to management interventions under different conditions. Such uncertainty needs to be accommodated in the development of recommendations for farm management. This requires an increased emphasis on the effective synthesis of complex and often apparently contradictory information and on field-based adaptive research, monitoring and social learning by farmer/researcher collaborations.
Keywords: sustainable agriculture; disease suppression; diversity; weeds, biological control; social learning
1. Introduction
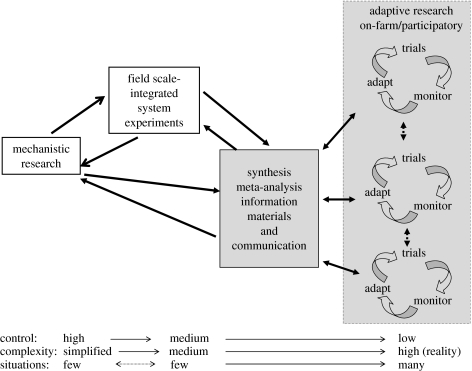
Manipulating biotic interactions to provide desired services and thus reduce or eliminate the need for external inputs is fundamental to the practice of ecologically sound agriculture. The challenge is how to encourage positive, while reducing negative, interactions. Here, I focus on how knowledge of biotic interactions can be used in the design and management of sustainable farming systems. For a farming system to be sustainable in human and ecological terms, it needs to sustain an acceptable level of production and the resource based upon which productivity depends; maintain environmental and human health and; provide desirable rural livelihoods and an accessible food and fibre supply for society. Embedded in these goals is the recognition that a sustainable system has to meet broadly held societal values and needs, which requires placing our efforts to achieve ecological sustainability firmly in the socioeconomic, political and cultural contexts of agro-food systems. However, the primary scope of this paper is the biotic dimensions of agroecosystems and for brevity I will only focus on crop-based agriculture. The paper is not intended to be a comprehensive review, but rather an illustration of the debates, progress and limitations of our ability to understand and manipulate the myriad of biotic interactions found in agricultural systems. Finally, I argue that the complex nature of ecological interactions requires an increased emphasis on synthesis and bidirectional information exchange between academic institution-based research and place-based adaptive research done in partnership with farmers and communities.
2. A word about terminology
Throughout the paper, I avoid using the terms ‘conventional’ and ‘sustainable’ as though they are opposites. This terminology was useful in the past for identifying ideas that challenged the existing research paradigm, with its focus on maximizing yield by compensating for limiting resources with additions of external inputs; and argued instead for a more holistic view of agriculture as managed ecosystems. At this point, however, maintaining a simple dichotomy contradicts the spectrum of farming systems currently practised, and can contribute to polarization of attitudes and reduce openness to new ideas. Farming systems vary along many dimensions (fertilizer, pesticide and energy use, organic matter inputs, complexity, biodiversity, etc.); making it difficult to know what constitutes conventional agriculture or to imply there is a simple way to identify sustainable systems. Indeed the sustainable agriculture literature has been criticized for contrasting ecological practices against an extreme negative caricature of what is called conventional, rather than against more representative management practices (Trewavas 2004). This criticism can also be raised about how organic and alternative agriculture is often portrayed in various media. Regardless, it points to the problem of what we mean by conventional, and equally what we mean by sustainable? Seeking clarification of what constitutes sustainable agriculture and how this can be evaluated is an important, evolving and necessary debate, but it falls beyond the scope of this paper. Here the term conventional will be used only in the context of specific studies where it refers to treatments that represent how a particular system is typically managed, and sustainable refers to management approaches thought to move the system towards the goals outlined in §1.
3. What does ecological knowledge have to offer for management of agricultural systems?
Can ecology provide insights into issues facing contemporary agriculture such as pollution, land degradation and loss of biodiversity? Weiner argues that current problems with agriculture relate to higher levels of organization, the domain of ecology, and that an emphasis on applied ecology or ecological engineering is needed (Weiner 2003). This call is echoed by Ormerod and colleagues who discuss the need for better ways of communicating ecological ideas and to more effectively integrate ecologists into agricultural research and extension (Ormerod et al. 2003). Greater reliance on manipulation of biotic interactions, as opposed to their replacement with inputs of energy and agrochemicals, increases agroecosystem complexity. Further, because biotic interactions are sensitive to changes in the physical and chemical environment, site- and season- specific characteristics such as microclimate, soil type and management history will impact the ways biota interact. The discipline of ecology constantly grapples with such complexity, but has been criticized for failing to produce unifying principles that can be broadly applied. However, ecosystems are middle number systems making their behaviour inherently less predictable than large number systems (the realm of physics) or small number systems where only a limited number of interactions can occur (O'Neill et al. 1986). In designing more ecologically based agricultural systems, dealing with complexity and uncertainty are inevitable challenges.
Ecological concepts emerging from the study of natural systems provide insights into how biotic interactions may determine agroecosystem function. Ecosystem function is the combination of ecosystem properties, services and goods (Hooper et al. 2005). Ecosystem properties include the sizes of compartments of materials and the fluxes of materials and energy among compartments. Particular levels or rates of ecosystem properties are not inherently ‘good’ or ‘bad’, in contrast to ecosystem goods and services to which humans attach great value. Ecosystem goods are ecosystem properties that have direct market value, such as production of food, fibre etc., whereas ecosystem services are those properties that directly or indirectly benefit human endeavours, such as regulating climate, cleansing air and water, pollination, storing and cycling nutrients. When considering agricultural sustainability, we focus on the provision of goods and services, and much of the debate and contention about what constitutes a sustainable system boils down to which goods or services should be given the greatest priority.
4. The diversity issue
The idea that more diverse ecosystems sustain greater productivity and stability in ecosystem functions is an intuitively appealing idea. It seems logical that if more than one species can perform the same function, then overall ecosystem functions will be less vulnerable to changes in the populations of a particular species due to environmental stress or pest attack (the insurance hypothesis). Similarly if farmers only produce a single type of crop, they would be more vulnerable to a disease outbreak, drop in prices or bad weather than if they had a number of crops with different susceptibilities to pests and weather conditions. Indeed diverse cropping systems are typical of many traditional agricultural systems found around the world, particularly in risk-prone environments (Abate et al. 2000). It is also in these regions, primarily in the developing world, where the greatest emphasis on improving diverse production systems through intercropping and agroforestry is found. Economic modelling studies also support the notion that under uncertain conditions, crop diversity is a mechanism for risk reduction, although this benefit is lost if agricultural policies compensate for inherent system risk by providing crop insurance or other financial support mechanisms (Wossink & Rossing 1998; Di Falco & Perrings 2005; Tilman et al. 2005).
Conway has argued that agroecosystem diversification should be a top priority in agricultural development owing to greater stability and less risk to resource poor farmers (Conway 1998), but others have challenged Conway's assumptions and argue that a focus on diversification in agricultural systems is not warranted (Wood 1998). It is clear that there are trade-offs with more diverse agricultural systems, and that the kind of diversity matters greatly (see below), but the question is how to design diverse systems that can meet multiple goals in an acceptable way. For example, in the humid tropics the inclusion of perennial tree and shrub species in cropland have important benefits, such as reduced erosion and more efficient nutrient cycling; benefits that are key for reversing problems of land degradation. The trade-off is that increased shading and microclimate changes may negatively impact the productivity of important annual crop species (Rao et al. 1997; Kho 2000).
On closer inspection, questions emerge about the relationships between diversity, stability and ecosystem functions: how much diversity is needed; does diversity always increase stability; is there a point at which further increases in diversity have no effect and is species richness most important or is it species composition? Ecologists are engaged in a lively debate over these issues; however, areas of general agreement are summarized in a recent review (Hooper et al. 2005). The main points of agreement can be summarized as follows: (i) increased diversity can lead to greater community stability, due in part to fluctuations of individual species' populations within the community; (ii) it is difficult to unequivocally demonstrate complementarity (positive effects of diversity such as increased productivity or stability due to niche separation and compensatory responses by different species); (iii) in small number systems and over short-time frames the sampling effect (species composition) is more important than species richness; (iv) factors other than diversity can be important for stability, such as facultative interactions; (v) as spatial and temporal scales increase, more biotic diversity is needed to sustain ecosystem functions; and (vi) little theoretical or empirical work has considered multiple trophic level interactions, but initial findings suggest that in multitrophic systems effects of diversity will be variable (Hooper et al. 2005).
These findings illuminate why species richness is often less important for agroecosystem function than the presence of a small subset of species. The primary concern in agriculture is the productivity of a few species, not the stability of the entire community. Further intensively managed agricultural systems are species poor relative to natural habitat, and can be considered as small number systems where sampling effects (species composition) typically dominate. Farming systems also experience frequent disturbance (tillage, harvest, crop rotation and pesticide use) and hence diversity effects are limited to short-time frames, again a circumstance when specific species effects tend to dominate. Some also question whether complementarity effects are important in fertile conditions as found in many agricultural systems. Loomis and Connor argue that resource capture in a well-managed corn monoculture is as effective as for any intercrop (Loomis & Connor 1992), given the high leaf area index and root densities that can be achieved in corn monocrops when provided with adequate fertility and water availability.
In managing diversity in agriculture, species selection is pivotal, and deliberate efforts are needed to encourage beneficial interactions and minimize undesired interactions. For intercropping systems, species are usually chosen owing to known niche separation (such as different rooting patterns, canopy types, phenology, etc.) or facultative interactions such as with the introduction of a legume. Well-designed intercrops can increase overall productivity (Mead et al. 1986; Vandermeer 1989) and potentially reduce risk to farmers (see §10). Even a small increase in diversity can have a large impact on system function owing to the specific properties of the introduced species. A remarkable example of this emerged in a regional-scale field experiment with rice production in China. Here simply interplanting two varieties of rice, rather than planting them in separate fields, led to a dramatic reduction in pest problems and pesticide use (Zhu et al. 2000). The effect was thought to be due to changes in canopy structure and microclimate with the new arrangement. Similarly, the addition of one or two species to fulfil particular functions, such as trees or shrubs for slope stabilization or a cover crop for erosion control, can affect the ability of a system to resist a major stress as in the case of Hurricane Mitch (Holt-Gimenez 2002). Further, there are numerous studies showing that increased vegetational diversity enhances biological pest control (Andow 1991a,b), but counter examples also exist where pests or disease levels increase due to the provision of highly palatable species or changes in canopy microclimate (Brown & Ewel 1987; Andow 1991a,b; Prieur-Richard et al. 2002).
While species composition is very important, it is premature to suggest that species richness may not play a role in agroecosystem function and stability. As scale increases so does the relative importance of species richness because greater numbers of species are needed for the maintenance of ecosystem functions (Hooper et al. 2005). Loss of diversity in agricultural landscapes has been linked to the disruption of ecological functions such as pest management (Settle et al. 1996; Wilby & Thomas 2002; Shennan et al. 2004; Tscharntke et al. 2005), pollination services, resistance to plant invasion (Tscharntke et al. 2005) and increased non-point source pollution by runoff, sediment loss and leaching of nutrients and pesticides into groundwater (Boody et al. 2005; Lovell & Sullivan 2006). Another circumstance where species richness may be important is in low disturbance agroecosystems such as orchards, perennial pastures, agroforestry and reduced tillage systems. Here the effects of diversity develop over many years, and species richness is expected to become more important (Hooper et al. 2005). Such agroecosystems provide interesting, but understudied, experimental opportunities to test this hypothesis.
Finally, most information on diversity effects have come from studies of a single trophic level, notably plants (Thebault & Loreau 2006), yet the limited information available suggest that the effects of diversity in multi-trophic systems are likely to be highly variable and difficult to predict (Hooper et al. 2005). This appears to be the case where natural enemy diversity has been experimentally manipulated. Resultant effects on herbivores and plants differed depending upon the study (Cardinale et al. 2003; Lang 2003; Finke & Denno 2005; Snyder et al. 2006; Straub & Snyder 2006). Studies with small numbers of natural enemies found that predator–predator facilitation can increase pest suppression (Cardinale et al. 2003), or alternatively that predator interference either reduced overall pest suppression (Hodge 1999; Snyder & Ives 2001; Prasad & Snyder 2004) or was present but did not affect pest suppression (Lang 2003). Most studies involved the addition or removal of a single generalist predator, but a few studies have looked at the impacts of greater diversity among predators. Species composition was more important than species richness in two studies (Finke & Denno 2005; Straub & Snyder 2006), whereas a positive effect of species richness on pest suppression was found in another (Snyder et al. 2006). Also, the lack of a relationship found between parasitoid diversity and the levels of herbivore parasitism may be because parasitoids are all part of the same functional group, limiting complementarity effects, and that parasitoids are largely under bottom-up control (Rodriguez & Hawkins 2000). In contrast, another study showed that differences in food web structure and the richness of herbivores in 19 plant–herbivore–parasitoid food webs did affect parasitism rates on hosts, with the parasitoids functioning better in simple food webs than in complex ones (Montoya et al. 2003).
Not only have multitrophic interactions not been factored into most diversity studies, but what are small number systems in terms of plant diversity may be large number systems at other trophic levels. Simple rice monocultures can support large numbers of arthropods (765 species in Javanese rice fields (Settle et al. 1996) that are important for biocontrol. When spatial and temporal habitat heterogeneity (hence arthropod diversity) is maintained by asynchronous planting and flooding, key pest populations are kept in check by natural enemy populations sustained during the absence of rice pests by the consumption of alternate food sources from detrital food webs in nearby flooded areas (Settle et al. 1996).
Vegetationally simple cropping systems also become large number systems when soil food webs are considered. Recent data from the soil biodiversity programme in the UK found >100 species of bacteria, 350 species of protozoa, 140 species of nematodes and 24 distinct types of arbuscular mycorrhizae in an unremarkable agricultural soil (Fitter et al. 2005). Below-ground biodiversity is thought to be higher than above-ground (Susilo et al. 2004), although there is debate about appropriate measures of diversity in soil micro-organisms. Only a limited amount of work has examined the role of diversity in maintaining soil functions, but we know that management measures such as tillage, pesticide use, crop type and fertility inputs all affect below-ground biotic communities, and that management impacts on specific organisms can affect ecosystem functions (see §13 for examples). The role of diversity per se, however, is unclear. In experiments that removed key taxonomic groups, little change in the rates of soil respiration or above-ground productivity were found (Liiri et al. 2002), perhaps because the relatively low degree of specialization among detritivores implies considerable redundancy among this functional group (Bradford et al. 2002). Others caution that it may take exposure to multiple types of stresses before there is sufficient loss of buffering capacity due to redundancy within functional groups for changes in ecosystem properties to become apparent (Griffiths et al. 2000). van Bruggen and Semenov argue that soil microbial communities are an excellent model system for experimental testing of relationships between diversity, stability and ecosystem functions such as disease suppression or nutrient cycling (van Bruggen & Semenov 2000). Given the rapid turnover rates of soil microbes, effects of diversity can be detected over weeks or months, rather than years. Other works emphasize the need to look beyond diversity per se to consider community structure and seasonal population dynamics. For example, soil food web studies suggest that the shape of the food web pyramid is a better indicator of stability than diversity per se or food chain length (Susilo et al. 2004).
5. Issues of scale
From the preceding discussion, it is apparent that considerations of spatial and temporal scale are important in understanding how ecosystem functions (O'Neill et al. 1986). These concepts have been applied to land management (King 1992), plant disease suppression (van Bruggen & Semenov 2000) and ecological approaches to pest management (Shennan et al. 2004). However, efforts to scale from small to larger scales, or vice versa, have had limited success in soil science due either to a key process being overlooked or the interaction of multiple factors creating idiosyncratic behaviour (Wagenet 1998; Shirmohammadi et al. 2005).
The importance of scale is illustrated in the context of pest management, where a great deal of research has been done. Spatial scale is particularly important in pest management because landscape features affect species interactions, microclimates and weather patterns and can have notable effects on pests by changing habitat patterns and immigration rates (Colunga-G et al. 1998). Initially attempts were made to apply the theory of island biogeography (MacArthur & Wilson 1963, 1967) to agricultural settings (Reeves et al. 2005). By treating crop fields as islands, the theory predicts that the number of species inhabiting a field is the result of immigration and extinction rates, leading over time to equilibrium in species richness. Larger islands should support more species and have lower extinction rates than smaller islands; and islands closer to the sources of colonists have higher immigration rates and hence a greater number of species than islands far from the sources of colonists. The theory implies a need to reduce island size and increase the distance from source pools for agricultural pests, while simultaneously increasing the island size and decreasing the distance from colonizer sources for natural enemies. This can be achieved by manipulating patterns of vegetation diversity in the field, farm and landscape. Island biogeography theory may be useful as a general guideline for ecological pest management, but the theory fails to take into account behavioural differences among colonizing species and species–trophic structure relationships (Letourneau 1998).
