Abstract
Developing technologies to reduce the rate of increase of atmospheric concentration of carbon dioxide (CO2) from annual emissions of 8.6 Pg C yr–1 from energy, process industry, land-use conversion and soil cultivation is an important issue of the twenty-first century. Of the three options of reducing the global energy use, developing low or no-carbon fuel and sequestering emissions, this manuscript describes processes for carbon (CO2) sequestration and discusses abiotic and biotic technologies. Carbon sequestration implies transfer of atmospheric CO2 into other long-lived global pools including oceanic, pedologic, biotic and geological strata to reduce the net rate of increase in atmospheric CO2. Engineering techniques of CO2 injection in deep ocean, geological strata, old coal mines and oil wells, and saline aquifers along with mineral carbonation of CO2 constitute abiotic techniques. These techniques have a large potential of thousands of Pg, are expensive, have leakage risks and may be available for routine use by 2025 and beyond. In comparison, biotic techniques are natural and cost-effective processes, have numerous ancillary benefits, are immediately applicable but have finite sink capacity. Biotic and abiotic C sequestration options have specific nitches, are complementary, and have potential to mitigate the climate change risks.
Keywords: climate change, greenhouse effect, soil management, geological sequestration, chemical sequestration, oceanic sequestration
1. Introduction
Global surface temperatures have increased by 0.8°C since the late nineteenth century, and 11 out of the 12 warmest years on record have occurred since 1995 (IPCC 2007). Earth's mean temperature is projected to increase by 1.5–5.8°C during the twenty-first century (IPCC 2001). The rate of increase in global temperature has been 0.15°C per decade since 1975. In addition to the sea-level rise of 15–23 cm during the twentieth century (IPCC 2007), there have been notable shifts in ecosystems (Greene & Pershing 2007) and frequency and intensity of occurrence of wild fires (Running 2006; Westerling et al. 2006). These and other observed climate changes are reportedly caused by emission of greenhouse gases (GHGs) through anthropogenic activities including land-use change, deforestation, biomass burning, draining of wetlands, soil cultivation and fossil fuel combustion. Consequently, the concentration of atmospheric GHGs and their radiative forcing have progressively increased with increase in human population, but especially so since the onset of industrial revolution around 1850. The concentration of carbon dioxide (CO2) has increased by 31% from 280 ppmv in 1850 to 380 ppmv in 2005, and is presently increasing at 1.7 ppmv yr−1 or 0.46% yr−1 (WMO 2006; IPCC 2007). Concentrations of methane (CH4) and nitrous oxide (N2O) have also increased steadily over the same period (IPCC 2001, 2007; Prather et al. 2001; WMO 2006). Total radiative forcing, externally imposed perturbation in the radiative energy budget of the Earth's climate system (Ramaswamy et al. 2001), of all GHGs since 1850 is estimated at 2.43 W m−2 (IPCC 2001, 2007).
There is a strong interest in stabilizing the atmospheric abundance of CO2 and other GHGs to mitigate the risks of global warming (Kerr 2007; Kintisch 2007b; Kluger 2007; Walsh 2007). There are three strategies of lowering CO2 emissions to mitigate climate change (Schrag 2007): (i) reducing the global energy use, (ii) developing low or no-carbon fuel, and (iii) sequestering CO2 from point sources or atmosphere through natural and engineering techniques. Between 1850 and 1998, anthropogenic emissions are estimated at 270±30 Pg by fossil fuel combustion and at 136±30 Pg by land-use change, deforestation and soil cultivation (IPCC 2001). Presently, approximately 7 Pg C yr−1 is emitted by fossil fuel combustion (Pacala & Socolow 2004) and 1.6 Pg C yr−1 by deforestation, land-use change and soil cultivation. Of the total anthropogenic emissions of 8.6 Pg C yr−1, 3.5 Pg C yr−1 is absorbed by the atmosphere, 2.3 Pg C yr−1 by the ocean and the remainder by an unidentified terrestrial sink probably in the Northern Hemisphere (Tans et al. 1990; Fan et al. 1998).
The objective of this paper is to discuss the process and technological options of CO2–C sequestration in one of the long-lived global C pools so as to reduce the net rate of increase of atmospheric concentration of CO2. While CO2–C sequestration is discussed in general, specific attention is given to the terrestrial C sequestration in forests and soils.
2. The global carbon cycle
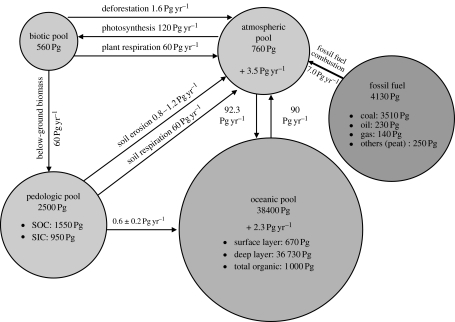
The importance of atmospheric concentration of CO2 on global temperature was recognized by Arrhenius (1896) towards the end of the nineteenth century, whereas anthropogenic perturbation of the global C cycle during the twentieth century has been an historically unprecedented phenomenon. Understanding the global C cycle and its perturbation by anthropogenic activities is important for developing viable strategies for mitigating climate change. The rate of future increase in atmospheric CO2 concentration will depend on the anthropogenic activities, the interaction of biogeochemical and climate processes on the global C cycle and interaction among principal C pools. There are five global C pools, of which the largest oceanic pool is estimated at 38 000 Pg and is increasing at the rate of 2.3 Pg C yr−1 (figure 1). The geological C pool, comprising fossil fuels, is estimated at 4130 Pg, of which 85% is coal, 5.5% is oil and 3.3% is gas. Proven reserves of fossil fuel include 678 Pg of coal (3.2 Pg yr−1 production), 146 Pg of oil (3.6 Pg yr−1 of production) and 98 Pg of natural gas (1.5 Pg yr−1 of production; Schrag 2007). Presently, coal and oil each account for approximately 40% of global CO2 emissions (Schrag 2007). Thus, the geological pool is depleting, through fossil fuel combustion, at the rate of 7.0 Pg C yr−1. The third largest pool is pedologic, estimated at 2500 Pg to 1 m depth. It consists of two distinct components: soil organic carbon (SOC) pool estimated at 1550 Pg and soil inorganic carbon (SIC) pool at 950 Pg (Batjes 1996). The SOC pool includes highly active humus and relatively inert charcoal C. It comprises a mixture of: (i) plant and animal residues at various stages of decomposition; (ii) substances synthesized microbiologically and/or chemically from the breakdown products; and (iii) the bodies of live micro-organisms and small animals and their decomposing products (Schnitzer 1991). The SIC pool includes elemental C and carbonate minerals such as calcite, dolomite and gypsum, and comprises primary and secondary carbonates. The primary carbonates are derived from the weathering of parent material. In contrast, the secondary carbonates are formed through the reaction of atmospheric CO2 with Ca+2 and Mg+2 brought in from outside the local ecosystem (e.g. calcareous dust, irrigation water, fertilizers, manures). The SIC is an important constituent in soils of arid and semi-arid regions. The fourth largest pool is the atmospheric pool comprising 760 Pg of CO2–C, and increasing at the rate of 3.5 Pg C yr−1 or 0.46% yr−1. The smallest among the global C pools is the biotic pool estimated at 560 Pg. The pedologic and biotic C pools together are called the terrestrial C pool estimated at approximately 2860 Pg. The atmospheric pool is also connected to the oceanic pool which absorbs 92.3 Pg yr−1 and releases 90 Pg yr−1 with a net positive balance of 2.3 Pg C yr−1. The oceanic pool will absorb approximately 5 Pg C−1 yr−1 by 2100 (Orr et al. 2001). The total dissolved inorganic C in the oceans is approximately 59 times that of the atmosphere. On the scales of millennia, the oceans determine the atmospheric CO2 concentration, not vice versa (Falkowski et al. 2000). The link between geological (fossil fuel) and atmospheric pool is in one direction: transfer of approximately 7.0 Pg C yr−1 from fossil fuel consumption to the atmosphere. The rate of fossil fuel consumption may peak by about 2025.