In managed landscapes, fields and non-agricultural areas form a structural mosaic of habitats with insects and other mobile organisms moving between them. The development of multitrophic arthropod communities depends on spatial processes (dispersal and foraging) that occur at larger scales than the farm, as well as temporal processes such as overwintering and reproduction. Habitat fragmentation caused by farming or urban development can disrupt both types of process and isolate small natural enemy populations from one another, increasing local extinctions (Kalkhoven 1993). Disturbance also takes place at multiple spatial and temporal scales, depending on the specific cropping systems and site characteristics that determine tillage regimes, resources for natural enemies and levels of chemical intervention (Landis & Menalled 1998). Thus farming practices and the quality and connectivity of habitat patches in the landscape will impact the maintenance of diverse communities of arthropods and other organisms. In a large study of arthropod biodiversity across Europe, Schweiger et al. found that land-use intensity explained most of the variability in species data, whereas landscape characteristics (especially connectivity) accounted for most of the variability in body size and trophic guilds (Schweiger et al. 2005). They recommend that management efforts to enhance diversity in agricultural landscapes should focus on reducing land-use intensity and enhancing habitat connectivity. A similar conclusion was reached in other studies targeting different organisms (Purtauf et al. 2005; Roschewitz et al. 2005a,b; Schmidt et al. 2005; Tscharntke et al. 2005).
6. The effects of disturbance
The degree of disturbance varies greatly among agroecosystems in terms of frequency, intensity and types of disturbance. Dominant disturbances are tillage operations, application of pesticides and herbicides and crop harvest. Vegetable production systems, in particular, are highly disturbed due to frequent tillage, short crop cycles often with multiple crops per year, and typically higher pesticide and fertilizer use. Many high-value vegetable crops are small seeded and require a well-tilled homogeneous seed bed to ensure good soil contact for germination and seedling establishment; subsequent tillage is also needed to reduce weed competition, since many are also poor competitors.
Disturbance through tillage disrupts complex trophic food webs above and below ground and increases the vulnerability of the ecosystem. Intensive tillage exposes bare soil to erosive forces, and also disrupts soil structure reducing microsite diversity reduced along with the structural and functional diversity of their associated microbial communities (Welbaum et al. 2004). The disruption of complex food webs in soils can contribute to reduced disease suppressive ability, loss of arbuscular mycorrhizal associations and reduced efficiency of microbially mediated processes such as nutrient recycling, degradation of toxic residues, maintenance of soil structure and aggregation (Hedlund et al. 2004; Welbaum et al. 2004; Garbeva et al. 2006). The bacterial organic matter decomposition pathway is more resistant to disturbance than the fungal pathway and becomes more dominant in highly disturbed systems (Hedlund et al. 2004). The effects of disturbance are illustrated by a study that followed the bacterial and fungal communities during the course of one year in a wheat field (Girvan et al. 2004). Populations were stable during later stages of the crop growth period, but the fungal community in particular fluctuated widely following harvesting and plowing. Fertilization increased microbial biomass and changed both the active bacterial and fungal community structures, whereas pesticide application had no effect on active bacterial numbers or heterogeneity, but had a major impact on community structure.
Reduced tillage systems help avoid many of the problems associated with frequent physical disturbance such as soil erosion and disruption of soil food webs and also reduce fuel usage. However, higher amounts of herbicide are typically used for weed control raising serious concerns about groundwater contamination and the evolution of herbicide tolerant weeds (Murphy & Lemerle 2006). In recent years, the loss of ecosystem services such as nutrient retention, pollination and biological control in intensively tilled agricultural landscapes has resulted in efforts to reintroduce diversity and areas of undisturbed habitat. Examples include planting of hedgerows and windbreaks, restoration of wetland and riparian areas, vegetated buffer strips and use of in-field insectary plantings (Shennan et al. 2004; Lovell & Sullivan 2006).
Shifting cultivation is a common form of agriculture practised throughout the tropics and subtropics. In its traditional form, small patches of forest vegetation were cut down and burned to provide nutrients for a period of crop production before the land was abandoned and allowed to regenerate. Here disturbance is used to shift mature forest into an early successional state, enabling fast-growing annual crops to grow. The regeneration of forest during the fallow subsidizes the cropping phase by providing nutrients for crop growth. With increasing population pressure, the trend has been to shorten the fallow period and increase the time land is in crop production. Further, secondary forest fallows are being replaced by managed fallows that can also provide food or income (Sanchez 1995; Drechsel et al. 1996; Tian et al. 2005). The general belief is that increasing the frequency of disturbance through shorter fallows reduces the long-term sustainability of the system by decreasing biomass accumulation during forest regeneration and thus reducing nutrient availability for the subsequent cropping cycle (Bruun et al. 2006), or by allowing insufficient time for invasive weeds to be shaded out by the forest canopy. Upland rice yields were positively correlated with the length of fallow, possibly due to reduced availability of N and P in sites where fallow lengths were reduced; however, there was no evidence of long-term degradation of soil organic carbon as a result of decreasing fallow periods (Bruun et al. 2006). In addition, increasing the size of cleared areas relative to regenerating fallows reduces seed sources of colonizing species and exposes more of the landscape to problems such as soil erosion and invasive weed establishment. In a Brazilian study, the effects of long and short fallow periods on the spatial and temporal dynamics of forest patches and cropland were characterized (Metzger 2003). Shorter fallow cycles (2–4 years as compared with more than 10 years) failed to produce a sustainable equilibrium of landscape types, and under this management the amount of secondary forest cover was steadily declining. However, others have cautioned against an oversimplification of the relationship between length of fallow and sustainability (Mertz 2002; Obale-Ebanga et al. 2003).
7. Understanding multitrophic interactions
Trophic interactions are a key element of community dynamics in agroecosystems. Interactions occur not only between adjacent tropic levels, such as crop and herbivore, but also as indirect effects across multiple trophic levels. For example, effects of predation can extend to lower trophic levels—a phenomenon referred to as a trophic cascade. Effects of top predators on lower trophic levels have been demonstrated in aquatic (Paine 1974; Power 1990) and terrestrial systems (Letourneau & Dyer 1997; Dyer & Letourneau 2003; Wardle et al. 2005). In fact, enhancing natural enemy populations to reduce crop pests, resulting in a positive ‘top-down’ effect on crop productivity, is a fundamental tenet of biological control (Letourneau 1998). Interactions can also work in the other direction, referred to as bottom-up effects. For example, the chemical composition of plant tissue can affect the behaviour of natural enemies directly through chemical cues for finding prey or indirectly through the effects on herbivore populations (Kagata & Ohgushi 2006). While there is increased recognition of the importance of both top-down and bottom-up processes, a conceptual synthesis is lacking due to a paucity of experimental data, failure to utilize pre-existing datasets and a tendency to emphasize one process over the other (Walker & Jones 2001).
Studies have also shown that foliar herbivory can have consequences for the functioning of soil food webs (Wardle 2006). In constructed grassland communities, aphid species treatments did not affect total plant biomass or productivity, but did impact the relative abundance of the three plant species, which in turn affected the abundances of secondary consumers in the soil food web (bacterial and fungal-feeding nematodes, and enchytraeids) but not primary consumers (microbes, herbivorous nematodes) or tertiary consumers (predatory nematodes). In this case species identity of the aphid combinations impacted multiple trophic levels in soil food webs, but diversity per se had few effects.
Understanding the interplay of trophic interactions has major implications for the management of agricultural systems. Top-down forces will be affected by pesticide use and the presence and quality of refugia for natural enemies, whereas changes in plant quality due to fertility management, choice of crop variety and the composition and abundance of weed communities could have bottom-up impacts on herbivores and natural enemies. We already know that such tritropic interactions can lead to unexpected effects of tactics used to control one type of pest (arthropods, nematodes, pathogens or weeds) on another type, giving rise to a call for interdisciplinary approaches to develop truly integrated pest management strategies that consider interactions among multiple components of the pest complex present (Norris & Kogan 2005; Schroeder et al. 2005; Thomas et al. 2005).
8. Weed management and crop productivity
Can manipulation of interactions among weeds, crops and other biota produce adequate weed suppression to reduce or eliminate herbicide use? The answer is a qualified yes. There is widespread agreement that effective long-term ecological weed management strategies involve the use of multiple tactics, sometimes referred to as the ‘many little hammers’ approach (Barberi 2002; Anderson 2004, 2005; Westerman et al. 2005). In this way, multiple interaction points among weeds, their microenvironment and other organisms are targeted to reduce weed biomass production, seed production and seed survival. In developing weed management strategies, the following must be taken into account: (i) not only do crops and weeds compete directly for resources (light, water and nutrients), but indirect effects mediated through changes in pest or disease dynamics can also be important (Norris 2005; Norris & Kogan 2005); (ii) the presence of weed seedbanks in the soil requires long-term management strategies to reduce both the annual input of new seeds and pre-existing seedbank numbers by increased seed predation or loss of viability and (iii) if selection pressures are sufficiently strong over extended periods of time, weed populations can evolve into more competitive populations better adapted to agricultural field conditions than populations from non-agricultural areas (Weinig 2005). The strongest selection pressure acting upon weed populations in recent years is herbicide use, and not surprisingly herbicide-resistant weeds now pose major challenges for growers, especially in reduced or no-till systems (D'Emden & Llewellyn 2006; Murphy & Lemerle 2006).
A holistic approach to weed management should include both short- and long-term strategies. Tactics fall into two main categories: those that reduce weed growth and fecundity during the growth cycle and those that reduce weed seed survival. Examples of the first group include the use of competitive crop varieties, manipulating crop seeding density and spatial arrangement, tillage, intercropping, use of allelopathic residues and suppressive mulches, and targeted use of biocontrol agents. The second group includes various soil management techniques (e.g. reduced tillage, residue management and organic matter inputs) and the use of weed-suppressive crop rotations that reduce seed survival due to enhanced seed predation or infection by pathogens.
9. Increasing crop competitiveness
Crop competitiveness can be increased by the use of weed-suppressive cultivars, manipulation of seeding density and spatial arrangement, and fertility or water management. Relatively little work has been done to breed more competitive crop varieties. It is time consuming to do large screenings in the presence/absence of weed competition, but comparative competition studies done with a wide range of varieties can help identify easily measurable traits associated with higher competitive ability and enable indirect screening for competitiveness, a much less time consuming option (Gibson et al. 2003). Although there is still debate, competitive traits include early plant vigour and canopy development (Barberi 2002; Caton et al. 2003; Bertholdsson 2005), allelopathy (Bertholdsson 2005; Ni & Zhang 2005), the ability to deplete a given resource and/or plasticity in root and shoot growth to respond rapidly to temporal and spatial patchiness in resource availability (Craine 2005; Fargione & Tilman 2006). Early plant vigour is particularly important in annual crop production. Extensive research has identified the stage(s) in which crops are most susceptible to weed competition, the so-called critical weed-free period, and this is often during the crop establishment and early growth stages (Knezevic et al. 2002; Seem et al. 2003).
There has been some reluctance to pursue the development of weed-suppressive varieties for fear of a negative trade-off between resource allocation to growth traits that confer competitiveness and resource allocation to seed or fruit production. However, studies show that this trade-off does not always occur. For example, rice traits that suppressed watergrass (Echinochloa oryzoides) were not inversely related to yields even though some cultivars were able to reduce watergrass biomass by as much as 40–80% (Gibson et al. 2003). Competitiveness in this case was related to early season vigour as measured by height growth rates, tiller production and specific leaf area (Caton et al. 2003; Gibson et al. 2003). Evaluation of wheat cultivars for weed-suppressive ability also found some variation, but competitive ability was strongly affected by environmental conditions (Lemerle et al. 2001). In a subsequent study, modification of wheat seeding density was much more effective at weed suppression than differences among cultivars (Lemerle et al. 2004). In another work, selection of rice varieties for their allelopathic potential is being pursued (Ni & Zhang 2005) and initial results from India suggest there is potential for improving lentil root systems to increase nutrient uptake ability in low fertility soils, which may improve competitiveness against weeds (Gahoonia et al. 2005, 2006). Furthermore, some weed species produce fewer and less dormant seeds when grown among a highly competitive crop; however, seed input from less competitive areas of the field such as field margins or areas of poor crop growth could still be problematic (Nurse & DiTommaso 2005).
The crop–weed competitive balance can also be affected by nitrogen availability depending upon the responsiveness of each species to increased N supply (Liebman & Davis 2000). Highly N-responsive crop such as maize typically show lower weed pressure with increased N availability (Evans et al. 2003a,b). Modifying crop seeding density and/or spatial arrangement can also shift the competitive balance in favour of the crop. Higher crop densities are needed under weedy than non-weedy conditions, and planting crops in rows can be less effective at suppressing weeds than random or evenly spaced arrangements (Baumann et al. 2001; Kristensen et al. 2006). Superior weed suppression by spring wheat was found at high seeding density planted in a uniform grid, versus conventional row spacing (Olsen et al. 2005a). Subsequent work showed that the more easily achieved random spreading of seed was almost as effective as the uniform grid pattern (Olsen et al. 2005b). However, changing planting density and spatial arrangement may have unforeseen consequences. For example, in no-till wheat, powdery mildew severity is related to nitrogen application, crop phenological stage, row spacing and seeding rate. Narrow row spacing restricts early season disease spread by reducing air movement along the rows, whereas high seeding rates increase later season disease severity by increasing canopy density and humidity (Tompkins et al. 1992). Further, the use of random or grid patterns eliminates the possibility of mechanical weed control, which may be critical for some crops; however, it could be a useful strategy for improving weed suppression in green manures and cover crops.
10. Intercropping and residue management
Extensive research has focused on the ability of intercrops (more than one crop growing together) to demonstrate over yielding, thought to be achieved by some combination of weed or pest suppression; increased resource use efficiency due to crop complementarity (different rooting depths, phenology, etc.); or facilitation (such as inclusion of a legume; Vandermeer 1989). Unfortunately, most intercrop studies are limited to the observation of short-term effects, and we know little about the impacts of long-term use of intercrops on weed suppression, soil food webs, disease suppression or other ecosystem processes. A recent review of 50 research articles found that few studies focused on issues of stability and sustainability and only half of the studies with annual species, and two-thirds of those including a perennial species, lasted for more than one year (Connolly et al. 2001). Clearly, longer-term and multi-site investigations are needed to determine whether intercropping improves system stability and to identify long-term impacts on the agroecosystem. A few studies have attempted to address stability and risk (Thiaw et al. 1993) and (Dapaah et al. 2003), and others have proposed methods and designs for assessing risk (Mead et al. 1986; Trenbath 1999; Connolly et al. 2001).
The challenge when designing intercrop systems is how to optimize the system (in terms of productivity, economics, risk, etc.) given the many possible permutations and combinations of seeding rates, spacing, timing of planting, fertility management, etc. for each crop. The most common designs are additive or replacement series, although response model designs are increasingly used (Baumann et al. 2001; Connolly et al. 2001). With the development of ecophysiological models for many crops, both mechanistic and descriptive models are being used to optimize intercrops for yield and crop quality (Baumann et al. 2002b). It is not always clear, however, exactly what intercrops are being compared against and why. Are both intercrops and comparison monocrops managed in the same way, or is each system optimized in terms of planting density, fertility management, etc.? The appropriate choice depends upon whether the goal is to understand mechanisms underlying intercrop performance or to evaluate intercrops as practical alternatives to monocultures. In the first case, it is important to minimize confounding factors, and ideally both intercrop and monocrops should be compared across a range of conditions (the response model approach), whereas in the latter case it is critical to compare intercrops against the best managed monocrops for a specific situation.
Intercrop systems are varied; multiple crops can be planted at the same time, or staggered (relay intercropping); crops can also be mixed in random or structured arrangements (e.g. rows, strips, contour plantings). Increased weed suppression is a goal for many intercrop systems to reduce the need for costly herbicides or labour. For example, a study of chickpea and wheat intercrops in India found significant weed suppression as compared with the monocrops, and this resulted in higher net income and more efficient resource use in the intercrop system despite the need for some hand weeding (Banik et al. 2006). In a barley–pea intercrop study, the weed community in the intercrop was similar, but more stable than in the barley monoculture and than the pea monocrop that was less suppressive and had highly variable weed communities (Poggio 2005). Work with vegetable intercrops has demonstrated increased weed suppression, but maintaining crop quality (often related to size) is also critical (Baumann et al. 2001, 2002a). The use of strips of rye–vetch cover crops when growing pumpkins also shows promise, provided the cover crops are planted after the pumpkin to reduce early competition and at sufficiently high seeding densities to suppress weeds (Vanek et al. 2005). In contrast, interseeded hairy vetch cover in cabbage production did not improve weed suppression or affect yield (Brainard et al. 2004). Others have found benefits of intercrops for weed suppression alone or in combination with reduced herbicide use (Szumigalski & Van Acker 2005).
11. Use of weed-suppressive mulches and allelopathy
Residue mulches can control weeds by reducing light transmission as found in cherry orchards where a suppressive mulch layer was shown to inhibit weed growth and increase yields by 20% over conventional herbicide tree row management (Landis et al. 2002). Clover planted into winter wheat and subsequently killed also effectively controls common ragweed (Snapp et al. 2005). Rather than using crop residue as mulches, there is also the potential to select species that can be used as ‘living mulches’ (Hartwig & Ammon 2002), but it can be difficult to kill or remove the living mulch at the right time to prevent competition with the crop. However, the use of legumes, particularly velvetleaf (Mucuna deeringiana (Bort) Merr) as a living mulch in corn fields in Mexico, proved to be very effective at weed suppression and increased corn yields (Caamal-Maldonado et al. 2001).