Figure 1.
Principal global C pools and fluxes between them. The data on C pools among major reservoirs are from Batjes (1996), Falkowski et al. (2000) and Pacala & Socolow (2004), and the data on fluxes are from IPCC (2001).
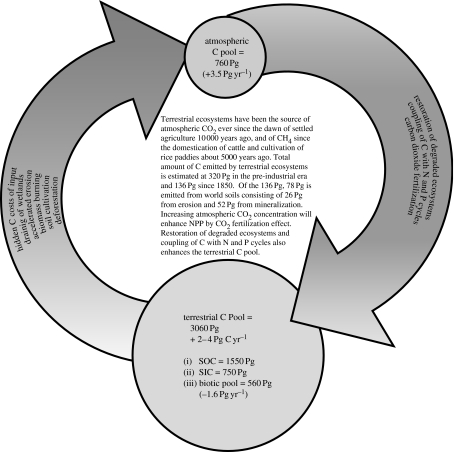
The terrestrial and atmospheric C pools are strongly interacting with one another (figure 2). The annual rate of photosynthesis is 120 Pg C, most of which is returned back to the atmosphere through plant and soil respiration. The terrestrial C pool is depleted by conversion from natural to managed ecosystems, extractive farming practices based on low external input and soil degrading land use. The pedologic pool loses 0.4–0.8 Pg C yr−1 to the ocean through erosion-induced transportation to aquatic ecosystems. The terrestrial sink is presently increasing at a net rate of 1.4±0.7 Pg C yr−1. Thus, the terrestrial sink absorbs approximately 2–4 Pg C yr−1 and its capacity may increase to approximately 5 Pg C yr−1 by 2050 (Cramer et al. 2001; Scholes & Noble 2001). Increase in the terrestrial sink capacity may be due to CO2 fertilization effect and change in land use and management. The biotic pool also contributes to increase in atmospheric CO2 concentration through deforestation and biomass burning.
Figure 2.
The atmospheric C pool is increasing at the rate of 3.5 Pg C yr−1. The terrestrial C pool contributes approximately 1.6 Pg C yr−1 through deforestation, biomass burning, draining of wetlands, soil cultivation including those of organic soils, accelerated erosion and hidden C costs of input (e.g. fertilizers, tillage, pesticides, irrigation). Terrestrial C pools are presently sink of 2–4 Pg C yr−1. Conversion to a judicious land use and adoption of recommended practices in managed ecosystems can make these important sinks especially due to CO2 fertilization effects.
3. Carbon sequestration
Emission rates from fossil fuel combustion increased by 40% between 1980 and 2000 (Wofsy 2001). Yet, the amount of CO2 accumulating in the atmosphere remained the same over this period because the excess CO2 released is being removed by oceans, forests, soils and other ecosystems (Battle et al. 2000). Atmospheric CO2 increased at a rate of 2.8–3.0 Pg C yr−1 during the 1980s and 1995, and between 3.0 and 3.5 Pg C yr−1 during 1995–2005. Considering the total anthropogenic emissions of between 6 and 8 Pg C yr−1, while the atmospheric increase has been 2.8–3.5 Pg yr−1, implies the existence of large global terrestrial sinks (Fung 2000; Pacala 2001). Fan et al. (1998) estimated a mean annual uptake of 1.7±0.5 Pg C yr−1 in North America, mostly south of 51° N. In comparison, the Eurasia–North Africa sink was relatively small. This process of transfer and secure storage of atmospheric CO2 into other long-lived C pools that would otherwise be emitted or remain in the atmosphere is called ‘carbon sequestration’. Therefore, in this context, C sequestration may be a natural or an anthropogenically driven process. The objective of an anthropogenically driven C sequestration process is to balance the global C budget such that future economic growth is based on a ‘C-neutral’ strategy of no net gain in atmospheric C pool. Such a strategy would necessitate sequestering almost all anthropogenically generated CO2 through safe, environmentally acceptable and stable techniques with low risks of leakage. Lackner (2003) estimated that if a C-neutral strategy is based mainly on sequestration rather than emission reduction, total C storage during the twenty-first century will exceed 600 Pg with residence time of centuries to millennia. However, even a small leakage of 2–3 Pg C yr−1 from the C sequestered in one of the pools can adversely impact the strategic planning for future generations. Pacala & Socolow (2004) outlined 15 options of stabilizing the atmospheric concentration of CO2 by 2050 at approximately 550 ppm. Of the 15 options, three were based on C sequestration in terrestrial ecosystems.
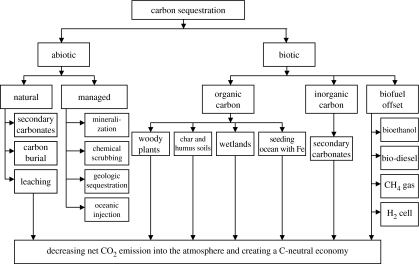
There are several technological options for sequestration of atmospheric CO2 into one of the other global pools (figure 3). The choice of one or a combination of several technologies is important for formulating energy policies for future economic growth and development at national and global scales. These options can be grouped into two broad categories: abiotic and biotic sequestration.
Figure 3.
A wide range of processes and technological options for C sequestration in agricultural, industrial and natural ecosystems.
(a) Abiotic sequestration
Abiotic sequestration is based on physical and chemical reactions and engineering techniques without intervention of living organisms (e.g. plants, microbes). The abiotic strategy of C sequestration in oceanic and geological structures has received considerable attention (Freund & Ormerod 1997) because theoretically abiotic sequestration has a larger sink capacity than biotic sequestration. Rapid progress is being made in developing/testing technologies for CO2 capture, transport and injection (Kerr 2001).
(i) Oceanic injection
Injection of a pure CO2 stream deep in the ocean is a possibility that has been widely considered by engineers for about three decades. Following the initial proposal in the late 1970s, there has been considerable progress in oceanic injection of CO2. To be stable and minimize outgassing, CO2 must be injected at great depths. Therefore, liquefied CO2 separated from industrial sources can be injected into the ocean by one of the following four techniques: (i) it is injected below 1000 m from a manifold lying at the ocean floor, and being lighter than water, it rises to approximately 1000 m depth forming a droplet plume; (ii) it is also injected as a denser CO2–seawater mixture at 500–1000 m depth, and the mixture sinks into the deeper ocean; (iii) it is discharged from a large pipe towed behind a ship; and (iv) it is pumped into a depression at the bottom of the ocean floor forming a CO2 lake. Liquefied CO2 injected at approximately 3000 m depth is believed to remain stable (O'Connor et al. 2001). The oceanic sink capacity for CO2 sequestration is estimated at 5000–10 000 Pg C, exceeding the estimated fossil fuel reserves (Herzog et al. 1997, 2002). However, CO2 injection may also have some adverse effects on deep sea biota (Auerbach et al. 1997; Caulfield et al. 1997; Seibel & Walsh 2001). In addition to the economics, the issue of stability of such an injection must be addressed owing to the increased stratification of the ocean water column and its turn over through natural processes.
(ii) Geological injection
This involves capture, liquefaction, transport and injection of industrial CO2 into deep geological strata. The CO2 may be injected in coal seams, old oil wells (to increase yield), stable rock strata or saline aquifers (Tsang et al. 2002; Klara et al. 2003; Baines & Worden 2004; Gale 2004). Saline aquifers are underground strata of very porous sediments filled with brackish (saline) water. In general, saline aquifers are located below the freshwater reservoirs with an impermeable layer in between. Industrial CO2 can be pumped into the aquifer, where it is sequestered hydrodynamically and by reacting with other dissolved salts to form carbonates. Carbon dioxide is injected in a supercritical state that has much lower density and viscosity than the liquid brine that it displaces. In situ, it forms a gas-like phase and also dissolves in the aqueous phase, creating a multiphase multicomponent environment. Injecting CO2 into reservoirs in which it displaces oil or gas could be an economic strategy of enhanced oil recovery (EOR). Production from oil and gas fields, which has been in decline, is raised by CO2-enhanced recovery (Klusman 2003). This strategy of CO2 sequestration is used in Texas, USA, to inject 20 million Mg of CO2 yr−1 at a price of $10–$15 Mg−1 (Lackner 2003). The technique is also used in offshore oil wells in Norway. In a strict sense, however, this is not sequestration when the CO2 injected is extracted from underground wells. The CO2 can also be injected into unmineable coal seams where CH4 is absorbed. Injected CO2 is absorbed onto coal twice as much as CH4, and the process enhances the gas recovery of coal bed CH4 (CBM). Principal concerns about geological sequestration, similar to that of the oceanic, are (Kintisch 2007a; Schrag 2007): (i) reliability of storage of vast quantities of CO2 in geological strata and (ii) the cost. Some have argued that risks of leakage are low. However, to date, a few direct injection of CO2 have been made on a commercial scale although the Regional Carbon Sequestration Partnership project of US DOE is planning for several demonstrations during 2008–2009. Similar to oceanic injection, cost and leakage are principal issues of geological sequestration which need to be resolved. Owing to the low density and viscosity and injection under supercritical conditions, the risks of CO2 leakage through confining strata may be higher than currently injected liquid wastes (Tsang et al. 2002). In addition, the chemical interactions of CO2 with the geological formations may have to be considered in formulating guidelines for appropriate regulatory and monitoring controls.
(iii) Scrubbing and mineral carbonation
Mineral carbonation is achieved through mimicry of natural inorganic chemical transformation of CO2 (Fan & Park 2004). It involves transformation of industrial strength CO2 emissions into CaCO3, MgCO3 and other minerals in the form of geologically and thermodynamically stable mineral carbonates. It is a two-stage process: scrubbing and mineral carbonation. Scrubbing, the process of chemical absorption of CO2 using an amine or carbonate solvent, is the most widely used method of carbon capture. The CO2 is purified by passing through an absorption column containing amine solvent. Other solvents used are K2CO3, lithium silicate, ceramic and nickel-based compounds. Pure CO2 gas, recovered by heating the CO2-rich amine, is re-precipitated through mineral carbonation. Carbonates thus formed are stable rock in which CO2 is sequestered forever. Aqueous mineral carbonation reactions leading to the formation of magnesite (MgCO3), olivine (Mg2SiO4) and serpentine (Mg3Si2O5(OH)4) are as follows (Gerdemann et al. 2003):
| \n\n\n\n \n |
All these reactions occur in nature and can be replicated in industrial settings. The industrial process of mineral carbonation has been described by Lackner et al. (1996, 1997) and O'Connor et al. (2000). The process uses a slurry of fine particle-sized mineral in water, at solid concentrations of 15–30%. Dissolution of the mineral and subsequent carbonation occur in a single operation as per the following theorized reactions (O'Connor et al. 2000; Gerdemann et al. 2004):
| \n\n\n\n \n |
Geological studies have shown that reserves of ultramafic (ultrabasic) minerals are sufficient to provide raw materials for mineral carbonation of industrial emissions for long time (Goff et al. 1997, 2000). However, these are geological reactions and occur at slow rate. The challenge lies in increasing the rate of reaction by decreasing the particle size, increasing temperature and pressure and using catalytic agents (Fan & Park 2004). Increasing the rate of reactions, however, requires energy and is expensive.
(b) Biotic sequestration
Biotic sequestration is based on managed intervention of higher plants and micro-organisms in removing CO2 from the atmosphere. It differs from management options which reduce emission or offset emission. Increasing use efficiency of resources (e.g. water, energy) is another option for managing the terrestrial C pool (table 1). Some biotic sequestration options are briefly described below.
Table 1.