In addition to light reduction, suppression of weeds can also be enhanced by using residues from plants with allelopathic properties. Allelopathy is defined as ‘the effect(s) of one plant (including micro-organisms) on another plant(s) through the release of a chemical compound(s) into the environment’ (see review by Bhowmik & Inderjit (2003)). The hope is to use allelopathy to achieve good weed suppression without stunting crop growth. While the use of allelopathic residues as surface mulch is most common, the options of breeding allelopathic crops, extracting allelopathic compounds to use as ‘natural herbicides’, or increasing allelopathic abilities through biotechnology are also being pursued (Bhowmik & Inderjit 2003). Some authors believe that the use of allelopathic mulches cannot eliminate the need for herbicides, but can only reduce the amount needed (Bhowmik & Inderjit 2003), whereas others are more optimistic (Khanh et al. 2005). In a study of no-till cotton, the use of black oat or rye as a cover crop eliminated the need for post-emergence herbicide but not pre-emergence application (Reeves et al. 2005). It is too early to say if allelopathy combined with other short- and long-term weed suppression tactics can be sufficiently effective to eliminate herbicide use in the absence of other incentives, such as organic certification or environmental regulations.
12. Integrated weed management
Clearly, there is no single ecological silver bullet to replace herbicide use, but an increasing numbers of studies are testing suites of tactics together; including modified crop rotations, planting arrangements, residue management and tillage (Menalled et al. 2001; Anderson 2004, 2005; Murphy et al. 2006; Sosnoskie et al. 2006). In one case, a combination of crop rotation, crop sequence, no-till, residue management and competitive crop canopies has enabled wheat growers in the Central Great Plains of the USA to reduce pesticide use by 50% (Anderson 2005). In this work, researchers used an empirical life cycle simulation based on demographic knowledge of major weed species to identify the best rotations and crop sequences for reducing weed growth and seed production (Anderson 2004). Others have shown significant reductions in weed seedbanks under different management systems. For example, over a 6-year period weed seed density in the soil increased in conventional and no-till systems, but declined in reduced input and organic systems (Menalled et al. 2001); however, despite the seedbank declines weeds were not effectively controlled in the reduced input and organic systems over a 12-year period (Davis et al. 2005, 2006). Elsewhere after six years reduced tillage in combination with crop rotation had increased weed diversity but reduced seed density by 80% (Murphy et al. 2006).
There are many options for further improvement in developing weed-suppressive cropping systems, especially, if more competitive crop varieties become commercially available, we increase our knowledge of weed and seedbank ecology and better utilize modelling tools such as life cycle simulations, and further refine cover crop and residue management strategies. Weed suppression goals need to be linked to the management of the whole pest complex; to do this will require stronger collaborations among weed scientists, entomologists, pathologists, nematologists, plant breeders and soil scientists. Other pests and diseases are impacted by changes in tillage, crop rotation, fertility, etc., and weeds themselves can be hosts for crop pests or provide refugia for natural enemies that can enhance biological control (Norris & Kogan 2005; Thomas et al. 2005; Wisler & Norris 2005). Unravelling the effects of different components of the pest complex will require innovative research designs and statistical analysis (Kranz 2005).
This section has focused on how to reduce weed competition with crops; however, there are also concerns about losing positive services that weeds can provide. There is evidence that weeds can be good hosts of arbuscular mycorrhizal fungi (AMF) that can play important roles in improving nutrient availability, suppressing pathogens and perhaps in the biocontrol of weeds (Vatovec et al. 2005). Others have demonstrated the role of weeds in sustaining biodiversity. For example, in Europe weeds found in stubble after grain harvest greatly affected the number of birds using the field, notably linnets (Carduelis cannabina; Moorcroft et al. 2002), and loss of weeds is thought be one of a number of causes in the overall decline of birds in farmland (Marshall et al. 2003). Work in the UK also revealed that arable weeds support high insect diversity reinforcing the need to balance weed control and biodiversity conservation (Marshall et al. 2003).
13. Management of plant diseases
The ability of soils to suppress plant diseases is due to a combination of general suppression (related to overall microbial biomass and microbial activity) and specific suppression (effects of individual or select groups of micro-organisms on a specific pathogen; Weller et al. 2002). General disease suppression is thought to be caused by increased competition for nutrients, especially soil carbon, when microbial activity is increased (Reeleder 2003). In a comparison of organic and conventional farms, suppression of corky root on tomato was greater in the organic than the conventional fields. Enhanced suppression in the organic fields was related to higher microbial activity and lower soil nitrate levels, whereas the conventional fields had a combination of higher soil nitrate levels and lower soil microbial activity (Drinkwater et al. 1995.). Further, take-all disease (Gaeumannomyces graminis var. tritici) was suppressed in organic relative to conventional soils in wheat and barley production, due to both general and specific suppression (Hiddink et al. 2005). Others have examined the role of soil fauna in the general suppression of fungal pathogens and conclude that facultative saprophytes may be most affected by mycelial-grazing soil animals, while obligate parasites may be more influenced by animals that ingest spores and other types of propagules (Friberg et al. 2005).
The ability of non-pathogenic strains of a disease causing fungi and AMF to act as biocontrol agents are examples of specific suppression. Non-pathogenic strains of Fusarium oxysporum can reduce the ability of the pathogenic strain to cause Fusarium wilt due to a combination of increased competition for resources, competition for infection sites and the ability of the non-pathogenic strains to induce plant resistance (Fravel et al. 2003; Bao et al. 2004). Colonization of strawberry plant roots by AMF induced resistance (IR) to Phytopthora fragariae in strawberry, but the effect was variety specific (Norman et al. 1996). Root necrosis was reduced 30–60% by AM colonization depending on the variety, and similar reductions were observed in a subsequent study (Vigo et al. 2000). The presence of AM colonization reduced the number of P. fragariae infection sites, and exudates from AM plants reduced sporulation of P. fragariae by 70% after 72 hours of exposure (Norman & Hooker 2000). Tomato plants colonized by AMF prior to exposure to Phytophthora nicotianae var. parasitica also experienced less root damage than un-colonized plants. In this case, the number of Phytophthora hyphae in the root tissue was decreased and cell necrosis around infected cells was reduced by the presence of the AM fungus (Cordier et al. 1996).
The best known example of specific suppression is the decline in take-all of wheat. The disease declines after years of continuous wheat cropping and is caused by a build-up of antagonistic fluorescent Pseudomonas spp. in the wheat root rhizosphere that produce antibiotic compounds and induce plant resistance (Weller et al. 2002). Interestingly, selected wheat cultivars reduced the incidence of root infection of apple seedlings, again apparently by increasing the populations of Pseudomonas spp. in the soil (Gu & Mazzola 2003). However, there are instances when take-all suppression was not affected by the presence of Pseudomonas spp. (Hiddink et al. 2005). In addition to Pseudomonas spp., a number of other soil micro-organisms produce antibiotic compounds capable of suppressing a range of pathogenic fungi under laboratory conditions, but due to technical limitations it has only recently been possible to demonstrate the presence of these antibiotics under field conditions (Raaijmakers et al. 2002). However, unequivocal demonstration that the levels and timing of production of antibiotics are correlated to observed disease suppression is still lacking.
Since the discovery of the role of rhizobacteria in take-all decline, an increasing body of literature has documented the ability of one organism to stimulate plant defence mechanisms and thus confer resistance to other pest or disease organisms, referred to as IR. Research on the physiological and biochemical bases of IR has resulted in the identification of chemical and biological elicitors of IR, some of which are now commercially available for use in agriculture (Mazzola 2004). Recent reviews summarize the current state of this field (Mazzola 2004; Vallad & Goodman 2004). The stimulation of IR has the potential to be a cornerstone of integrated pest and disease management, and may be particularly significant for non-chemical control of foliar diseases, for which few other options exist.
A variety of plant-associated bacteria have been found to enhance plant growth and elicit plant defence mechanisms; many are from the rhizosphere (called rhizobacteria), others from the phyllosphere (leaf surface) and from inside tissues of healthy plants (Kloepper et al. 1999). Some have found that compatible mixtures of rhizobacteria are more effective than a single strain (Ramamoorthy et al. 2001; Jetiyanon & Kloepper 2002; Jetiyanon et al. 2003); also a mix of antagonistic bacteria as a seed treatment in combination with foliar biocontrol agents improved the control of bacterial speck (Pseudomonas syringae pv. tomato), and bacterial spot of tomato caused by Xanthomonas campestris pv. vesicatoria and Xanthomonas vesicatoria (Ji et al. 2006). In theory, it should be possible to induce resistance to soil-borne and foliar diseases as well as pathogenic nematodes and arthropod pests and virus vectors (Kloepper et al. 2004). Specific strains of Bacillus spp. have been found to elicit IR in 11 different host plants and cause reductions in a spectrum of diseases (foliar, stem and soil-borne fungal diseases), viruses, root-knot nematodes as well as reducing populations of three insect vectors of viral diseases. Two formulations have been developed for commercial use, one as a plant growth promoter and the other to control diseases of soybean (Kloepper et al. 2004). In another study, Zehnder et al. found that plant growth promoting rhizobacteria caused IR in cucumber to bacterial wilt (Erwinia traechiphila) by reducing the production of a compound that stimulated feeding by the cucumber beetle vectors of bacterial wilt and by triggering other defence mechanisms once the bacteria enter the plant (Zehnder et al. 2001).
While formulations of antagonistic bacteria have been developed for commercial use as biocontrol agents, there are a number of issues that currently limit their use. (Note: sales of biocontrol products in general are only 1% of total agricultural chemical sales (Fravel 2005).) A major problem is lack of consistent and predictable control under field situations, as opposed to simple soil-less potting media where biocontrol has been more successful. The variable results in the field could be due to application problems (physiological state of the bacteria, timing and dosage) or differences in microclimate, crop genotypes, weed communities and soil ecology (Fravel 1999, 2005; Sabaratnam & Traquair 2002). Product registration is a significant barrier to the commercialization of AM fungal biocontrol formulations (Whipps 2004), and costs of growing and formulating mixtures of organisms may still be too high to make biocontrol economically attractive currently (Fravel 1999, 2005). Concerns regarding the potential non-target effects of any biocontrol agent applied in the field and the paucity of information available to assess ecological risks of using specific microbes (and other organisms) as biocontrol agents are increasingly being raised (Wajnberg et al. 2001). This concern is particularly acute for genetically engineered bacteria (Timms-Wilson et al. 2004). Augmenting indigenous antagonists in the soil through use of organic amendments, intercropping or crop rotations could be a more ecologically sound alternative to the introduction of specific antagonists that does not raise many of the concerns regarding non-target effects of introduced organisms (Kloepper et al. 1999).
Foliar diseases present a major challenge to the development of non-chemical management alternatives. IR has the potential to be an important strategy for the control of foliar pathogens and this is a very active research area. Another strategy being investigated is increasing competition for nutrients on leaf surfaces by enhancing saprophytic fungal, bacterial and/or yeast populations. This approach shows promise for controlling grey mould, Botrytis cinerea, on grapes, tomato and potted plants (Farber et al. 2006), but is limited to pathogens that require nutrients to grow and infect the plant. For other pathogens that penetrate the leaf rapidly and do not require nutrients from the leaf surface, enhancing rates of mycoparasitism could be more effective and this has been used to successfully control powdery mildew on grapes in coastal California (Farber et al. 2006). The bacterial disease fireblight on apple and pear, caused by Erwinia amylovora, is also controlled by increased populations of Pseudomonas flourescens on the leaf surface when applied as a spray or disseminated by honeybees prior to bloom (Wilson 1977). Finally, mites present on the leaf surface of woody perennials feed on fungi and other micro-organisms, and one (Orthotydeus lambi) has been found to suppress the development of powdery mildew on wild and cultivated grapes (English-Loeb et al. 1999; Norton et al. 2000).
Compost additions and cover crop residues have been found to reduce fungal, bacterial and nematode pathogens in a number of systems, although the effect can be highly variable depending on the specific crop/pathogen/amendment combination (Abawi & Widmer 2000). For example, compost was found to reduce certain fruit diseases of tomato but not others, increase foliar disease levels and had differential effects depending on the tomato cultivar and whether the plants were grown organically or not (Abassi et al. 2002). Incorporation of pest suppressive crop residues into soils (biofumigation) has been studied for a number of years, with particular attention being paid to species that produce compounds known to inhibit the growth of other organisms, such as Brassica spp. that produce glucosinalates, sudan grass and cereal rye (SAN 1998). Biofumigation with broccoli residue incorporation suppresses Sclerotinia minor in lettuce (Hao et al. 2003) and Verticillium wilt in cauliflower when disease pressure is moderate. One reason for the variable performance of Brassica spp. reported in a number of studies may be that under certain conditions the residue can stimulate saprophytic growth of Rhizoctonia solani and increase damping off in subsequent crops (Yulianti et al. 2006). However, for varieties with desirable glucosinilate profiles, consistent and repeatable suppression of pathogenic nematodes has been observed (Zasada & Ferris 2004).
Another technique that offers promise is to incorporate crop residues and force anaerobic decomposition to occur by tarping with oxygen impermeable silage plastic after residue incorporation. Products of anaerobic decomposition cause high levels of suppression of a number of plant pathogens and nematodes across a range of crops, but disappear rapidly after oxygen returns to the soil and thus do not reduce crop growth (Blok et al. 2000; Goud et al. 2004). This method is being developed for commercial use in The Netherlands and Japan.
14. Management of plant parasitic nematodes
Many of the approaches for increasing soil suppressiveness to plant pathogens can also be used for the suppression of plant parasitic nematodes. As with soil-borne diseases, both general and specific types of suppressiveness to pathogenic nematodes have been identified (see review Westphal (2005)). For example, combinations of plant-growth-promoting rhizobacteria, organic amendments and phytochemicals can be incorporated into transplant mixes and suppress root-knot nematodes in tomato transplants (Kokalis-Burelle et al. 2002). Nematode community structure is also known to shift in response to organic matter levels and quality with diversity increasing with organic matter inputs (Mikola & Sulkava 2001; Wardle 2006). It appears that shifts can happen over relatively short time frames; for example, the effects of long-term crop management on nematodes, other than plant feeders, disappeared within a year of disruptive soil management (Berkelmans et al. 2003). Nonetheless, crop rotations can be an effective nematode management tool through a combination of the inclusion of non-host crops or varieties, use of nematode-suppressive cover crops and residues, and stimulating changes in soil communities through organic matter management (Caamal-Maldonado et al. 2001; Vargas-Ayala & Rodriguez-Kabana 2001; Pyrowolakis et al. 2002; Zasada & Ferris 2004; Snapp et al. 2005; Westphal & Scott 2005). Similarly, the species composition of fallows in shifting cultivation and bush-fallow systems can also be manipulated to suppress key plant parasitic nematodes (Adediran et al. 2005).
15. Ecological management of arthropod pests
Highly visible plant species like trees generally have chemical defences that become more toxic over time, reducing leaf digestibility to herbivores (Feeny 1976; Rhoades & Gates 1976). Conversely, less conspicuous annual plants rely on escape in space and time as their main defence (Price 1997). In contemporary agriculture, crop plants are both highly conspicuous and abundant, and defensive toxins have typically been removed through breeding for crop quality. In monocultures, increased herbivory could be due to how concentrated the food source is, making it easier to find host plants; higher pest tenure time; higher herbivore feeding and reproductive rates; or changes in crop quality (Andow 1991b). In addition, the lack of plant diversity decreases the diversity and abundance of predator and parasitoid natural enemies of crop pests (Hooks & Johnson 2003; Landis et al. 2005; Lavandero et al. 2006). In the widely studied insect communities of cruciferous crops, research has shown that when planted as mixtures rather than sole crops, herbivore responses include reduced colonization, reduced adult tenure time in the marketable crop, and oviposition interference (Hooks & Johnson 2003). Further, making it more difficult for natural enemies to find their herbivore hosts by using mixture can avoid the boom/bust cycles in natural enemies seen in monocultures due to local extinctions of hosts by over predation (Gols et al. 2005).
Plant quality changes due to fertility and water management also affects herbivory (Awmack & Leather 2002), as shown for mites in apples (Walde 1995) and leafminers in bean (Kaneshiro & Jones 1996). Yet, despite many studies no clear principles relating nutrient levels and herbivory have emerged (Busch & Phelan 1999). In 60% of studies, herbivore populations increased at higher nitrogen additions, yet no effect or negative responses were observed in the other 40%. Recent work suggests that the ratios of nutrients can have stronger effects on herbivores than individual nutrient levels (Busch & Phelan 1999; Beanland et al. 2003).
While planting in mixtures and fertility practices affect herbivory, the direct manipulation of predator–prey population and community dynamics is the cornerstone of biological control of arthropod pests. Predation and parasitism can be increased by either artificially releasing natural enemies or pathogens (classical biocontrol) or by transforming the agroecosystem to create favourable conditions for natural enemies (conservation biocontrol; Dent 1991).
16. Classical biocontrol
Where exotic pests have colonized a region, classical biocontrol typically involves importation of a natural enemy from the area of pest origin. The successes and failures of biocontrol releases are analysed extensively in other reviews (King et al. 1985; Jacas et al. 2006; van Lenteren et al. 2006). Data from 87 studies of arthropod releases indicate that life-history traits found to predict success included host specificity, whether the agent was a predator or parasitoid, and the number of generations per year. From a more limited number of studies, possible traits for predicting non-target effects included sex ratio of progeny and the documented presence of native natural enemies (Kimberling 2004). However, there is an increasing focus on non-target effects of biocontrol agent releases (van Lenteren et al. 2006). A recent book addresses non-target effects for the spectrum of biocontrol agents and spotlights the lamentable paucity of information collected on non-target effects (Wajnberg et al. 2001).