Terrestrial carbon management options.
| management of terrestrial C pool | sequestration of C in terrestrial pool |
|---|---|
| reducing emissions | sequestering emissions as SOC |
| eliminating ploughing | increasing humification efficiency |
| conserving water and decreasing irrigation need | improving soil aggregation |
| using integrated pest management to minimize the use of pesticides | deep incorporation of SOC through establishing deep-rooted plants, promoting bioturbation and transfer of DOC into the ground water |
| biological nitrogen fixation to reduce fertilizer use | |
| offsetting emissions | sequestering emissions as SIC |
| establishing biofuel plantations | forming secondary carbonates through biogenic processes |
| biodigestion to produce CH4 gas | leaching of biocarbonates into the ground water |
| bio-diesel and bioethanol production | |
| enhancing use efficiency | |
| precision farming | |
| fertilizer placement and formulations | |
| drip, sub-irrigation or furrow irrigation |
(i) Oceanic sequestration
There are several biological processes leading to C sequestration in the ocean through photosynthesis. Phytoplankton photosynthesis is one such mechanism (Rivkin & Legendre 2001), which fixes approximately 45 Pg C yr−1 (Falkowski et al. 2000). Some of the particulate organic material formed by phytoplankton is deposited at the ocean floor and is thus sequestered (Raven & Falkowski 1999). Availability of Fe is one of the limiting factors on phytoplankton growth in oceanic ecosystems. Thus, several studies have assessed the importance of Fe fertilization on biotic CO2 sequestration in the ocean (Martin & Fitzwater 1988; Falkowski 1997; Martin et al. 2002; Boyd et al. 2004). It is also argued that incremental C could be sold as credits in the developing global C marketplace. Similar to deep injection, ocean fertilization may also change the ecology of the ocean (Chisholm et al. 2001). However, with the current state of knowledge, the topic of ocean fertilization remains a debatable issue (Johnson & Karl 2002).
(ii) Terrestrial sequestration
Transfer of atmospheric CO2 into biotic and pedologic C pools is called terrestrial C sequestration. Of the 8.6 Pg C yr−1 emitted into the atmosphere, only 3.5 Pg or 40% of the anthropogenically emitted CO2 remains in the atmosphere primarily owing to unspecified terrestrial sinks which sequester atmospheric CO2 and play an important role in the global C cycle. Terrestrial ecosystems constitute a major C sink owing to the photosynthesis and storage of CO2 in live and dead organic matter. Owing to its numerous ancillary benefits (e.g. improved soil and water quality, restoration of degraded ecosystems, increased crop yield), terrestrial C sequestration is often termed as win–win or no-regrets strategy (Lal et al. 2003). It offers multiple benefits even without the threat of global climate change. There are three principal components of terrestrial C sequestration: forests, soils and wetlands.
Forest ecosystems store C as lignin and other relatively resistant polymeric C compounds. Presently, the net rate of C sequestration in forest ecosystems (other than those being deforested) is 1.7±0.5 Pg C yr−1 (Fan et al. 1998). The forest C is sequestered not only in the harvestable timber, but also in woody debris, wood products and other woody plants encroaching upon grasslands (Wofsy 2001). The terrestrial NPP is not saturated at the current CO2 concentration and may increase with increase in atmospheric CO2 concentration through the CO2 fertilization effect. The NPP may be saturated at 800–1000 ppm of CO2 concentration (Falkowski et al. 2000). Thus, the forest sink may increase by CO2 fertilization effect (Krishnamurthy & Machavaram 2000). The potential CO2 fertilization effect may peak during the middle of the twenty-first century (Kohlmaier et al. 1998). However, at increasing CO2 concentration, the NPP may be limited by the deficiency of N, P, H2O and other factors. Interaction between cycles of C, N, P and H2O, if moderated through judicious management, may enhance terrestrial C sequestration.
Afforestation is one of the viable options of C sequestration in terrestrial ecosystems (IPCC 1999; Fang et al. 2001; Lamb et al. 2005). The potential of C sequestration through afforestation is estimated at 3 Tg C yr−1 in Norway, 6 Tg C yr−1 in New Zealand, 9 Tg C yr−1 in Sweden, 107 Tg C yr−1 in Russia and 117 Tg C yr−1 in the USA (IPCC 1999). The rate of C sequestration in US forests, considering all components, is 0.3–0.7 Pg C yr−1 (Pacala et al. 2001). Restoration of degraded tropical forest is another important option (Lamb et al. 2005). It is estimated that 350 Mha of tropical forest have been converted to other land uses, and another 500 Mha of forests have been degraded to varying extent (Lal 2005a,b,c). Thus, establishment of productive and monoculture plantations of Pinus, Eucalyptus and Acacia can enhance the terrestrial C pool in these ecosystems. There is also a potential for improving the management of secondary or regrowth forests in degraded tropical landscapes. Fang et al. (2001) estimated that in China, between 1970 and 1998, C sequestration in forests increased at an average rate of 21 Tg C yr−1 mainly due to afforestation and growth. The total C pool in Chinese forest decreased from 5.1 Pg in 1949 to 4.3 Pg in 1977–1981. Subsequently, it progressively increased during 1980s and 1990s to 4.7 Pg in 1998 (Fang et al. 2001). Townsend et al. (2002) suggested a sizeable CO2 uptake in C-3 dominated tropical regions in eight of the 10-year study period. Their data showed a possible existence of a large equatorial terrestrial CO2 sink.
Pacala & Socolow's (2004) estimated management of temperate and tropical forest is one of the 15 options to stabilize atmospheric CO2 concentration at 550 ppm by 2050. They estimated that 0.5 Pg C emission would be avoided if the current rate of clear cutting of primary tropical forest were reduced to zero over 50 years by 2050. Another 0.5 Pg C yr−1 would be sequestered by reforesting or afforesting approximately 250 Mha in the tropics or 400 Mha in the temperate zone. An additional 0.3 Pg C yr−1 can be sequestered by establishing approximately 300 Mha of plantations of non-forested lands (Pacala & Socolow 2004). The afforestation may account for a total of 25 Pg C sequestration between 2000 and 2050.
However, afforestation on a large scale can impact water resources. Jackson et al. (2005) documented substantial losses in stream flow, and increased soil salinization and acidification, with afforestation. They observed that establishment of tree plantations decreased stream flow by 227 mm yr−1 globally (52%), with 13% of streams drying completely for at least one year. Thus, any plans of large-scale afforestation for C sequestration must consider the possible adverse impact on availability of water.
Bunker et al. (2005) addressed the concern of decline in tropical forest biodiversity due to expansion of monoculture plantations. Thus, resulting effects of monoculture plantations on key ecosystem services (e.g. biodiversity, water resources, elemental cycling, C sequestration) must be critically assessed. There is a strong need for developing regulatory policies, including the transaction costs of trading C credits and obtaining the permits. The cost of C sequestration vis-à-vis the opportunity cost must also be considered (McCarl & Schneider 2001).