Where natural enemies are present, augmenting local populations with mass releases can speed up pest suppression and provide greater suppression early in the season. In the USA, augmentative releases are used on an estimated 19% of fruit and nut acreage, and 3% of vegetable acreage (Office of Technology Assessment 1995), and predatory mites are released on 50–70% of California's strawberry acreage to control the two-spotted spider mite, Tetranychus urticae (Parella et al. 1992; Hoffman et al. 1998). However, augmentation is rarely used on anything other than high-value crops (Collier & Van Steenwyk 2004), probably due to variable effectiveness and high costs as compared with pesticides. Augmentation releases achieved target pest densities in about 15% of case studies, failed in 64% and were often less effective than pesticide applications but frequently more expensive. A number of factors limit the efficacy of augmentation including unfavourable environmental conditions, mortality, inadequate dispersal and predation of released agents (Collier & Van Steenwyk 2004).
17. Conservation biological control
The goal of conservation biological control is to restore or enhance indigenous populations of beneficial insects by providing food resources (host prey, pollen and nectar, alternate prey) and shelter for overwintering. Habitat management involves vegetation diversification at multiple scales (Landis et al. 2005). Use of insectary plantings or leaving strips of unharvested plants are examples of in-field strategies, whereas wildflower borders, grassy buffer strips, windbreaks and hedgerows are examples of field margin diversification techniques. Larger-scale distribution and connectivity of landscape features such as hedgerows, habitat fragments and riparian vegetation can also impact levels of biological control as well as provide biodiversity conservation benefits.
Interplanting crops with flowering herbaceous plants is promoted as a farmscaping technique, since pollen and nectar are essential to the fecundity and longevity of several natural enemy species (Jervis et al. 1993; Idris & Grafius 1995). Chaney (1998) found that sweet alyssum, Lobularia maritima, had consistently higher natural enemy to pest ratios than other plants tested. Natural enemy densities were high and aphid populations were low within 11 m of the insectary (Collins et al. 2002), suggesting that alyssum planted every 20th bed would maintain effective biological control in lettuce fields. This technique has been adopted by a number of lettuce growers in coastal California, but they tend to interplant at smaller intervals (C. Shennan 2004, personal observation). Maintenance of varied successional stages of perennials may also be important. For example in California, strip harvested alfalfa fields retained Lygus hesperus populations in the unharvested alfalfa where it is not a pest, whereas completely harvested alfalfa fields caused survivors to migrate into other crop fields where they did become pests (Stern et al. 1964).
Planting of multispecies hedgerows along the edges of farm fields can provide stable habitat and resources for beneficials while fields are bare, or crops are young, but there are still many gaps in our knowledge of how well this vegetation diversification actually enhances biological control and under what circumstances. Numerous studies demonstrate increased abundance and diversity of natural enemy populations in hedgerows, for example, but few have identified the extent to which pests or natural enemies migrate from the hedgerows into adjacent fields, and even fewer have attempted to quantify the impacts on biological control (Letourneau 1998). Biological control may not be enhanced by hedgerows if the availability of pollen and nectar is so high within the hedgerows so that natural enemies do not disperse into adjacent agricultural fields to feed on crop pests (Bugg et al. 1987); or if the hedgerow attracts new pests, non-pest prey that natural enemies prefer over the crop pest; or top predators that prey on the natural enemies of interest (Pollard 1971; Bugg & Pickett 1998; Rosenheim et al. 1999; Nicholls et al. 2001). Natural enemy dispersal ranges, which can vary from a few metres to over a kilometre for some parasitoid species (Corbett 1998), will determine the effectiveness of various habitat patterns at enhancing biological control. Blackberry and prune trees provide habitat for alternative hosts of the parasitic wasp, Anagros epos, which later preys upon the vineyard leafhopper pest, Erythroneura elegantula (Doutt & Nakata 1973; Murphy et al. 1998), but connecting border plantings to in-field floral corridors may encourage greater natural enemy movement and biological control in vineyards (Nicholls et al. 2001). However, even if parasitism rates are increased, it is unclear whether this leads to meaningful levels of biocontrol (English-Loeb et al. 2003)
Successful conservation biological control relies upon matching vegetational scale and pattern to the movement range of desired natural enemies in relation to their primary food sources. This requires an expansion beyond habitat management at the field level to incorporate larger landscape patterns and processes, a still relatively unexplored area. In addition to the size and distance between habitat patches, we are beginning to realize the importance of the ‘matrix’ between patches for insect movement (Ricketts et al. 2001). Many species that live in habitat patches also utilize resources outside the habitat patch, a desirable attribute for biological control since we want natural enemies to migrate into agricultural fields. Structurally complex landscapes have been found to lead to higher levels of parasitism and lower crop damage (Thies & Tscharntke 1999; Pullaro et al. 2006); but this is not always the case even within the same region if parasitism rates also depend upon the presence of particular species or plant communities (Menalled et al. 1999; Landis et al. 2005).
18. Mediating nutrient availability
Biotic interactions are also at the heart of nutrient cycling and important mediators of nutrient availability. The identification of soil organisms and the structure and composition of soil food webs has increased greatly in recent times, as has our ability to measure fluxes of nutrients as they cycle through different components of the ecosystem. Unfortunately, our understanding of the interactions between soil food web decomposers and patterns of nutrient cycling is still sparse (Ruess et al. 2002), reflecting the continual challenge of how to connect population and community ecology with fluxes of material and energy (O'Neill et al. 1986; van Bruggen & Grunwald 1994). For example, the significance of nematode population and community dynamics for the mineralization of nitrogen in the soil has only recently been recognized (Ferris et al. 2004). A full discussion of soil ecology and nutrient cycling is beyond the scope of this paper and I refer the reader to other excellent texts on the topic (Brussaard & Ferrera-Cerrato 1997; Schlesinger 1997; Lavelle & Spain 2001; Coleman et al. 2004).
The mutualistic relationship between N-fixing bacteria and certain plants is a major driver of ecosystem processes. The most important mutualism for agriculture is between rhizobium bacteria and legumes, with legumes providing 25–35% of the world's protein (Finan et al. 2002). Limitations on the amount of biological nitrogen fixation (BNF) in agriculture are predominantly related to management and environment, leading some to argue that any impacts of genetically engineered N-fixing non-legume plants are likely to be small (Peoples et al. 2002). Limiting factors for BNF are mainly inadequate moisture, unfavourable temperature regimes, nutrient limitations and less than optimal nodulation from lack of appropriate inocula. Addressing these issues and expanding legumes into areas where they are not currently grown could have a large impact on global BNF and fertilizer use in the future (Peoples et al. 2002).
Another important mutualism is the relationship between AMF and plant roots. The ability of AM associations to suppress soil-borne diseases was discussed earlier, but they can also affect P and micronutrient uptake (Lekberg & Koide 2005) and increase plant drought tolerance (Subramanian et al. 2006). Most horticultural and crop plants are symbiotic with AMF, but exploration of how to best utilize this relationship in crop management is in its infancy (Plenchette et al. 2005; Gosling et al. 2006). In general, the beneficial effects of AMF through improved P nutrition are seen at low-to-moderate soil P levels (Lekberg & Koide 2005; Plenchette et al. 2005). A meta-analysis of 290 published studies found that inoculation with AMF increased root colonization by 29% and that some management practices also increased colonization such as shortened fallow (by 20%) and reduced soil disturbance (by 7%). Increased colonization generally raised yields by 23%, whereas a negative effect of increased colonization on plant biomass production was found in only 2% of all trials (Lekberg & Koide 2005). One benefit of rapid colonization of crop roots with AMF is improved P uptake early in the season, when P deficiency can be a problem, but colonization is delayed and the P benefit lost if the previous crop grown was non-mycorrhizal (Miller 2000).
The significance of the complex interplay between plant roots, the substrates they excrete, rhizobacteria, AMF and nutrient availability in the rhizosphere is beginning to be appreciated. It is clear that rhizobacteria and AMF can have synergistic effects on plant growth, but the underlying mechanisms are unclear (Artursson et al. 2006). Mycorrhizal infection is, however, known to increase N-fixation by rhizobium bacteria. Further, the presence of plant roots and the associated rhizosphere can greatly increase the rate of soil organic matter decomposition (the priming effect) by more than 300% for some plants during certain phonological stages (Cheng et al. 2003), and some rhizosphere bacteria release potassium from insoluble forms in the soil (Sheng & He 2006). Plants can also change root structure and physiology in response to perceived nutrient deficiency, and there is the possibility of breeding plants more capable of secreting compounds that increase the availability of nutrients in the rhizophere as well as make them better hosts of rhizosphere organisms (Rengel & Marschner 2005).
19. Whole system management
From the preceding sections, it is evident that managing diversity and disturbance at multiple spatial and temporal scales is at the core of using biotic interactions to provide desired agroecosystem services. Management approaches such as reduced tillage have somewhat predictable effects on soil biota; it favours the more readily disrupted fungal food webs, supports higher populations of AMF and can increase weed seed predation. Similarly, increased habitat diversity generally increases the abundance and diversity of natural enemies, but in neither case is the effect on crop production easy to predict. Crop growth and yield depend upon a complex balance of these and other interactions that can be species specific, affected by previous cropping history and highly dependent on environmental conditions. The design and management of this complexity thus require an understanding of general system behaviour combined with species- and site-specific knowledge.
The performance of ecological management could be improved, if the crop plants themselves are better adapted to the conditions likely to occur in a reduced input system. Characteristics such as increased competitiveness to weeds, disease and pest resistance, enhanced ability to support beneficial rhizosphere micro-organisms and improved capacity to access soil nutrients could all potentially benefit crop growth. However, selecting for such a mix of characteristics would be difficult and time consuming, so alternative approaches to plant breeding have been proposed based upon building diverse composite cross populations that are then subjected to natural and artificial selections in varied environments (Phillips & Wolfe 2005). Others advocate the use of mixtures of cultivars within a field to improve crop production (Sarandon & Sarandon 1995) and disease management (Mundt 2002).
It is increasingly possible to design crop rotations to enhance beneficial biotic interactions. These include crop rotation and other tactics to enhance the populations of beneficial rhizobacteria and thus soil suppressiveness to diseases and nematodes (Welbaum et al. 2004); maintenance of AM populations by reduced tillage (Gosling et al. 2006); and suppression of weed populations by using cover crops in fallows and designing rotations based on weed life cycle simulations (Anderson 2004). Nonetheless, more interdisciplinary, collaborative work will improve our ability to identify successful integrated weed, disease and arthropod pest management strategies (Norris & Kogan 2005). Crop diversification through intercropping is mostly being researched in the tropics and subtropics and can be highly productive low input options for farmers in many countries (Thiaw et al. 1993; Trenbath 1999; Dapaah et al. 2003); although research is being done in developed countries (Baumann et al. 2002a), there is little evidence of adoption by farmers (personal observation). In the past the same could be said of agroforestry, but interest in alley cropping and other types of agroforestry is increasing in the USA and Canada as an option for marginal land not only to sustain good crop productivity and provide organic matter inputs for organic production but also to increase carbon sequestration and improve the efficiency of nutrient cycling (Zinkhan & Mercer 1997; Jordan 2004; Thevathasan & Gordon 2004). Managing tree crop competition is critical in agroforestry, especially in alley cropping, and it has proved challenging to develop systems that work in low fertility and water limited environments, prompting the development of predictive tools to help in the design of agroforestry systems under different environmental conditions (Kho 2000; Ong et al. 2004).
Agricultural landscapes are important for providing other benefits beyond food and fibre production, such as the maintenance of good water and air quality, as well as the conservation of biodiversity. These attributes are increasingly being scrutinized, and deliberate efforts through policy interventions are being made to conserve and enhance these ecosystem services. As discussed previously, we know that distribution of habitats and their connectivity across the landscape are very important for maintaining viable populations of different organisms, as is the timing and intensity of agricultural management. For example, asynchronous tillage is important for maintaining beetle populations in arable cropland (Holland et al. 2005), just as asynchronous flooding is important for sustaining natural enemy populations in rice fields (Settle et al. 1996). Initial studies also found that creating a mosaic of crop fields and wetlands in difference successional stages had great promise as a strategy for improving waterfowl habitat and sustaining crop production in a multi-use landscape (Shennan & Bode 2002). Interestingly, maintaining rice fields flooded though the winter for waterfowl foraging habitat also provided beneficial agronomic impacts by increasing decomposition of rice straw and reducing grassy weed biomass (van Groenigen et al. 2003). Further, numerous studies have shown the interactive effects of landscape complexity and the impacts of agricultural management practices, with more benign practices (such as organic farming) having the greatest effects on increased biodiversity in simple landscapes (Bengtsson et al. 2005; Tscharntke et al. 2005).
20. Managing complexity and uncertainty
It is, however, pertinent to ask how widespread the use of ecological management strategies is, but the question is hard to answer because information on adoption is sparse. Some practices such as reduced tillage have increased (D'Emden & Llewellyn 2006), as has the acreage in organic production. However, estimates of organic acreage reflect more about which practices are not being used than those that are, since certified organic management can vary from simple input substitution to system diversification and use of multiple cultural and biological strategies (Guthman 2000). Some studies have tried to get at the use of ecological practices for pest control through farmer interviews (Shennan et al. 2001) or by tracking the sales of biocontrol agents (Fravel 2005; Georgis et al. 2006). Biocontrol agents still account for less than 1% of total agrichemical sales (Fravel 2005) and recent pesticide use reduction in northern Europe is largely attributable to improved forecasting and pesticide management (Finch & Collier 2000). From my personal experience, it can be said that a significant barrier to the wider adoption of ecological agriculture is increased management complexity and perceived higher risk relative to the continued use of chemical inputs.
Complexity and variability of responses to management is a common theme throughout this review. Current research and extension institutions are not well suited to deal with complexity. They were developed based upon a model of mechanistic research leading to management recommendations that were extended to different growing regions. This model is most successful when management technologies work across a broad range of locations and only require minor adjustments for specific contexts. (Note: management based on inputs of fertilizers, pesticides and water attenuates variation in resource availability, whereas ecologically based approaches need to accommodate variability.) Indeed, high input agriculture is most successful where environments are relatively uniform, well suited for crop production and large areas can be planted to similar crops, namely: irrigated rice in the lowlands of Asia; irrigated rice–wheat in South Asia; temperate maize-based cropping systems of the North American plains and the rain-fed wheat systems of northwest and central Europe (Cassman 1999). However, extension of the high input model into more heterogeneous, risk-prone and resource-limited systems as found in Africa and other areas in the developing world, has not worked well. This is not only due to a lack of capital but also to difficulty in developing broad recommendations for highly heterogeneous environments and associated diversity of crops grown. It is unlikely, therefore, that the same research–extension model would be effective for more diverse production systems that rely heavily on ecological processes rather than chemical inputs.
We face a conundrum: on the one hand to understand mechanisms that drive ecological interactions, researchers can only study a few variables at a time and try to control others; but in real farming situations, multiple variables interact in site-specific ways. On the other hand, on-farm research encompasses complexity but is both site- and farmer specific, and has to address attendant problems of uncontrolled confounding variables (Drinkwater et al. 1995). Clearly, it is impossible to research the effects of every combination of variables, and besides we already know from theoretical and empirical work that the outcomes of complex biotic interactions can be unpredictable and idiosyncratic (O'Neill et al. 1986; Hooper et al. 2005; Wardle et al. 2005; Thebault & Loreau 2006). This means that mechanistic research can only take us so far in the development of ecologically based management systems, and that adaptive experimentation and monitoring through farm-based trials are critical. Further, better monitoring of farmer experimentation could provide invaluable feedback and information to identify broad patterns of responses to managment (figure 1) and contribute to new theory (Deugd et al. 1998). Integrated systems experiments are intermediately controlled and replicated hybrids with aspects of mechanistic and on-farm research. A whole system approach is taken, but researchers are still limited to testing a few combinations of management strategies (chosen based on both researchers' mechanistic and farmer's experiential knowledge) and they are rarely done in multiple locations.
Figure 1.
A conceptual framework for systems of knowledge generation and information exchange to improve the success and implementation of ecologically based agricultural systems.
The bulk of the information discussed in this paper is derived from the first two boxes in figure 1. Synthesis, communication and adaptive experimentation and implementation all represent areas where greater investment of people and resources is needed. Owing to their inherent complexity, farmers themselves need to increase their understanding of ecological processes to better adapt management approaches for their own situations. This requires a greater emphasis on researcher/farmer partnerships to conduct field-based adaptive research and a greater investment in monitoring system performance as adaptations are made. Others have also recognized their need for innovative interdisciplinary partnerships and a shift from a research–extension–diffusion model to an interactive social learning process that incorporates both farmer and researcher knowledge (Barberi 2002; Roling et al. 2004; Anderson 2005; Cherr et al. 2006; Hobbs & Hilborn 2006; Warner 2006). Participatory research integrated with farmer education has more than 20 years of history in agricultural development, with some notable success such as IPM use in Asian rice systems (Matteson 2000). In Cameroon, lack of adoption of alley cropping led to a study where researcher-managed trials were compared against farmer- managed trials. Once farmers began to adapt the alley crop system, interest increased and the number of farmers testing the technology rose from 15 to 236 within 6 years (Kanmegne & Degrande 2002). A similar movement towards farmer/researcher teams has happened in the USA with some success. Interestingly, in California the greatest success at adoption of ecological practices has been in perennial systems (e.g. wine grapes, nut crops), but it has proved more difficult for annual crops where many growers lease land rather than own it (Warner 2006).
A current weakness, however, is that little monitoring of the performance of systems after they have been implemented and adapted is being done. This is a missed opportunity to get important feedback that could inform theory, research and extension. The depth of data collection could range from basic descriptive information to field-based data collection and experimentation, but if data is collected using agreed general protocols, it could more easily be used for meta-analysis and synthesis. Clearly, this would require a new level of communication and coordination, but is a task worth undertaking.
The importance of increased synthesis of information and its translation into effective communication tools for different audiences cannot be overstated. There are a number of ways to approach this. Use of meta-analysis provide invaluable information on the patterns observed across multiple studies (Lekberg & Koide 2005; Andow 1991b; Connolly et al. 2001) as do good synthetic review articles (Collier & Van Steenwyk 2004; Hol & Cook 2005; Hooper et al. 2005). These avenues target the research community, but materials better suited to farmers, the public and policy makers are also needed. The Ecological Society of America recognized this need and now produces a successful series of publications ranging from the in-depth ‘Ecological Monographs’, to the more populist ‘Issues in Ecology’ series (http://www.esa.org/publications/). There is, however, a particular need for improved avenues of bidirectional communication between (and among) those involved in farmer-based adaptive experimentation and institution-based academic researchers.
21. Conclusions
Our understanding of biotic interactions taking place in agroecosystems is growing rapidly, aided by the integration of ecological methodologies and ecologists into agricultural research. Important elements of understanding biotic interactions include the consideration of the effects of diversity, species composition and food web structure on ecosystem processes; the impacts of timing, frequency and intensity of disturbance; and the importance of multitrophic interactions. All of these elements need to be considered at multiple scales that depend in part on the range of the movement of the organisms involved. The potential for a greater use of ecological management approaches is high; however owing to the nature of ecosystems as medium number systems, there is some inherent unpredictability about responses to different management interventions that needs to be accommodated in the development of recommendations for farm management. This requires an increased emphasis on the effective synthesis of complex and often apparently contradictory information and a greater emphasis on field-based adaptive research that includes monitoring performance as adaptations are made, along with social learning mediated by farmer/researcher collaborations.
Footnotes
One contribution of 15 to a Theme Issue ‘Sustainable agriculture II’.
References
- Abassi P.A, Al-Dahmani J, Sahin F, Hoitink H.A.J, Miller S.A. Effect of compost amendments on disease severity and yield of tomato in conventional and organic production systems. Plant Dis. 2002;18:156–161. doi: 10.1094/PDIS.2002.86.2.156. doi:10.1094/PDIS.2002.86.2.156 [DOI] [PubMed] [Google Scholar]
- Abate T, van Huis A, Ampofo J.K.O. Pest management strategies in traditional agriculture, an African perspective. Annu. Rev. Entomol. 2000;45:631–659. doi: 10.1146/annurev.ento.45.1.631. doi:10.1146/annurev.ento.45.1.631 [DOI] [PubMed] [Google Scholar]
- Abawi G.S, Widmer T.L. Impact of soil health management practices on soilborne pathogens, nematodes and root diseases of vegetable crops. Appl. Soil Ecol. 2000;15:37–47. doi:10.1016/S0929-1393(00)00070-6 [Google Scholar]
- Adediran J.A, Adegbite A.A, Akinlosotu T.A, Agbaje G.O, Taiwo L.B, Owolade O.F, Oluwatosin G.A. Evaluation of fallow and cover crops for nematode suppression in three agroecologies of south western Nigeria. Afr. J. Biotechnol. 2005;4:1034–1039. [Google Scholar]
- Anderson R.L. Sequencing crops to minimize selection pressure for weeds in the central Great Plains. Weed Technol. 2004;18:157–164. doi:10.1614/WT-03-090R [Google Scholar]
- Anderson R.L. A multi-tactic approach to manage weed population dynamics in crop rotations. Agr. J. 2005;97:1579–1583. doi:10.2134/agronj2005.0194 [Google Scholar]
- Andow D.A. Yield loss to arthropods in vegetationally diverse agroecosystems. Environ. Entomol. 1991a;20:1228–1235. [Google Scholar]
- Andow D.A. Vegetational diversity and arthropod population response. Annu. Rev. Entomol. 1991b;36:561–586. doi:10.1146/annurev.en.36.010191.003021 [Google Scholar]
- Artursson V, Finlay R.D, Jansson J.K. Interactions between arbuscular mycorrhizal fungi and bacteria and their potential for stimulating plant growth. Environ. Microbiol. 2006;8:1–10. doi: 10.1111/j.1462-2920.2005.00942.x. doi:10.1111/j.1462-2920.2005.00942.x [DOI] [PubMed] [Google Scholar]
- Awmack C.S, Leather S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002;47:817–844. doi: 10.1146/annurev.ento.47.091201.145300. doi:10.1146/annurev.ento.47.091201.145300 [DOI] [PubMed] [Google Scholar]
- Banik P, Midya A, Sarkar B.K, Ghose S.S. Wheat and chickpea intercropping systems in an additive series experiment, advantages and weed smothering. Eur. J. Agron. 2006;24:325–332. [Google Scholar]
- Bao J, Fravel D, Lazarovits G, Chellemi D, van Berkum P, O'Neill N. Biocontrol genotypes of Fusarium oxysporum from tomato fields in Florida. Phytoparasitica. 2004;32:9–20. [Google Scholar]
- Barberi P. Weed management in organic agriculture, are we addressing the right issues? Weed Res. 2002;42:177–193. doi:10.1046/j.1365-3180.2002.00277.x [Google Scholar]
- Baumann D.T, Bastiaans L, Kropff M.J. Competition and crop performance in a leek–celery intercropping system. Crop Sci. 2001;41:764–774. [Google Scholar]
- Baumann D.T, Bastiaans L, Kropff M.J. Intercropping system optimization for yield, quality, and weed suppression combining mechanistic and descriptive models. Agron. J. 2002a;94:734–742. [Google Scholar]
- Baumann D.T, Bastiaans L, Goudriaan J, van Laar H.H, Kropff M.J. Analysing crop yield and plant quality in an intercropping system using an eco-physiological model for interplant competition. Agr. Syst. 2002b;73:173–203. doi:10.1016/S0308-521X(01)00084-1 [Google Scholar]
- Beanland L, Phelan L, Saliminen S. Micronutrient interactions on soybean growth and the developmental performance of three insect herbivores. Environ. Entomol. 2003;32:641–651. [Google Scholar]
- Bengtsson J, Ahnstrom J, Weibull A.C. The effects of organic agriculture on biodiversity and abundance, a meta-analysis. J. Appl. Ecol. 2005;42:261–269. doi:10.1111/j.1365-2664.2005.01005.x [Google Scholar]
- Berkelmans R, Ferris H, Tenuta M, van Bruggen A.H.C. Effects of long-term crop management on nematode trophic levels other than plant feeders disappear after 1 year of disruptive soil management. Appl. Soil Ecol. 2003;23:223–235. doi:10.1016/S0929-1393(03)00047-7 [Google Scholar]
- Bertholdsson N.O. Early vigour and allelopathy—two useful traits for enhanced barley and wheat competitiveness against weeds. Weed Res. 2005;45:94–102. doi:10.1111/j.1365-3180.2004.00442.x [Google Scholar]
- Bhowmik P.C, Inderjit S. Challenges and opportunities in implementing allelopathy for natural weed management. Crop Prot. 2003;22:661–671. doi:10.1016/S0261-2194(02)00242-9 [Google Scholar]
- Blok W.J, Lamers J.G, Termorshuizen A.J, Bollen G.J. Control of soilborne plant pathogens by incorporating fresh organic amendments followed by tarping. Phytopathology. 2000;90:253–259. doi: 10.1094/PHYTO.2000.90.3.253. doi:10.1094/PHYTO.2000.90.3.253 [DOI] [PubMed] [Google Scholar]
- Boody G, Vondracek B, Andow D.A, Krinke M, Westra J, Zimmerman J, Welle P. Multifunctional agriculture in the United States. Bioscience. 2005;55:27–38. doi:10.1641/0006-3568(2005)055[0027:MAITUS]2.0.CO;2 [Google Scholar]
- Bradford M.A, et al. Impacts of soil faunal community composition on model grassland ecosystems. Science. 2002;298:615–618. doi: 10.1126/science.1075805. doi:10.1126/science.1075805 [DOI] [PubMed] [Google Scholar]
- Brainard D.C, Bellinder R.R, Miller A.J. Cultivation and interseeding for weed control in transplanted cabbage. Weed Technol. 2004;18:704–710. doi:10.1614/WT-03-157R [Google Scholar]
- Brown B.J, Ewel J.J. Herbivory in complex and simple tropical successional ecosystems. Ecology. 1987;68:108–116. doi:10.2307/1938810 [Google Scholar]
- Brussaard L, Ferrera-Cerrato R. CRC/Lewis Publishers; Boca Raton, FL: 1997. Soil ecology in sustainable agricultural systems. [Google Scholar]
- Bruun T.B, Mertz O, Elberling B. Linking yields of upland rice in shifting cultivation to fallow length and soil properties. Agr. Ecosyst. Environ. 2006;113:139–149. doi:10.1016/j.agee.2005.09.012 [Google Scholar]
- Bugg R.L, Pickett C.H. Introduction, enhancing biological control—habitat management to promote natural enemies of agricultural pests. In: Pickett C.H, Bugg R.L, editors. Enhancing biological control. Habitat management to promote natural enemies of agricultural pests. The Regents of the University of California; Berkeley, CA: 1998. pp. 1–23. [Google Scholar]
- Bugg R.L, Ehler L.E, Wilson L.T. Effect of common knotweed (Polygonum aviculare) on abundance and efficiency of insect predators of crop pests. Hilgardia. 1987;55:1–51. [Google Scholar]
- Busch J.W, Phelan P.L. Mixture models of soybean growth and herbivore performance in response to nitrogen–sulphur–phosphorous interactions. Ecol. Entomol. 1999;24:132–145. doi:10.1046/j.1365-2311.1999.00185.x [Google Scholar]
- Caamal-Maldonado J.A, Jimenez-Osornio J.J, Torres-Barragan A, Anaya A.L. The use of allelopathic legume cover and mulch species for weed control in cropping systems. Agron. J. 2001;93:27–36. [Google Scholar]
- Cardinale B.J, Harvey C.T, Gross K, Ives A.R. Biodiversity and biocontrol, emergent impacts of a multi-enemy assemblage on pest suppression and crop yield in an agroecosystem. Ecol. Lett. 2003;6:857–865. doi:10.1046/j.1461-0248.2003.00508.x [Google Scholar]
- Cassman K.G. Ecological intensification of cereal production systems, yield potential, soil quality, and precision agriculture. Proc. Natl Acad. Sci. USA. 1999;96:5952–5959. doi: 10.1073/pnas.96.11.5952. doi:10.1073/pnas.96.11.5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton B.P, Cope A.E, Mortimer M. Growth traits of diverse rice cultivars under severe competition, implications for screening for competitiveness. Field Crop. Res. 2003;83:157–172. doi:10.1016/S0378-4290(03)00072-8 [Google Scholar]
- Chaney W. Biological control of aphids in lettice using in-field insectaries. In: Pickett C.H, Bugg R.L, editors. Enhancing biological control: habitat management to promote natural enemies of agricultural pests. The Regents of the University of California; Berkeley, CA: 1998. pp. 73–83. [Google Scholar]
- Cheng W.X, Johnson D.W, Fu S.L. Rhizosphere effects on decomposition, controls of plant species, phenology, and fertilization. Soil Sci. Soc. Am. J. 2003;67:1418–1427. [Google Scholar]
- Cherr C.M, Scholberg J.M.S, McSorley R. Green manure approaches to crop production, a synthesis. Agron. J. 2006;98:302–319. doi:10.2134/agronj2005.0035 [Google Scholar]
- Coleman D.C, Crossley D.A, Hendrix P.F. 2nd edn. Elsevier Academic Press; Amsterdam, The Netherlands; Boston, MA: 2004. Fundamentals of soil ecology. [Google Scholar]
- Collier T, Van Steenwyk R. A critical evaluation of augmentative biological control. Biol. Control. 2004;31:245–256. doi:10.1016/j.biocontrol.2004.05.001 [Google Scholar]
- Collins K.L, Boatman N.D, Wilcox A, Holland J.M, Chaney K. Influence of beetle banks on cereal, aphid predation in winter wheat. Agr. Ecosyst. Environ. 2002;93:337–350. doi:10.1016/S0167-8809(01)00340-1 [Google Scholar]
- Colunga-G M, Gage S.H, Dyer L.E. The insect community. In: Cavigelli M.A, Deming S.R, Probyn L.K, Harwood R.R, editors. Michigan field crop ecology, managing biological processes for productivity and environmental quality. Michigan State University Extension Bulletin E-2646. Michigan State University; East Lansing, MI: 1998. pp. 59–70. [Google Scholar]
- Connolly J, Goma H.C, Rahim K. The information content of indicators in intercropping research. Agr. Ecosyst. Environ. 2001;87:191–207. doi:10.1016/S0167-8809(01)00278-X [Google Scholar]
- Conway G. Comstock Publication Associates; Ithaca, NY: 1998. The doubly green revolution, food for all in the twenty-first century. [Google Scholar]
- Corbett A. The importance of movement in the response of natural emeies to habitat manipulation. In: Pickett C.H, Bugg R.L, editors. Enhancing biological control, habitat management to promote natural enemies of agricultural pests. University of California Press; Berkeley, CA: 1998. pp. 25–48. [Google Scholar]
- Cordier C, Gianinazzi S, Pearson V. Colonisation patterns of root tissues by Phytophthora nicotianae var. parasitica related to reduced disease in mycorrhizal tomato. Plant Soil. 1996;185:223–232. doi:10.1007/BF02257527 [Google Scholar]
- Craine J.M. Reconciling plant strategy theories of Grime and Tilman. J. Ecol. 2005;93:1041–1052. doi:10.1111/j.1365-2745.2005.01043.x [Google Scholar]
- Dapaah H.K, Asafu-Agyei J.N, Ennin S.A, Yamoah C. Yield stability of cassava, maize, soya bean and cowpea intercrops. J. Agr. Sci. 2003;140:73–82. doi:10.1017/S0021859602002770 [Google Scholar]
- Davis A.S, Renner K.A, Gross K.L. Weed seedbank and community shifts in a long-term cropping systems experiment. Weed Sci. 2005;53:296–306. doi:10.1614/WS-04-182 [Google Scholar]
- Davis A.S, Kathleen A.I, Hallett S.G, Renner K.A. Weed seed mortality in soils with contrasting agricultural management histories. Weed Sci. 2006;54:291–297. [Google Scholar]
- D'Emden F.H, Llewellyn R.S. No-tillage adoption decisions in southern Australian cropping and the role of weed management. Field Crop. Res. 2006;46:563–569. [Google Scholar]
- Dent D. CAB International; Wallingford, UK: 1991. Insect pest management. [Google Scholar]
- Deugd M, Roling N, Smaling E.M.A. A new praxeology for integrated nutrient management, facilitating innovation with and by farmers. Agr. Ecosyst. Environ. 1998;71:269–274. doi:10.1016/S0167-8809(98)00146-7 [Google Scholar]
- Di Falco S, Perrings C. Crop biodiversity, risk management and the implications of agricultural assistance. Ecol. Econ. 2005;55:459–466. doi:10.1016/j.ecolecon.2004.12.005 [Google Scholar]
- Doutt R.L, Nakata J. The rubus leafhopper and its egg parasitoid, an endemic biotic system useful in grape pest mangement. Environ. Entomol. 1973;2:381–386. [Google Scholar]
- Drechsel P, Steiner K.G, Hagedorn F. A review on the potential of improved fallows and green manure in Rwanda. Agroforest. Syst. 1996;33:109–136. doi:10.1007/BF00213645 [Google Scholar]
- Drinkwater L.E, Letourneau D.K, Workneh F, van Bruggen A.H.C, Shennan C. Fundamental differences between conventional and organic tomato agroecosystems in California. Ecol. Appl. 1995;5:1098–1112. doi:10.2307/2269357 [Google Scholar]