Wetlands and the associated soils or histosols constitute a large pedologic pool estimated at approximately 450 Pg (Gorham 1991; Warner et al. 1993). Wetland soils may contain as much as 200 times more C than the associated vegetation (Milne & Brown 1997; Garnett et al. 2001). Gorham (1991) and Kobak et al. (1998) estimated that C sequestration in wetlands/peatsoils since the post-glaciation period resulted in the C accumulation at the rate of 0.1 Pg C yr−1 over 10 000–18 000 years. However, drainage of peatlands and their subsequent cultivation made these ecosystems a net source of CO2. Large areas of wetlands have been drained worldwide for agriculture (Armentano & Menges 1986) and forestry (Paavilainen & Paivanen 1995). Drained wetland soils decompose and subside at the rate of approximately 1–2 cm yr−1 primarily due to oxidation (Rojstaczer & Deverel 1995; Hillman 1997; Wosten et al. 1997). Restoration of wetlands can lead to reversal of the process and make restored wetlands once again a sink of atmospheric CO2. However, there is a long time lag after the restoration until processes in restored wetlands become similar to those of natural wetlands.
Soil C sequestration. Implies enhancing the concentration/pools of SOC and SIC as secondary carbonates through land-use conversion and adoption of recommended management practices (RMPs) in agricultural, pastoral and forestry ecosystems and restoration of degraded and drastically disturbed soils. Formation of charcoal and use of biochar as a fertilizer is another option (Fowles 2007). In contrast to geological sequestration that implies injecting CO2 at 1–2 km depth, the SOC sequestration involves putting C into the surface layer of 0.5–1 m depth through the natural processes of humification. Most soils under the managed ecosystems contain a lower SOC pool than their counterparts under natural ecosystems owing to the depletion of the SOC pool in cultivated soils. The most rapid loss of the SOC pool occurs in the first 20–50 years of conversion of natural to agricultural ecosystems in temperate regions and 5–10 years in the tropics (Lal 2001). In general, cultivated soils normally contain 50–75% of the original SOC pool. The depletion of the SOC pool is caused by oxidation/mineralization, leaching and erosion.
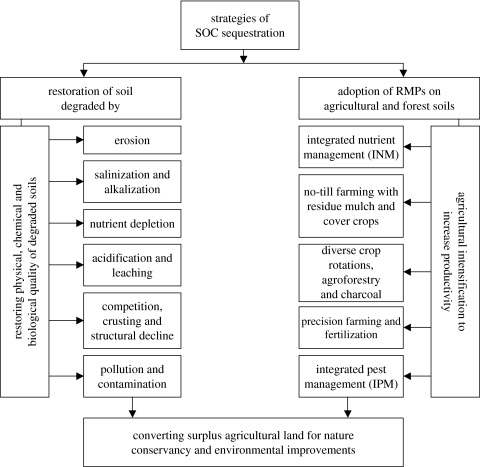
Strategies for increasing the SOC pool are outlined in figure 4. There is a wide range of degraded soils with a depleted SOC pool. Important among these are those degraded by erosion, nutrient depletion, acidification and leaching, structural decline and pollution/contamination (figure 4). Restoring degraded soils and ecosystems is a strategy with multiple benefits for water quality, biomass productivity and for reducing net CO2 emission. Grainger (1995) estimated that there are approximately 750 Mha of degraded lands in the tropics with potential for afforestation and soil quality enhancement. With a sequestration potential of approximately 0.5 Mg ha−1 yr−1 as SOC and an additional 1.0 Mg ha−1 yr−1 as biomass, a terrestrial C sequestration potential of 750 Mha is approximately 1.1 Pg C yr−1. Lal (2001) estimated the SOC sequestration potential of 0.4–0.7 Pg C yr−1 through desertification control in soils of arid and semi-arid regions. Similar estimates were provided by Squires et al. (1995).
Figure 4.
Management strategies for soil carbon sequestration through restoration of degraded soils and adoption of RMPs on agricultural soils.
West & Post (2002) assessed SOC sequestration rate upon conversion of plough tillage to no-till farming through analyses of data of 67 long-term experiments from around the world. They reported the mean rate of SOC sequestration of 570±140 Kg C ha−1 yr−1, which may lead to the new equilibrium SOC pool in 40–60 years. Lal (2004a,b) estimated the global SOC sequestration potential, 0.4–1.2 Pg C yr−1 or 5–15%, of the global fossil fuel emissions. Pacala & Socolow (2004) estimated that conversion of plough tillage to no-till farming on 1600 Mha of cropland along with adoption of conservation-effective measures could lead to sequestration of 0.5–1 Pg C yr−1 by 2050.
Integrated nutrient management (INM) is also essential to SOC sequestration. The humification process can be severely constrained by the lack of N, P, S and other building blocks of soil humus (Himes 1998). The efficiency of C sequestration is reduced when C and N are not adequately balanced (Paustian et al. 1997). Therefore, the SOC sequestration rate is enhanced by an increase in the application of biomass C (Campbell et al. 1991; Janzen et al. 1998) and N (Halvorson et al. 1999, 2002). Liebig et al. (2002) observed that high N rate treatments increased SOC sequestration rates by 1.0–1.4 Mg C ha−1yr−1 compared with unfertilized controls. Similar observations were made by Dumanski et al. (1988), Schjonning et al. (1994), Gregorich et al. (1996), Bowman & Halvorson (1998), Studdert & Echeverria (2000), and Jacinthe et al. (2002). Malhi et al. (1997) reported that SOC sequestration depended both on the rate and the source of N application. In Victoria, Australia, Ridley et al. (1990) observed that application of P and lime increased the SOC pool in the 0–10 cm layer by 11.8 Mg ha−1 over a 68-year period at an average rate of 0.17 Mg C ha−1 yr−1.
Application of manures and other organic amendments is another important strategy of SOC sequestration. Several long-term experiments in Europe have shown that the rate of SOC sequestration is greater with application of organic manures than with chemical fertilizers (Jenkinson 1990: Witter et al. 1993; Christensen 1996; Korschens & Muller 1996; Smith et al. 1997). Increase in the SOC pool in the 0–30 cm depth by long-term use of manure compared to chemical fertilizers was 10% over 100 years in Denmark (Christensen 1996), 22% over 90 years in Germany (Korschens & Muller 1996), 100% over 144 years at Rothamsted, UK (Jenkinson 1990) and 44% over 31 years in Sweden (Witter et al. 1993). The data from Morrow plots in Illinois indicated that manured plots contained 44.6 Mg ha−1 more SOC than unmanured control (Anderson et al. 1990). In Hungary, Arends & Casth (1994) observed an increase in SOC concentration by 1.0–1.7% by manuring. Smith et al. (1997) estimated that application of manure at the rate of 10 Mg ha−1 to cropland in Europe would increase the SOC pool by 5.5% over 100 years. In Norway, Uhlen (1991) and Uhlen & Tveitnes (1995) reported that manure application would increase SOC sequestration at the rate of 70–227 Kg ha−1 yr−1 over 37–74-year period.