- Dyer L.A, Letourneau D. Top-down and bottom-up diversity cascades in detrital vs. living food webs. Ecol. Lett. 2003;6:60–68. doi:10.1046/j.1461-0248.2003.00398.x [Google Scholar]
- English-Loeb G, Norton A.P, Gadoury D.M, Seem R.C, Wilcox W.F. Control of powdery mildew in wild and cultivated grapes by a tydeid mite. Biol. Control. 1999;14:97–103. doi:10.1006/bcon.1998.0681 [Google Scholar]
- English-Loeb G, Rhainds M, Martinson T, Ugine T. Influence of flowering cover crops on Anagrus parasitoids (Hymenoptera, Mymaridae) and Erythroneura leafhoppers (Homoptera, Cicadellidae) in New York vineyards. Agr. Forest Entomol. 2003;5:173–181. doi:10.1046/j.1461-9563.2003.00179.x [Google Scholar]
- Evans S.P, Knezevic S.Z, Lindquist J.L, Shapiro C.A. Influence of nitrogen and duration of weed interference on corn growth and development. Weed Sci. 2003a;51:546–556. doi:10.1614/0043-1745(2003)051[0546:IONADO]2.0.CO;2 [Google Scholar]
- Evans S.P, Knezevic S.Z, Lindquist J.L, Shapiro C.A, Blankenship E.E. Nitrogen application influences the critical period for weed control in corn. Weed Sci. 2003b;51:408–417. doi:10.1614/0043-1745(2003)051[0408:NAITCP]2.0.CO;2 [Google Scholar]
- Farber S, et al. Linking ecology and economics for ecosystem management. Bioscience. 2006;56:121–133. doi:10.1641/0006-3568(2006)056[0121:LEAEFE]2.0.CO;2 [Google Scholar]
- Fargione J, Tilman D. Plant species traits and capacity for resource reduction predict yield and abundance under competition in nitrogen-limited grassland. Funct. Ecol. 2006;20:533–540. doi:10.1111/j.1365-2435.2006.01116.x [Google Scholar]
- Feeny P. Plant apparency and chemical defenses. In: Wallace J.W, Mansell R.L, editors. Biochemical interaction between plants and insects. Plenum Press; New York, NY: 1976. pp. 1–40. [Google Scholar]
- Ferris H, Venette R.C, Scow K.M. Soil management to enhance bacterivore and fungivore nematode populations and their nitrogen mineralisation function. Appl. Soil Ecol. 2004;25:19–35. doi:10.1016/j.apsoil.2003.07.001 [Google Scholar]
- Finan T.M, O'Brian M.R, Layzell D.B, Vessey J.K, Newton W, editors. Nitrogen fixation, global perspectives. CABI International; Wallingford, UK: 2002. [Google Scholar]
- Finch S, Collier R.H. Integrated pest management in field vegetable crops in northern Europe—with focus on two key pests. Crop Prot. 2000;19:817–824. doi:10.1016/S0261-2194(00)00109-5 [Google Scholar]
- Finke D.L, Denno R.F. Predator diversity and the functioning of ecosystems, the role of intraguild predation in dampening trophic cascades. Ecol. Lett. 2005;8:1299–1306. doi:10.1111/j.1461-0248.2005.00832.x [Google Scholar]
- Fitter A.H, Gilligan C.A, Hollingworth K, Kleczkowski A, Twyman R.M, Pitchford J.W. Biodiversity and ecosystem function in soil. Funct. Ecol. 2005;19:369–377. doi:10.1111/j.0269-8463.2005.00969.x [Google Scholar]
- Fravel D. Hurdles and bottlenecks on the road to biocontrol of plant pathogens. Aust. Plant Pathol. 1999;28:53–56. doi:10.1071/AP99007 [Google Scholar]
- Fravel D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005;43:337–359. doi: 10.1146/annurev.phyto.43.032904.092924. doi:10.1146/annurev.phyto.43.032904.092924 [DOI] [PubMed] [Google Scholar]
- Fravel D, Olivain C, Alabouvette C. Fusarium oxysporum and its biocontrol. New Phytol. 2003;157:493–502. doi: 10.1046/j.1469-8137.2003.00700.x. doi:10.1046/j.1469-8137.2003.00700.x [DOI] [PubMed] [Google Scholar]
- Friberg H, Lagerlof J, Ramert B. Influence of soil fauna on fungal plant pathogens in agricultural and horticultural systems. Biocontrol Sci. Technol. 2005;15:641–658. doi:10.1080/09583150500086979 [Google Scholar]
- Gahoonia T.S, Ali O, Sarker A, Rahman M.M, Erskine W. Root traits, nutrient uptake, multi-location grain yield and benefit–cost ratio of two lentil (Lens culinaris, Medikus.) varieties. Plant Soil. 2005;272:153–161. doi:10.1007/s11104-004-4573-x [Google Scholar]
- Gahoonia T.S, Ali O, Sarker A, Nielsen N.E, Rahman M.M. Genetic variation in root traits and nutrient acquisition of lentil genotypes. J. Plant Nutr. 2006;29:643–655. doi:10.1080/01904160600564378 [Google Scholar]
- Garbeva P, Postma J, van Veen J.A, van Elsas J.D. Effect of above-ground plant species on soil microbial community structure and its impact on suppression of Rhizoctonia solani AG3. Environ. Microbiol. 2006;8:233–246. doi: 10.1111/j.1462-2920.2005.00888.x. doi:10.1111/j.1462-2920.2005.00888.x [DOI] [PubMed] [Google Scholar]
- Georgis R, et al. Successes and failures in the use of parasitic nematodes for pest control. Biol. Control. 2006;38:103–123. doi:10.1016/j.biocontrol.2005.11.005 [Google Scholar]
- Gibson K.D, Fischer A.J, Foin T.C, Hill J.E. Crop traits related to weed suppression in water-seeded rice (Oryza sativa L.) Weed Sci. 2003;51:87–93. doi:10.1614/0043-1745(2003)051[0087:CTRTWS]2.0.CO;2 [Google Scholar]
- Girvan M.S, Bullimore J, Ball A.S, Pretty J.N, Osborn A.M. Responses of active bacterial and fungal communities in soils under winter wheat to different fertilizer and pesticide regimens. Appl. Environ. Microbiol. 2004;70:2692–2701. doi: 10.1128/AEM.70.5.2692-2701.2004. doi:10.1128/AEM.70.5.2692-2701.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gols R, Bukovinszky T, Hemerik L, Harvey J.A, Van Lenteren J.C, Vet L.E.M. Reduced foraging efficiency of a parasitoid under habitat complexity, implications for population stability and species coexistence. J. Anim. Ecol. 2005;74:1059–1068. doi:10.1111/j.1365-2656.2005.01003.x [Google Scholar]
- Gosling P, Hodge A, Goodlass G, Bending G.D. Arbuscular mycorrhizal fungi and organic farming. Agr. Ecosyst. Environ. 2006;113:17–35. doi:10.1016/j.agee.2005.09.009 [Google Scholar]
- Goud J.K.C, Termorshuizen A.J, Blok W.J, van Bruggen A.H.C. Long-term effect of biological soil disinfestation on Verticillium wilt. Plant Dis. 2004;88:688–694. doi: 10.1094/PDIS.2004.88.7.688. doi:10.1094/PDIS.2004.88.7.688 [DOI] [PubMed] [Google Scholar]
- Griffiths B.S, et al. Ecosystem response of pasture soil communities to fumigation-induced microbial diversity reductions, an examination of the biodiversity–ecosystem function relationship. Oikos. 2000;90:279–294. doi:10.1034/j.1600-0706.2000.900208.x [Google Scholar]
- Gu Y.-H, Mazzola M. Modification of fluorescent pseudomonad community and control of apple replant disease induced in a wheat cultivar-specific manner. Appl. Soil Ecol. 2003;24:57–72. doi:10.1016/S0929-1393(03)00066-0 [Google Scholar]
- Guthman J. Raising organic, an agroecological assesment of grower practices in California. Agr. Human Values. 2000;17:257–266. doi:10.1023/A:1007688216321 [Google Scholar]
- Hao J.J, Subbarao K.V, Koike S.T. Effects of broccoli rotation on lettuce drop caused by Sclerotinia minor and on the population density of sclerotia in soil. Plant Dis. 2003;87:159–166. doi: 10.1094/PDIS.2003.87.2.159. doi:10.1094/PDIS.2003.87.2.159 [DOI] [PubMed] [Google Scholar]
- Hartwig N.L, Ammon H.U. 50th Anniversary—invited article—cover crops and living mulches. Weed Sci. 2002;50:688–699. doi:10.1614/0043-1745(2002)050[0688:AIACCA]2.0.CO;2 [Google Scholar]
- Hedlund K, Griffiths B, Christensen S, Scheu S, Setala H, Tscharntke T, Verhoef H. Trophic interactions in changing landscapes, responses of soil food webs. Basic Appl. Ecol. 2004;5:495–503. doi:10.1016/j.baae.2004.09.002 [Google Scholar]
- Hiddink G.A, Bruggen A.H.C.v, Termorshuizen A.J, Raaijmakers J.M, Semenov A.V. Effect of organic management of soils on suppressiveness to Gaeumannomyces graminis var. tritici and its antagonist, Pseudomonas fluorescens. Eur. J. Plant Pathol. 2005;113:417–435. doi:10.1007/s10658-005-5402-7 [Google Scholar]
- Hobbs N.T, Hilborn R. Alternatives to statistical hypothesis testing in ecology, a guide to self teaching. Ecol. Appl. 2006;16:5–19. doi: 10.1890/04-0645. doi:10.1890/04-0645 [DOI] [PubMed] [Google Scholar]
- Hodge M.A. The implications of intraguild predation for the role of spiders in biological control. J. Arachnol. 1999;27:351–362. [Google Scholar]
- Hoffman, M. P., Ridgway, R. L., Show, E. D. & Matteoni, J. 1998 Practical application of mass-reared natural enemies, selected case histories. In Mass-reared natural enemies, application, regulation, and needs, (eds R. L. Ridgway, M. Hoffmann, M. Inscoe & C. Glenister), pp. 268–293. Thomas Say Publications in Entomology. Lanham, MD: Entomological Society of America.
- Hol W.H.G, Cook R. An overview of arbuscular mycorrhizal fungi–nematode interactions. Basic Appl. Ecol. 2005;6:489–503. doi:10.1016/j.baae.2005.04.001 [Google Scholar]
- Holland J.M, Thomas C.F.G, Birkett T, Southway S, Oaten H. Farm-scale spatiotemporal dynamics of predatory beetles in arable crops. J. Appl. Ecol. 2005;42:1140–1152. [Google Scholar]
- Holt-Gimenez E. Measuring farmers' agroecological resistance after Hurricane Mitch in Nicaragua, a case study in participatory, sustainable land management impact monitoring. Agr. Ecosyst. Environ. 2002;93:87–105. doi:10.1016/S0167-8809(02)00006-3 [Google Scholar]
- Hooks C.R.R, Johnson M.W. Impact of agricultural diversification on the insect community of cruciferous crops. Crop Prot. 2003;22:223–238. doi:10.1016/S0261-2194(02)00172-2 [Google Scholar]
- Hooper D.U, et al. Effects of biodiversity on ecosystem functioning, a consensus of current knowledge. Ecol. Monogr. 2005;75:3–35. doi:10.1890/04-0922 [Google Scholar]
- Idris A.B, Grafius E. Wildflowers as nectar sources for Diadegma insulare (Hymenoptera, Ichneumonidae), a parasitoid of diamondback moth (Lepidoptera, Yponomeutidae) Environ. Entomol. 1995;24:1726–1735. [Google Scholar]
- Jacas J.A, Urbaneja A, Vinuela E. History and future of introduction of exotic arthropod biological control agents in Spain, a dilemma? Biocontrol. 2006;51:1–30. doi:10.1007/s10526-005-5808-3 [Google Scholar]
- Jervis M.S, Kidd M.A.C, Fitton M.D, Huddleson T, Dawah H.A. Flower visiting by hymenopteran parasitoids. J. Nat. Hist. 1993;27:287–294. doi:10.1080/00222939300770051 [Google Scholar]
- Jetiyanon K, Kloepper J.W. Mixtures of plant growth-promoting rhizobacteria for induction of systemic resistance against multiple plant diseases. Biol. Control. 2002;24:285–291. doi:10.1016/S1049-9644(02)00022-1 [Google Scholar]
- Jetiyanon K, Fowler W.D, Kloepper J.W. Broad-spectrum protection against several pathogens by PGPR mixtures under field conditions in Thailand. Plant Dis. 2003;87:1390–1394. doi: 10.1094/PDIS.2003.87.11.1390. doi:10.1094/PDIS.2003.87.11.1390 [DOI] [PubMed] [Google Scholar]
- Ji P, Campbell H.L, Kloepper J.W, Jones J.B, Suslow T.V, Wilson M. Integrated biological control of bacterial speck and spot of tomato under field conditions using foliar biological control agents and plant growth-promoting rhizobacteria. Biol. Control. 2006;36:358–367. doi:10.1016/j.biocontrol.2005.09.003 [Google Scholar]
- Jordan C.F. Organic farming and agroforestry, alleycropping for mulch production for organic farms of southeastern United States. Agroforest. Syst. 2004;61:79–90. doi:10.1023/B:AGFO.0000028991.86647.35 [Google Scholar]
- Kagata H, Ohgushi T. Bottom-up trophic cascades and material transfer in terrestrial food webs. Ecol. Res. 2006;21:26–34. doi:10.1007/s11284-005-0124-z [Google Scholar]
- Kalkhoven J.T.R. Survival of populations and the scale of the fragmented agricultural landscape. In: Bunce R.G.H, Ryszkowski L, Paoletti M.G, editors. Landscape ecology and agroecosystems. Lewis Publishers; Boca Raton, FL: 1993. pp. 83–90. [Google Scholar]
- Kaneshiro L.N, Jones M.W. Tritrophic effects of leaf nitrogen on Liriomyza trifolii (Burgess) and an associated parasitoid Chrysocharis oscinidis (Ashmead) on bean. Biol. Control. 1996;6:186–192. doi:10.1006/bcon.1996.0023 [Google Scholar]
- Kanmegne J, Degrande A. From alley cropping to rotational fallow, farmers' involvement in the development of fallow management techniques in the humid forest zone of Cameroon. Agroforest. Syst. 2002;54:115–120. doi:10.1023/A:1015095320293 [Google Scholar]
- Khanh T.D, Chung M.I, Xuan T.D, Tawata S. The exploitation of crop allelopathy in sustainable agricultural production. J. Agr. Crop Sci. 2005;191:172–184. [Google Scholar]
- Kho R.M. A general tree–environment–crop interaction equation for predictive understanding of agroforestry systems. Agr. Ecosyst. Environ. 2000;80:87–100. doi:10.1016/S0167-8809(00)00136-5 [Google Scholar]
- Kimberling D.N. Lessons from history, predicting successes and risks of intentional introductions for arthropod biological control. Bio. Invas. 2004;6:301–318. doi:10.1023/B:BINV.0000034599.09281.58 [Google Scholar]
- King A.W. Considerations of scale and hierarchy. In: Woodley S, Kay J, Francis G, editors. Ecological integrity and the management of ecosystems. St Lucie Press; Delray, FL: 1992. pp. 19–46. [Google Scholar]
- King E.G, Hopper K.R, Powell J.E. Analysis of systems for biological control of crop arthropod pests in the U.S. by augmentation of predators and parasites. In: Hoy M.A, Herzog D.C, editors. Biological control in agricultural IPM systems. Academic Press; Orlando, FL; London, UK: 1985. pp. 201–228. [Google Scholar]
- Kloepper J.W, Rodriguez-Kabana R, Zehnder G.W, Murphy J.F, Sikora E, Fernandez C. Plant root–bacterial interactions in biological control of soilborne diseases and potential extension to systemic and foliar diseases. Aust. Plant Pathol. 1999;28:21–26. doi:10.1071/AP99003 [Google Scholar]
- Kloepper J.W, Ryu C.M, Zhang S.A. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 2004;94:1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259. doi:10.1094/PHYTO.2004.94.11.1259 [DOI] [PubMed] [Google Scholar]
- Knezevic S.Z, Evans S.P, Blankenship E.E, Van Acker R.C, Lindquist J.L. Critical period for weed control, the concept and data analysis. Weed Sci. 2002;50:773–786. doi:10.1614/0043-1745(2002)050[0773:CPFWCT]2.0.CO;2 [Google Scholar]
- Kokalis-Burelle N, Martinez-Ochoa N, Rodriguez-Kabana v, Kloepper J.W. Development of multi-component transplant mixes for suppression of Meloidogyne incognita on tomato (Lycopersicon esculentum) J. Nemat. 2002;34:362–369. [PMC free article] [PubMed] [Google Scholar]
- Kranz J. Interactions in pest complexes and their effects on yield. Zeitschrift Fur Pflanzenkrankheiten Und Pflanzenschutz-J. Plant Dis. Prot. 2005;112:366–385. [Google Scholar]
- Kristensen L, Olsen J, Weiner J, Griepentrog H.W, Norremark M. Describing the spatial pattern of crop plants with special reference to crop–weed competition studies. Field Crop. Res. 2006;96:207–215. doi:10.1016/j.fcr.2005.07.004 [Google Scholar]
- Landis D.A, Menalled F.D. Ecological considerations in the conservation of effective parasitoid communities in agricultural systems. In: Barbosa P, editor. Conservation biological control. Academic Press; San Diego, CA: 1998. pp. 101–122. [Google Scholar]
- Landis J.N, Sanchez J.E, Bird G.W, Edson C.E, Isaacs R, Lehnert R.H, Schilder A.M.C, Swinton S.M. Michigan State University; East Lansing, MI: 2002. Fruit crop ecology and management extension bulletin E-2759. [Google Scholar]