Soils under diverse cropping systems generally have a higher SOC pool than those under monoculture (Dick et al. 1986; Buyanoski et al. 1997; Drinkwater et al. 1998; Buyanoski & Wagner 1998). Elimination of summer fallow is another option for minimizing losses of the SOC pool (Delgado et al. 1998; Rasmussen et al. 1998). Growing a winter cover crop enhances soil quality through SOC sequestration. In the UK, Fullen & Auerswald (1998) reported that grass leys set aside increased SOC concentration by 0.02% yr−1 for 12 years. In Australia, Grace & Oades (1994) observed that the SOC pool in the 0–10 cm layer increased linearly with increase in frequency of pasture in the crop rotation cycle. In comparison with continuous cropping, incorporation of cover crops in the rotation cycle enhanced SOC concentration in the surface layer by 15% in Sweden (Nilsson 1986), 23% in The Netherlands (Van Dijk 1982) and 28% in the UK (Johnston 1973) over 12–28-year period. Similar results were reported by Lal et al. (1998) for the US cropland.
The rate of sequestration ranges from negative or zero under arid and hot climates to approximately 1000 Kg C ha−1yr−1 under humid and temperate climates (Lal 2004b, 2005a,b,c). Normal rates of SOC sequestration on agricultural soils are 300–500 Kg C ha−1 yr−1. High rates are obtained with no-till farming, crop residue retention as mulch, growing cover crops in the rotation cycle and adopting complex farming systems including agroforestry, INM including manuring and through restoration of degraded soils by afforestation.
Adoption of RMPs on agricultural and forest soils is a win–win strategy (Follett 2001; Lal et al. 2003; Lal 2004a,b, 2006). While improving soil quality and agronomic productivity, agricultural intensification through adoption of RMPs also improves water quality, reduces non-point source pollution by decreasing dissolved and sediment loads, and reduces net rate of CO2 emission through SOC sequestration. It is a very natural process (Morris 2006). However, for the SOC sequestered to be stabilized, it is important to understand the stabilizing mechanisms (Six et al. 2002), limitations of biophysical ecosystems in C sequestration (Schlesinger 1999; Sauerbeck 2001), and relevant economic and policy issues (McCarl & Schneider 2001). Trading of C credits may require several permits (at federal, state and local levels) and marketing procedures.
(iii) Secondary carbonates
Soil C sequestration may also occur in SIC as secondary carbonates, and leaching of bicarbonates into the ground water. Secondary carbonates occur in various forms as films, threads, concretions and pedants. They also occur as laminar caps, caliche and calcrate (Gile 1993). In skeletal soils with high gravel concentration, secondary carbonates occur as coatings on lower surfaces of stones and pebbles. There are four principal mechanisms of formation of secondary carbonates (Monger 2002; Mermut & Landi 2006). Marion et al. (1985) proposed that secondary carbonates are formed through dissolution of CO2 in the surface layer followed by translocation and re-precipitation with Ca+2 and Mg+2 in the subsoil. In contrast, Sobecki & Wilding (1983) described the second mechanism based on capillary rise of Ca+2 from shallow ground water and its re-precipitation in the surface layer. The third mechanism of in situ dissolution and re-precipitation was proposed by Rabenhorst & Wilding (1986). Monger (2002) observed that pedogenic/secondary carbonates are of biogenic origin and are formed by activity of soil fauna (e.g. termites). Chemical reactions describing the dissolution of CO2 are shown in the following equations described by Mermut & Landi (2006):
| \n\n\n\n \n |
Secondary carbonates are formed in the soil pH range of 7.3–8.5. There must, however, be sufficient quantity of Ca+2 and Mg+2 present in the soil system. Decreasing water content, decreasing partial pressure of CO2 or in soil air, and increasing the products of Ca+2 or in soil favour the precipitation of secondary carbonates.
In contrast to SOC, the rate of formation of secondary carbonates is low. In the deserts of southwestern USA, Marion et al. (1985) estimated that during the Pleistocene the rate of deposition of secondary carbonates was 1.2–6 Kg C ha−1 yr−1. In another study for the same region, Schlesinger (1985) estimated the rate of formation of secondary carbonates as being between 1.2 and 4.2 Kg C ha−1 yr−1. Also in the southwestern USA, Monger & Gallegos (2000) reported the rates of formation of secondary carbonates at 1–14 Kg C ha−1 yr−1. In Saskatchewan, Canada, Landi (2002) estimated the rate of deposition of secondary carbonates ranging between 9.9 and 13.4 Kg C ha−1 yr−1. For a non-calcareous parent material, Machette (1985) estimated the rate of deposition ranging between 1.7 and 6.1 Kg C ha−1 yr−1, and concluded that rates are generally high in calcareous soils.
Leaching of biocarbonates into ground water is another mechanism of transfer of SIC into a less active pool, especially in soils irrigated with good quality water (Nordt et al. 2000). The rate of leaching of HCO3- in irrigated soils may be as much as 0.25–1.0 Mg C ha−1 yr−1 (Wilding 1999). There are 250 Mha of irrigated land area globally. The potential of leaching of in these soils may be 62.5–250 Tg C yr−1. With the exception of irrigated soils, there are not many technological options to enhance the rate of deposition of secondary carbonates. Use of organic amendments (e.g. crop residue mulch, manure and other biosolids) may enhance activity of termites and other soil fauna, and also increase the formation of secondary carbonates through biogenic processes. Use of good quality irrigation water to enhance leaching of is another relevant option for irrigated soils.
4. Biofuels
Converting biomass-derived sugars to ethanol and plant-derived oils and fats into bio-diesel is a viable strategy to reduce use of fossil fuels and develop alternate/sustainable sources of energy (Himmel et al. 2007; Stephanopoulos 2007; Wald 2007). Of the world's total primary energy supply of 11.2 Pg of oil equivalent in 2004, 35.03% came from oil, 24.6% from coal, 20.44% from gas, 6.33% from nuclear and 13.61% from renewable sources (Goldemberg 2007). Of the renewable sources, 2.48% was contributed by the traditional biofuels (e.g. animal dung, crop residues, wood products), and 1.91% by modern biofuels. Only 3.22% of the primary energy was contributed by hydro, solar, wind and geothermal sources.