- Landis D.A, Menalled F.D, Costamagna A.C, Wilkinson T.K. Manipulating plant resources to enhance beneficial arthropods in agricultural landscapes. Weed Sci. 2005;53:902–908. doi:10.1614/WS-04-050R1.1 [Google Scholar]
- Lang A. Intraguild interference and biocontrol effects of generalist predators in a winter wheat field. Oecologia. 2003;134:144–153. doi: 10.1007/s00442-002-1091-5. doi:10.1007/s00442-002-1091-5 [DOI] [PubMed] [Google Scholar]
- Lavandero B, Wratten S.D, Didham R.K, Gurr G. Increasing floral diversity for selective enhancement of biological control agents, a double-edged sward? Basic Appl. Ecol. 2006;7:236–243. doi:10.1016/j.baae.2005.09.004 [Google Scholar]
- Lavelle P, Spain A.V. Kluwer Academic Publishers; Dordrecht, The Netherlands; Boston, MA: 2001. Soil ecology. [Google Scholar]
- Lekberg Y, Koide R.T. Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol. 2005;168:189–204. doi: 10.1111/j.1469-8137.2005.01490.x. doi:10.1111/j.1469-8137.2005.01490.x [DOI] [PubMed] [Google Scholar]
- Lemerle D, Verbeek B, Orchard B. Ranking the ability of wheat varieties to compete with Lolium rigidum. Weed Res. 2001;41:197–209. doi:10.1046/j.1365-3180.2001.00232.x [Google Scholar]
- Lemerle D, Cousens R.D, Gill G.S, Peltzer S.J, Moerkerk M, Murphy C.E, Collins D, Cullis B.R. Reliability of higher seeding rates of wheat for increased competitiveness with weeds in low rainfall environments. J. Agr. Sci. 2004;142:395–409. doi:10.1017/S002185960400454X [Google Scholar]
- Letourneau D.K. Conserving biology, lessons for conserving natural enemies. In: Barbosa P, editor. Conservation biological control. Academic Press; San Diego, CA: 1998. pp. 9–38. [Google Scholar]
- Letourneau D.K, Dyer L.E. Experimental test in lowland tropical forest shows top-down effects through four trophic levels. Ecology. 1997;79:1678–1687. [Google Scholar]
- Liebman M, Davis A.S. Integration of soil, crop and weed management in low-external-input farming systems. Weed Res. 2000;40:27–47. doi:10.1046/j.1365-3180.2000.00164.x [Google Scholar]
- Liiri M, Setala H, Haimi J, Pennanen T, Fritze H. Soil processes are not influenced by the functional complexity of soil decomposer food webs under disturbance. Soil Biol. Biochem. 2002;34:1009–1020. doi:10.1016/S0038-0717(02)00034-2 [Google Scholar]
- Loomis R.S, Connor D.J. Cambridge University Press; Cambridge, UK; New York, NY: 1992. Crop ecology, productivity and management in agricultural systems. [Google Scholar]
- Lovell S.T, Sullivan W.C. Environmental benefits of conservation buffers in the United States, evidence, promise, and open questions. Agr. Ecosyst. Environ. 2006;112:249–260. doi:10.1016/j.agee.2005.08.002 [Google Scholar]
- MacArthur R.H, Wilson E.O. An equilibrium theory of insular zoogeography. Evolution. 1963;17:373–387. doi:10.2307/2407089 [Google Scholar]
- MacArthur R.H, Wilson E.O. Princeton University Press; Princeton, NJ: 1967. The theory of island biogeography. [Google Scholar]
- Marshall E.J.P, Brown V.K, Boatman N.D, Lutman P.J.W, Squire G.R, Ward L.K. The role of weeds in supporting biological diversity within crop fields. Weed Res. 2003;43:77–89. doi:10.1046/j.1365-3180.2003.00326.x [Google Scholar]
- Matteson P.C. Insect pest management in tropical Asian irrigated rice. Annu. Rev. Entomol. 2000;45:549–574. doi: 10.1146/annurev.ento.45.1.549. doi:10.1146/annurev.ento.45.1.549 [DOI] [PubMed] [Google Scholar]
- Mazzola M. Assessment and management of soil microbial community structure for disease suppression. Annu. Rev. Phytopathol. 2004;42:35–59. doi: 10.1146/annurev.phyto.42.040803.140408. doi:10.1146/annurev.phyto.42.040803.140408 [DOI] [PubMed] [Google Scholar]
- Mead R, Riley J, Dear K, Singh S.P. Stability comparison of intercropping and monocropping systems. Biometrics. 1986;42:253–266. doi:10.2307/2531048 [Google Scholar]
- Menalled F.D, Marino P.C, Gage S.H, Landis D. Does agricultural landscape structure affect parasitism and parasitoid diversity? Ecol. Appl. 1999;9:634–641. [Google Scholar]
- Menalled F.D, Gross K.L, Hammond M. Weed aboveground and seedbank community responses to agricultural management systems. Ecol. Appl. 2001;11:1586–1601. [Google Scholar]
- Mertz O. The relationship between length of fallow and crop yields in shifting cultivation, a rethinking. Agroforest. Syst. 2002;55:149–159. doi:10.1023/A:1020507631848 [Google Scholar]
- Metzger J.P. Effects of slash-and-burn fallow periods on landscape structure. Environ. Conserv. 2003;30:325–333. doi:10.1017/S0376892903000341 [Google Scholar]
- Mikola J, Sulkava P. Responses of microbial-feeding nematodes to organic matter distribution and predation in experimental soil habitat. Soil Biol. Biochem. 2001;33:811–817. doi:10.1016/S0038-0717(00)00229-7 [Google Scholar]
- Miller M.H. Arbuscular mycorrhizae and the phosphorus nutrition of maize, a review of Guelph studies. Can. J. Plant Sci. 2000;80:47–52. [Google Scholar]
- Montoya J.M, Rodriguez M.A, Hawkins B.A. Food web complexity and higher-level ecosystem services. Ecol. Lett. 2003;6:587–593. doi:10.1046/j.1461-0248.2003.00469.x [Google Scholar]
- Moorcroft D, Whittingham M.J, Bradbury R.B, Wilson J.D. The selection of stubble fields by wintering granivorous birds reflects vegetation cover and food abundance. J. Appl. Ecol. 2002;39:535–547. doi:10.1046/j.1365-2664.2002.00730.x [Google Scholar]
- Mundt C.C. Use of multiline cultivars and cultivar mixtures for disease management. Annu. Rev. Phytopathol. 2002;40:381–400. doi: 10.1146/annurev.phyto.40.011402.113723. doi:10.1146/annurev.phyto.40.011402.113723 [DOI] [PubMed] [Google Scholar]
- Murphy C.E, Lemerle D. Continuous cropping systems and weed selection. Euphytica. 2006;148:61–73. doi:10.1007/s10681-006-5941-9 [Google Scholar]
- Murphy B.C, Rosenheim J.A, Granett J, Pickett C.H, Dowell R.V. Measuring the impact of a natural enemy refuge, the prune tree/vineyard example. In: Pickett C.H, Bugg R.L, editors. Enhancing biological control, habitat management to promote natural enemies of agricultural pests. University of California Press; Berkeley, CA: 1998. pp. 297–309. [Google Scholar]
- Murphy S.D, Clements D.R, Belaoussoff S, Kevan P.G, Swanton C.J. Promotion of weed species diversity and reduction of weed seedbanks with conservation tillage and crop rotation. Weed Sci. 2006;54:69–77. doi:10.1614/WS-04-125R1.1 [Google Scholar]
- Ni H.W, Zhang C.X. Use of allelopathy for weed management in China—a review. Allelopath. J. 2005;15:3–11. [Google Scholar]
- Nicholls C.I, Parrella M, Altieri M.A. The effects of a vegetational corridor on the abundance and dispersal of insect biodiversity within a northern California organic vineyard. Lands Ecol. 2001;16:133–146. doi:10.1023/A:1011128222867 [Google Scholar]
- Norman J.R, Hooker J.E. Sporulation of Phytophthora fragariae shows greater stimulation by exudates of non-mycorrhizal than by mycorrhizal strawberry roots. Mycol. Res. 2000;104:1069–1073. doi:10.1017/S0953756299002191 [Google Scholar]
- Norman J.R, Atkinson D, Hooker J.E. Arbuscular mycorrhizal fungal-induced alteration to root architecture in strawberry and induced resistance to the root pathogen Phytophthora fragariae. Plant Soil. 1996;185:191–198. doi:10.1007/BF02257524 [Google Scholar]
- Norris R.E. Ecological bases of interactions between weeds and organisms in other pest categories. Weed Sci. 2005;53:909–913. doi:10.1614/WS-04-048R1.1 [Google Scholar]
- Norris R.F, Kogan M. Ecology of interactions between weeds and arthropods. Annu. Rev. Entomol. 2005;50:479–503. doi: 10.1146/annurev.ento.49.061802.123218. doi:10.1146/annurev.ento.49.061802.123218 [DOI] [PubMed] [Google Scholar]
- Norton A.P, English-Loeb G, Gadoury D, Seem R.C. Mycophagous mites and foliar pathogens, Leaf domatia mediate tritrophic interactions in grapes. Ecology. 2000;81:490–499. [Google Scholar]
- Nurse R.E, DiTommaso A. Corn competition alters the germinability of velvetleaf (Abutilon theophrasti) seeds. Weed Sci. 2005;53:479–488. doi:10.1614/WS-04-185R1 [Google Scholar]
- Obale-Ebanga F, Sevink J, de Groot W, Nolte C. Myths of slash and burn on physical degradation of savannah soils, impacts on Vertisols in north Cameroon. Soil Use Manage. 2003;19:83–86. doi:10.1079/SUM2003083 [Google Scholar]
- Office of Technology Assessment. United States Congress; Washington, DC: 1995. Biologically based technologies for pest control. [Google Scholar]
- Olsen J, Kristensen L, Weiner J. Effects of density and spatial pattern of winter wheat on suppression of different weed species. Weed Sci. 2005a;53:690–694. doi:10.1614/WS-04-144R2.1 [Google Scholar]
- Olsen J, Kristensen L, Weiner J, Griepentrog H.W. Increased density and spatial uniformity increase weed suppression by spring wheat. Weed Res. 2005b;45:316–321. doi:10.1111/j.1365-3180.2005.00456.x [Google Scholar]
- O'Neill R.V, DeAngelis D.L, Wade J.B, Allen T.F.H. Princeton University Press; Princeton, NJ: 1986. A hierarchical concept of ecosystems. [Google Scholar]
- Ong C.K, Kho R.M, Radersmaa S. Ecological interactions in multispecies agroecosystems, concepts and rules. In: van Noordwijk M, Cadish G, Ong C.K, editors. Below–ground interactions in tropical agroecosystems, concepts and models with multiple plant components. CABI Publications; Wallingford, UK; Cambridge, MA: 2004. pp. 1–15. [Google Scholar]
- Ormerod S.J, Marshall E.J.P, Kerby G, Rushton S.P. Meeting the ecological challenges of agricultural change, editors' introduction. J. Appl. Ecol. 2003;40:939–946. doi:10.1111/j.1365-2664.2003.00872.x [Google Scholar]
- Paine R.T. Intertidal community structure, experimental studies on the relationship between a dominant competitor and its principal predator. Oecologia. 1974;15:93–120. doi: 10.1007/BF00345739. doi:10.1007/BF00345739 [DOI] [PubMed] [Google Scholar]
- Parella M.P, Heintz K.M, Nunney L. Biological control through augmentative releases of natural enemies, a strategy whose time has come. Anim. Entomol. 1992;38:172–179. [Google Scholar]
- Peoples M.B, Giller K.E, Herridge D.F, Vessey J.K. Limitations of biological nitrogen fixation as a renewable source of nitrogen for agriculture. In: Finan T.M, O'Brian M.R, Layzell D.B, Vessey J.K, Newton W, editors. Nitrogen fixation, global perspectives. CABI International; Wallingford, UK: 2002. pp. 356–360. [Google Scholar]
- Phillips S.L, Wolfe M.S. Evolutionary plant breeding for low input systems. J. Agr. Sci. 2005;143:245–254. doi:10.1017/S0021859605005009 [Google Scholar]
- Plenchette C, Clermont-Dauphin C, Meynard J.M, Fortin J.A. Managing arbuscular mycorrhizal fungi in cropping systems. Can. J. Plant Sci. 2005;85:31–40. [Google Scholar]
- Poggio S.L. Structure of weed communities occurring in monoculture and intercropping of field pea and barley. Agr. Ecosyst. Environ. 2005;109:48–58. doi:10.1016/j.agee.2005.02.019 [Google Scholar]
- Pollard E. Hedges. VI. Habitat diversity and crop pests, a study of Brevicoryne brassica and its syrphid predators. J. Appl. Ecol. 1971;8:751–780. doi:10.2307/2402682 [Google Scholar]
- Power M.E. Effect of fish in river food webs. Science. 1990;250:411–415. doi: 10.1126/science.250.4982.811. doi:10.1126/science.250.4982.811 [DOI] [PubMed] [Google Scholar]
- Prasad R.P, Snyder W.E. Predator interference limits fly egg biological control by a guild of ground-active beetles. Biol. Control. 2004;31:428–437. doi:10.1016/j.biocontrol.2004.07.005 [Google Scholar]
- Price P.W. 3rd edn. Wiley; New York, NY: 1997. Insect ecology. [Google Scholar]
- Prieur-Richard A.H, Lavorel S, Linhart Y.B, Dos Santos A. Plant diversity, herbivory and resistance of a plant community to invasion in Mediterranean annual communities. Oecologia. 2002;130:96–104. doi: 10.1007/s004420100774. doi:10.1007/s004420100774 [DOI] [PubMed] [Google Scholar]
- Pullaro T.C, Marino P.C, Jackson D.M, Harrison H.F, Keinath A.P. Effects of killed cover crop mulch on weeds, weed seeds, and herbivores. Agr. Ecosyst. Environ. 2006;115:97–104. doi:10.1016/j.agee.2005.12.021 [Google Scholar]
- Purtauf T, Roschewitz I, Dauber J, Thies C, Tscharntke T, Wolters V. Landscape context of organic and conventional farms, Influences on carabid beetle diversity. Agr. Ecosyst. Environ. 2005;108:165–174. doi:10.1016/j.agee.2005.01.005 [Google Scholar]
- Pyrowolakis A, Westphal A, Sikora R.A, Becker J.O. Identification of root-knot nematode suppressive soils. Appl. Soil Ecol. 2002;19:51–56. doi:10.1016/S0929-1393(01)00170-6 [Google Scholar]
- Raaijmakers J.M, Vlami M, de Souza J.T. Antibiotic production by bacterial biocontrol agents. A. Van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2002;81:537–547. doi: 10.1023/a:1020501420831. doi:10.1023/A:1020501420831 [DOI] [PubMed] [Google Scholar]
- Ramamoorthy V, Viswanathan R, Raguchander T, Prakasam V, Samiyappan R. Induction of systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases. Crop Prot. 2001;20:1–11. doi:10.1016/S0261-2194(00)00056-9 [Google Scholar]
- Rao M.R, Nair P.K.R, Ong C.K. Biophysical interactions in tropical agroforestry systems. Agroforest. Syst. 1997;38:3–50. doi:10.1023/A:1005971525590 [Google Scholar]
- Reeleder R.D. Fungal plant pathogens and soil biodiversity. Can. J. Soil Sci. 2003;83:331–336. [Google Scholar]
- Reeves D.W, Price A.J, Patterson M.G. Evaluation of three winter cereals for weed control in conservation-tillage nontransgenic cotton. Weed Technol. 2005;19:731–736. doi:10.1614/WT-04-245R1.1 [Google Scholar]
- Rengel Z, Marschner P. Nutrient availability and management in the rhizosphere, exploiting genotypic differences. New Phytol. 2005;168:305–312. doi: 10.1111/j.1469-8137.2005.01558.x. doi:10.1111/j.1469-8137.2005.01558.x [DOI] [PubMed] [Google Scholar]
- Rhoades D.F, Gates R.G. Toward a general theory of plant anti-herbivore chemistry. In: Wallace J.W, Mansell R.L, editors. Biochemical interaction between plants and insects. Plenum Press; New York, NY: 1976. pp. 168–213. [Google Scholar]
- Ricketts T.H, Daily G.C, Ehrlich P.R, Fay J.P. Countryside biogeography of moths in a fragmented landscape, biodiversity in native and agricultural habitats. Conserv. Biol. 2001;15:378–388. doi:10.1046/j.1523-1739.2001.015002378.x [Google Scholar]
- Rodriguez M.A, Hawkins B.A. Diversity, function and stability in parasitoid communities. Ecol. Lett. 2000;3:35–40. doi:10.1046/j.1461-0248.2000.00115.x [Google Scholar]
- Roling N.G, Hounkonnou D, Offei S.K, Tossou R, Van Huis A. Linking science and farmers' innovative capacity, diagnostic studies from Ghana and Benin. Njas-Wageningen J. Life Sci. 2004;52:211–235. [Google Scholar]
- Roschewitz I, Gabriel D, Tscharntke T, Thies C. The effects of landscape complexity on arable weed species diversity in organic and conventional farming. J. Appl. Ecol. 2005a;42:873–882. doi:10.1111/j.1365-2664.2005.01072.x [Google Scholar]
- Roschewitz I, Hucker M, Tscharntke T, Thies C. The influence of landscape context and farming practices on parasitism of cereal aphids. Agr. Ecosyst. Environ. 2005b;108:218–227. doi:10.1016/j.agee.2005.02.005 [Google Scholar]
- Rosenheim J.A, Limburg D.D, Colfer R.G. Impact of generalist predators on a biological control agent, Chrysoperla carnea, direct observations. Ecol. Appl. 1999;9:409–417. [Google Scholar]
- Ruess L, Schmidt I.K, Michelsen A, Jonasson S. Responses of nematode species composition to factorial addition of carbon, fertiliser, bactericide and fungicide at two sub-arctic sites. Nematology. 2002;4:527–539. doi:10.1163/156854102760290509 [Google Scholar]
- Sabaratnam S, Traquair J.A. Formulation of a Streptomyces biocontrol agent for the suppression of Rhizoctonia damping-off in tomato transplants. Biol. Control. 2002;23:245–253. doi:10.1006/bcon.2001.1014 [Google Scholar]
- SAN. 2nd edn. National Agricultural Library; Beltsville, MD: 1998. Managing cover crops profitably. [Google Scholar]
- Sanchez P.A. Science in agroforestry. Agroforest. Syst. 1995;30:5–55. doi:10.1007/BF00708912 [Google Scholar]
- Sarandon S.J, Sarandon R. Mixture of cultivars—pilot field trial of an ecological alternative to improve production or quality of wheat (Triticum aestivum) J. Appl. Ecol. 1995;32:288–294. doi:10.2307/2405096 [Google Scholar]
- Schlesinger W.H. 2nd edn. Academic Press; San Diego, CA: 1997. Biogeochemistry, an analysis of global change. [Google Scholar]
- Schmidt M.H, Roschewitz I, Thies C, Tscharntke T. Differential effects of landscape and management on diversity and density of ground-dwelling farmland spiders. J. Appl. Ecol. 2005;42:281–287. doi:10.1111/j.1365-2664.2005.01014.x [Google Scholar]
- Schroeder J, Thomas S.H, Murray L.W. Impacts of crop pests on weeds and weed–crop interactions. Weed Sci. 2005;53:918–922. doi:10.1614/WS-04-052R1.1 [Google Scholar]
- Schweiger O, et al. Quantifying the impact of environmental factors on arthropod communities in agricultural landscapes across organizational levels and spatial scales. J. Appl. Ecol. 2005;42:1129–1139. doi:10.1111/j.1365-2664.2005.01085.x [Google Scholar]
- Seem J.E, Creamer N.G, Monks D.W. Critical weed-free period for ‘Beauregard’ sweetpotato (Ipomoea batatas) Weed Technol. 2003;17:686–695. doi:10.1614/WT02-089 [Google Scholar]
- Settle, W. H., Ariawan, H., Astuti, E. T., Cahyana, W., Hakim, A. L., Hindayana, D., Lestari, A. S., Pajarningsih & Sartanto 1996 Managing tropical rice pests through conservation of generalist natural enemies and alternative prey. Ecology77, 1975–1988. (doi:10.2307/2265694)
- Sheng X.F, He L.Y. Solubilization of potassium-bearing minerals by a wildtype strain of Bacillus edaphicus and its mutants and increased potassium uptake by wheat. Can. J. Microbiol. 2006;52:66–72. doi: 10.1139/w05-117. doi:10.1139/w05-117 [DOI] [PubMed] [Google Scholar]
- Shennan C, Bode C.A. Integrating wetland habitat with agriculture. In: Jackson L.L, Jackson D, editors. The farm as a natural habitat. Island Press; Washington, DC: 2002. pp. 189–204. [Google Scholar]
- Shennan C, Cecchettini C.L, Goldman G.B, Zalom F.G. Profiles of California farmers by degree of IPM use as indicated by self-descriptions in a phone survey. Agr. Ecosyst. Environ. 2001;84:267–275. doi:10.1016/S0167-8809(00)00248-6 [Google Scholar]
- Shennan C, Pisani Gareau T, Sirrine J.R. Agroecological approaches to pest management in the US. In: Pretty J, editor. The pesticide detox, solutions for safe agriculture. Earthscan Publications Ltd; London, UK: 2004. pp. 193–211. [Google Scholar]
- Shirmohammadi A, Djodjic F, Bergstrom L. Scaling issues in sustainable management of nutrient losses. Soil Use Manage. 2005;21:160–166. [Google Scholar]
- Snapp S.S, Swinton S.M, Labarta R, Mutch D, Black J.R, Leep R, Nyiraneza J, O'Neil K. Evaluating cover crops for benefits, costs and performance within cropping system niches. Agron. J. 2005;97:322–332. [Google Scholar]
- Snyder W.E, Ives A.R. Generalist predators disrupt biological control by a specialist parasitoid. Ecology. 2001;82:705–716. [Google Scholar]
- Snyder W.E, Snyder G.B, Finke D.L, Straub C.S. Predator biodiversity strengthens herbivore suppression. Ecol. Lett. 2006;9:789–796. doi: 10.1111/j.1461-0248.2006.00922.x. doi:10.1111/j.1461-0248.2006.00922.x [DOI] [PubMed] [Google Scholar]
- Sosnoskie L.M, Herms N.P, Cardina J. Weed seedbank community composition in a 35-yr-old tillage and rotation experiment. Weed Sci. 2006;54:263–273. [Google Scholar]
- Stern V.M, van den Bosch R, Leigh T.F. Strip cutting of alfalfa for lygus bug control. Calif. Agr. 1964;18:4–6. [Google Scholar]
- Straub C.S, Snyder W.E. Species identity dominates the relationship between predator biodiversity and herbivore suppression. Ecology. 2006;87:277–282. doi: 10.1890/05-0599. doi:10.1890/05-0599 [DOI] [PubMed] [Google Scholar]
- Subramanian K.S, Santhanakrishnan P, Balasubramanian P. Responses of field grown tomato plants to arbuscular mycorrhizal fungal colonization under varying intensities of drought stress. Sci. Hort. 2006;107:245–253. doi:10.1016/j.scienta.2005.07.006 [Google Scholar]
- Susilo F.X, Neutel A.M, van Noordwijk M, Hairiah K, Brown G, Swift M.J. Soil biodiversity and food webs. In: van Noordwijk M, Cadisch G, Ong C.K, editors. Below–ground interactions in tropical agroecosystems, concepts and models in multiple plant component systems. CABI Publishing; Wallingford, UK; Cambridge, MA: 2004. pp. 285–307. [Google Scholar]
- Szumigalski A, Van Acker R. Weed suppression and crop production in annual intercrops. Weed Sci. 2005;53:813–825. doi:10.1614/WS-05-014R.1 [Google Scholar]
- Thebault E, Loreau M. The relationship between biodiversity and ecosystem functioning in food webs. Ecol. Res. 2006;21:17–25. doi:10.1007/s11284-005-0127-9 [Google Scholar]
- Thevathasan N.V, Gordon A.M. Ecology of tree intercropping systems in the north temperate region, experiences from southern Ontario, Canada. Agroforest. Syst. 2004;61:257–268. doi:10.1023/B:AGFO.0000029003.00933.6d [Google Scholar]
- Thiaw S, Hall A.E, Parker D.R. Varietal intercropping and the yields and stability of cowpea production in semiarid Senegal. Field Crop. Res. 1993;33:217–233. doi:10.1016/0378-4290(93)90081-W [Google Scholar]
- Thies C, Tscharntke T. Landscape structure and biological control in agroecosystems. Science. 1999;285:893–895. doi: 10.1126/science.285.5429.893. doi:10.1126/science.285.5429.893 [DOI] [PubMed] [Google Scholar]
- Thomas S.H, Schroeder J, Murray L.W. The role of weeds in nematode management. Weed Sci. 2005;53:923–928. doi:10.1614/WS-04-053R.1 [Google Scholar]
- Tian G, Kang B.T, Kolawole G.O, Idinoba P, Salako F.K. Long-term effects of fallow systems and lengths on crop production and soil fertility maintenance in West Africa. Nutr. Cycl. Agroecosyst. 2005;71:139–150. doi:10.1007/s10705-004-1927-y [Google Scholar]
- Tilman D, Polasky S, Lehman C. Diversity, productivity and temporal stability in the economies of humans and nature. J. Environ. Econ. Manage. 2005;49:405–426. doi:10.1016/j.jeem.2004.03.008 [Google Scholar]
- Timms-Wilson T.M, Kilshaw K, Bailey M.J. Risk assessment for engineered bacteria used in biocontrol of fungal disease in agricultural crops. Plant Soil. 2004;266:57–67. doi:10.1007/s11104-005-2567-y [Google Scholar]
- Tompkins D.K, Wright A.T, Fowler D.B. Foliar disease development in no-till winter wheat, influence of agronomic practices on powdery mildew development. Can. J. Plant Sci. 1992;72:965–972. [Google Scholar]
- Trenbath B.R. Multispecies cropping systems in India—predictions of their productivity, stability, resilience and ecological sustainability. Agroforest. Syst. 1999;45:81–107. doi:10.1023/A:1006285319817 [Google Scholar]
- Trewavas A. A critical assessment of organic farming-and-food assertions with particular respect to the UK and the potential environmental benefits of no-till agriculture. Crop Prot. 2004;23:757–781. doi:10.1016/j.cropro.2004.01.009 [Google Scholar]
- Tscharntke T, Klein A.M, Kruess A, Steffan-Dewenter I, Thies C. Landscape perspectives on agricultural intensification and biodiversity—ecosystem service management. Ecol. Lett. 2005;8:857–874. doi:10.1111/j.1461-0248.2005.00782.x [Google Scholar]
- Vallad G.E, Goodman R.M. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci. 2004;44:1920–1934. [Google Scholar]
- van Bruggen A.H.C, Grunwald N.J. The need for a dual hierarchical approach to study plant disease suppression. Appl. Soil Ecol. 1994;1:91–95. doi:10.1016/0929-1393(94)90029-9 [Google Scholar]
- van Bruggen A.H.C, Semenov A.M. In search of biological indicators for soil health and disease suppression. Appl. Soil Ecol. 2000;15:13–24. doi:10.1016/S0929-1393(00)00068-8 [Google Scholar]
- van Groenigen J.W, Burns E.G, Eadie J.M, Horwath W.R, van Kessel C. Effects of foraging waterfowl in winter flooded rice fields on weed stress and residue decomposition. Agricult. Ecosys Environ. 2003;95:289–296. doi:10.1016/S0167-8809(02)00097-X [Google Scholar]
- van Lenteren J.C, Bale J, Bigler E, Hokkanen H.M.T, Loomans A.M. Assessing risks of releasing exotic biological control agents of arthropod pests. Annu. Rev. Entomol. 2006;51:609–634. doi: 10.1146/annurev.ento.51.110104.151129. doi:10.1146/annurev.ento.51.110104.151129 [DOI] [PubMed] [Google Scholar]
- Vandermeer J. Cambridge University Press; London, UK: 1989. The ecology of intercropping. [Google Scholar]
- Vanek S, Wien H.C, Rangarajan A. Time of interseeding of lana vetch and winter rye cover strips determines competitive impact on pumpkins grown using organic practices. Hortscience. 2005;40:1716–1722. [Google Scholar]
- Vargas-Ayala R, Rodriguez-Kabana R. Bioremediative management of soybean nematode population densities in crop rotations with velvetbean, cowpea, and winter crops. Nematropica. 2001;31:37–46. [Google Scholar]
- Vatovec C, Jordan N, Huerd S. Responsiveness of certain agronomic weed species to arbuscular mycorrhizal fungi. Renew. Agr. Food Syst. 2005;20:181–189. doi:10.1079/RAF2005115 [Google Scholar]
- Vigo C, Norman J.R, Hooker J.E. Biocontrol of the pathogen Phytophthora parasitica by arbuscular mycorrhizal fungi is a consequence of effects on infection loci. Plant Pathol. 2000;49:509–514. doi:10.1046/j.1365-3059.2000.00473.x [Google Scholar]
- Wagenet R.J. Scale issues in agroecological research chains. Nutr. Cycl. Agroecosyst. 1998;50:23–34. doi:10.1023/A:1009770312707 [Google Scholar]
- Wajnberg E, Scott J.K, Quimby P.C. CABI; Wallingford, UK; England, UK; New York, NY: 2001. Evaluating indirect ecological effects of biological control. [Google Scholar]
- Walde S.J. How quality of host plants affects a predator–prey interaction in biological control. Ecology. 1995;76:1206–1219. doi:10.2307/1940927 [Google Scholar]
- Walker M, Jones T.H. Relative roles of top-down and bottom-up forces in terrestrial tritrophic plant–insect herbivore–natural enemy systems. Oikos. 2001;93:177–187. doi:10.1034/j.1600-0706.2001.930201.x [Google Scholar]
- Wardle D.A. The influence of biotic interactions on soil biodiversity. Ecol. Lett. 2006;9:870–886. doi: 10.1111/j.1461-0248.2006.00931.x. doi:10.1111/j.1461-0248.2006.00931.x [DOI] [PubMed] [Google Scholar]
- Wardle D.A, Williamson W.M, Yeates G.W, Bonner K.I. Trickle-down effects of aboveground trophic cascades on the soil food web. Oikos. 2005;111:348–358. doi:10.1111/j.0030-1299.2005.14092.x [Google Scholar]
- Warner K.D. Extending agroecology, grower participation in partnerships is key to social learning. Renew. Agr. Food Syst. 2006;21:84–94. doi:10.1079/RAF2005131 [Google Scholar]
- Weiner, J. 2003 Ecology—the science of agriculture in the 21st century. Patent Part 3–4. Date Issued, Nov–Dec 2003.
- Weinig C. Rapid evolutionary responses to selection in heterogeneous environments among agricultural and nonagricultural weeds. Int. J. Plant Sci. 2005;166:641–647. doi:10.1086/429853 [Google Scholar]
- Welbaum G.E, Sturz A.V, Dong Z.M, Nowak J. Managing soil microorganisms to improve productivity of agro-ecosystems. Crit. Rev. Plant Sci. 2004;23:175–193. doi:10.1080/07352680490433295 [Google Scholar]
- Weller D.M, Raaijmakers J.M, McSpadden Gardener B.B, Thomashow L.S. Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu. Rev. Phytopathol. 2002;40:309–348. doi: 10.1146/annurev.phyto.40.030402.110010. doi:10.1146/annurev.phyto.40.030402.110010 [DOI] [PubMed] [Google Scholar]
- Westerman P, Liebman M, Menalled F.D, Heggenstaller A.H, Hartzler R.G, Dixon P.M. Are many little hammers effective?—velvetleaf (Abutilon theophrasti) population dynamics in two- and four-year crop rotation systems. Weed Sci. 2005;53:382–392. doi:10.1614/WS-04-130R [Google Scholar]
- Westphal A. Detection and description of soils with specific nematode suppressiveness. J. Nemat. 2005;37:121–130. [PMC free article] [PubMed] [Google Scholar]
- Westphal A, Scott A.W. Implementation of soybean in cotton cropping sequences for management of reniform nematode in South Texas. Crop Sci. 2005;45:233–239. [Google Scholar]
- Whipps J.M. Prospects and limitations for mycorrhizas in biocontrol of root pathogens. Can. J. Bot. - Revue Canadienne de Botanique. 2004;82:1198–1227. [Google Scholar]
- Wilby A, Thomas M.B. Natural enemy diversity and pest control, patterns of pest emergence with agricultural intensification. Ecol. Lett. 2002;5:353–360. doi:10.1046/j.1461-0248.2002.00331.x [Google Scholar]
- Wilson M. Biocontrol of aerial plant diseases in agriculture and horticulture, current approaches and future prospects. J. Ind. Microbiol. Biotech. 1977;19:188–191. doi:10.1038/sj.jim.2900436 [Google Scholar]
- Wisler G.C, Norris R.E. Interactions between weeds and cultivated plants as related to management of plant pathogens. Weed Sci. 2005;53:914–917. doi:10.1614/WS-04-051R.1 [Google Scholar]
- Wood D. Ecological principles in agricultural policy, but which principles? Food Policy. 1998;23:371–381. doi:10.1016/S0306-9192(98)00043-8 [Google Scholar]
- Wossink G.A.A, Rossing W.A.H. On increasing returns and discrete choice, integrating production ecological principles in economic analysis of crop management. J. Environ. Manage. 1998;54:233–247. doi:10.1006/jema.1998.0228 [Google Scholar]
- Yulianti T, Sivasithamparam K, Turner D.W. Saprophytic growth of Rhizoctonia solani Kuhn AG2-1 (ZG5) in soil amended with fresh green manures affects the severity of damping-off in canola. Soil Biol. Biochem. 2006;38:923–930. doi:10.1016/j.soilbio.2005.07.014 [Google Scholar]
- Zasada I.A, Ferris H. Nematode suppression with brassicaceous amendments, application based upon glucosinolate profile. Soil Biol. Biochem. 2004;36:1017–1024. doi:10.1016/j.soilbio.2003.12.014 [Google Scholar]
- Zehnder G.W, Murphy J.F, Sikora E.J, Kloepper J.W. Application of rhizobacteria for induced resistance. Eur. J. Plant Pathol. 2001;107:39–50. doi:10.1023/A:1008732400383 [Google Scholar]
- Zhu Y.Y, et al. Genetic diversity and disease control in rice. Nature. 2000;406:718–722. doi: 10.1038/35021046. doi:10.1038/35021046 [DOI] [PubMed] [Google Scholar]
- Zinkhan F.C, Mercer D.E. An assessment of agroforestry systems in the southern USA. Agroforest. Syst. 1997;35:303–321. doi:10.1007/BF00044460 [Google Scholar]