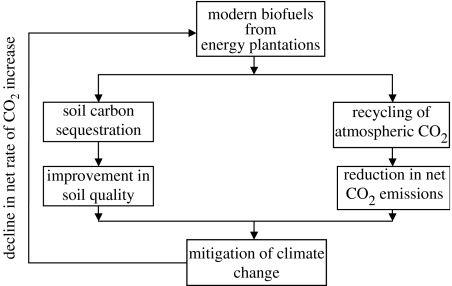
Biofuels, high on the political and scientific agenda, are related to C sequestration in two distinct but interrelated aspects (figure 5): (i) soil C sequestration through restoration of the depleted SOC pool, especially when agriculturally degraded/marginal soils are converted to energy plantations, and (ii) recycling of atmospheric CO2 into biomass-based biofuels. With choice of the appropriate species and prudent management, biofuels produced from energy plantations established by dedicated crops (e.g. poplar, willow, switch grass, miscanthus, karnal grass, Andropogan, Pennisetum) can sequester C in soil, offset fossil fuel emissions and reduce the rate of abundance of atmospheric CO2 and other GHGs. In contrast to the beneficial impacts, some have argued that there will be in increase in competition for land and water to establish energy plantations.
Figure 5.
Interactive effects of modern biofuels produced from energy plantations on terrestrial/biotic carbon sequestration.
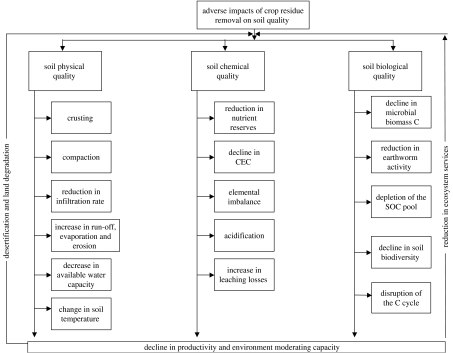
With the long-term goal of producing 1 Pg of lignocellulosic biomass in the USA and 4–5 Pg in the World, crop residues (e.g. corn, wheat, sorghum, millet, barley) are increasingly being considered as source of the biomass (Somerville 2006; Graham et al. 2007). It is also argued that using corn grain-based ethanol instead of gasoline reduces GHG emissions only by 18% (Service 2007). Thus, ethanol produced from cellulosic biomass rather than grains is a preferred alternative. Furthermore, the price of corn grains has increased to more than $330 Mg−1 in the USA, which has a snowballing impact on food prices in Mexico and elsewhere. Some countries (e.g. USA, China, India) are planning to use 300–400 million Mg of crop residue per annum as a source of renewable energy, which may have severe adverse consequences on soils and the environment. Indeed, indiscriminate removal of crop residues can severely jeopardize soil quality (figure 6). For example, it has been the perpetual removal of crop residues for numerous competing uses (e.g. fodder, household fuel, construction material) and use of animal dung for cooking fuel which have caused widespread and severe problems of soil degradation, low crop yields, and hunger and malnutrition in South Asia and sub-Saharan Africa (Lal 2006).
Figure 6.
Adverse impacts of crop residue of cellulosic bioethanol production on soil and environment quality.
Crop residues are not a waste. They are precious commodities and their use as soil amendments is essential for preserving soil quality (Wilhelm et al. 2004). Adverse impacts of residue removal on soil quality must be objectively and critically reviewed prior to installing large-scale cellulosic ethanol plants. Thus, cellulosic biomass must be produced on specifically established energy plantations. It is also possible to procure biomass from low-input high-density (LIHD) mixtures of native grassland perennials (Tilman et al. 2006).
5. Farming carbon
Carbon sequestered in soils and trees can be traded, just as any farm produce (e.g. corn, milk, meat). Trading C credits is a rapidly growing industry (Gressel 2007; McGowan 2007). The market is well established in Europe (Johnson & Heinen 2004; Brahic 2006) and the USA through Chicago Climate Exchange (Breslau 2006). The ‘cap and trade’ movement is gaining momentum in the USA (Schlesinger 2006; McGowan 2007). The discussion on this topic is now being focused on policy options for slowing emissions of GHGs (Kerr 2007; Kintisch 2007b). Important among these options are: (i) imposing tax on C emissions called C tax, (ii) providing government subsidies, and (iii) trading C credits. The cap and trade system would ensure the achievement of environment goals. While the market for trading C sequestered in soils and biota is being developed in North America and Europe, the strategy may be extremely beneficial to the resource-poor and small land holders of developing countries of Asia and Africa. Trading C credits can provide a much needed income stream for the farmer, and an essential incentive to invest in soil restoration (e.g. erosion control, irrigation, fertilizers), break the agrarian stagnation and advance food security.
6. Merits and limitations of biotic versus abiotic carbon sequestration
Biotic C sequestration, based on removal of atmospheric CO2 through photosynthesis, is a natural process. The magnitude of CO2 removal through photosynthesis, in woody plants of managed and natural ecosystems, is likely to increase in the future due to the CO2 fertilization effect. The process can be managed through input of essential nutrients (e.g. N, P, K, Ca, Mg, S, Zn, Cu, Mo) and management of water. There are numerous ancillary benefits of terrestrial/biotic C sequestration. Important among these are: (i) improved quality of soil and water resources, (ii) decreased nutrient losses from ecosystems, (iii) reduced soil erosion, (iv) better wildlife habitat, (v) increased water conservation, (vi) restored degraded soils, and (vii) increased use efficiency of input. Soil C sequestration, both as SOC and SIC, is also a natural process essential for recycling of elements and water. Similar to the terrestrial pool, increase in the SOC pool also has numerous ancillary benefits affecting local, regional and global processes. Principal benefits of SOC sequestration to soil quality are: (i) improvement in soil structure, (ii) reduction in soil erosion, (iii) decrease in non-point source pollution, (iv) increase in plant-available water reserves, (v) increase in storage of plant nutrients, (vi) denaturing of pollutants, (vii) increase in soil quality, (viii) increase in agronomic productivity of food security, (ix) moderation of climate, and (x) increase in aesthetic and economic value of the soil. Therefore, the process of biotic C sequestration strengthens and enhances ecosystem services while enhancing agronomic production. The process is cost-effective, and RMPs for adoption on agricultural and forest soils/ecosystem are available for most ecoregions of the world (IPCC 1999). However, the total sink capacity for biotic C sequestration especially that in terrestrial ecosystems is low at 50–100 Pg C over 25–50-year period (Lal 2004a,b). Further, C sequestered in soil and biota can be re-emitted with change in soil management (e.g. ploughing) and land use (e.g. deforestation) (table 2).
Table 2.
Comparative advantages and constraints of biotic versus abiotic options.
| parameters | biotic (terrestrial) | abiotic (engineering) |
|---|---|---|
| the process | natural (photosynthesis, humification) | engineering (capture and injection) |
| sink capacity | finite (50–100 Pg) | extremely large (thousands of Pg) |
| time horizon | immediate, for next 25–50 years | 10–20 years from now, for long period |
| cost | negative, none or low | High |
| risks | ||
| NPP and biomass yield reduction | minor or low | N.A. |
| human health | minor to low (agricultural chemicals) | High |
| environmental | positive effect (win-win, no-regret) | high |
| leakage | none or small (by ploughing, etc.) | complex and expensive methods |
| monitoring and verification | simple and routine methods | complex and expensive methods |
| regulatory measures | monetary incentives may be helpful | legislative and policy measures essential |
In contrast to biotic sequestration, abiotic sequestration is an engineering process. The technology for deep injection in the ocean, geological strata, coal mines and oil wells, etc. are being developed and may be routinely available by 2025 and beyond. At present, these techniques are expensive and injected CO2 is prone to leakage. In addition to high cost, the issues of measurement and monitoring, adverse ecological impacts and regulatory measures need to be developed and implemented. However, the sink capacity of abiotic techniques is extremely large at thousands of Pg C, and often estimated to exceed the fossil C reserves. Biotic and abiotic systems are complimentary to one another. Depending upon ecosystem characteristics, there may be site-specific ecological niches for biotic and abiotic sequestration options. Biotic sequestration options are immediately available. Use of such options buys us time, while C-neutral energy production technologies of alternatives to fossil fuels and techniques of abiotic sequestration take effect.
7. Conclusions
Although natural terrestrial and oceanic sinks are presently absorbing approximately 60% of the 8.6 Pg C yr−1 emitted, natural sink capacity and rate are not large enough to assimilate all the projected anthropogenic CO2 emitted during the twenty-first century or until the C-neutral energy sources take effect. The sink capacity of managed ecosystems (e.g. forest, soils and wetlands) can be enhanced through conversion to a judicious land use and adoption of RMPs of forestry, agricultural crops and pastures. Purposeful manipulation of biological processes can accelerate the CO2 sequestration process with adoption of regulatory measures and identification of policy incentives. However, for these management systems to be effective, there is a strong need for an integrated systems approach. While the global C cycle is affected by human activities, it is also coupled with cycles of H2O and other elements (e.g. N, P, S) and effectiveness of biotic/terrestrial sequestration depends on scientific understanding of these coupled cycles.
Abiotic sequestration of anthropogenically emitted CO2 through direct injection in oceanic and geological strata has a large sink capacity. Mineral carbonation of CO2 into stable carbonates of Ca and Mg is another option with a large capacity. These engineering techniques are being developed and may be available for routine use by 2025 and beyond. Additional research is needed to make these technologies cost-effective, reduce leakage risks, minimize any adverse impacts on the environment and enhance safety. In addition to economics, the human dimension issues need to be objectively and critically addressed for both biotic and abiotic CO2 sequestration options. Appropriate policy and regulatory measures need to be developed, especially with regards to measurement, monitoring, residence time and trading of carbon credits. While carbon sequestration is an important strategy, the significance of reducing emissions through development of carbon neutral technologies cannot be overemphasized. The latter may involve efficient energy production and usage measures, and finding alternatives to fossil fuel. Biofuels (e.g. bioethanol, bio-diesel, methane gas from biodigester and H2 cell from biomass) are an important component of alternative sources of energy.
Footnotes
One contribution of 15 to a Theme Issue ‘Sustainable agriculture II’.
References
- Anderson S.H, Gantzer C.J, Brown J.R. Soil physical properties after 100 years of continuous cultivation. J. Soil Water Conserv. 1990;45:117–121. [Google Scholar]
- Arends T, Casth P. The comparative effect of equivalent amounts of NPK applied in farmyard manure or in fertilizers, as a function of soil properties. Agrok mas Talajtan. 1994;43:398–407. [Google Scholar]
- Armentano T.V, Menges E.S. Patterns of change in the carbon balance of organic wetlands of the temperate zone. J. Ecology. 1986;74:755–774. doi:10.2307/2260396 [Google Scholar]
- Arrhenius S. On the influence of carbonic acid in the air upon the temperature of the ground. Phil. Mag. J. Sci. (London, Edinburgh, Dublin) 1896;41:237. [Google Scholar]
- Auerbach D.I, Caulfield J.A, Adams E.E, Herzog H.J. Impacts of ocean CO2 disposal on marine life: a toxicological assessment integrating constant-concentration laboratory assay data with variable concentration field exposure. Environ. Model. Assess. 1997;2:333–343. doi:10.1023/A:1019029931755 [Google Scholar]
- Baines S.J, Worden R.H, editors. Geological storage of carbon dioxide. The Geological Society; London, UK: 2004. [Google Scholar]
- Batjes N.H. Total C and N in soils of the world. Eur. J. Soil Sci. 1996;47:151–163. doi:10.1111/j.1365-2389.1996.tb01386.x [Google Scholar]
- Battle M, Bender M.L, Tans P.P, White J.W.C, Ellis J.T, Conway T, Francey R. Global carbon sinks and their variability inferred from O2 and δ13C. Science. 2000;287:2467–2470. doi: 10.1126/science.287.5462.2467. doi:10.1126/science.287.5462.2467 [DOI] [PubMed] [Google Scholar]
- Bowman R.A, Halvorson A.D. Soil chemical changes after nine years of differential N fertilization in no-till dryland wheat–corn–fallow rotation. Soil Sci. 1998;163:241–247. doi:10.1097/00010694-199803000-00009 [Google Scholar]
- Boyd P.W, et al. The decline and fate of an iron-induced sub-arctic phytoplankton bloom. Nature. 2004;428:549–553. doi: 10.1038/nature02437. doi:10.1038/nature02437 [DOI] [PubMed] [Google Scholar]
- Brahic C. Price crash rattles Europe's CO2 reduction scheme. Science. 2006;312:1123. doi: 10.1126/science.312.5777.1123. doi:10.1126/science.312.5777.1123 [DOI] [PubMed] [Google Scholar]
- Breslau, K. It can pay to be green: cleaner air means profits at the climate exchange. Newsweek, 22 May 2006, p. 45.
- Bunker D.E, DeClerck F, Bradford J.C, Colwell R.K, Perfecto I, Phillips O.L, Sankaran M, Naeem S. Species loss and aboveground carbon storage in a tropical forest. Science. 2005;310:1029–1031. doi: 10.1126/science.1117682. doi:10.1126/science.1117682 [DOI] [PubMed] [Google Scholar]
- Buyanoski H.A, Brown J.R, Wagner G.H. Sanborn field; effects of 100 years of cropping on soil parameters influencing crop productivity. In: Paul E.A, Paustian E, Elliot T, Cole C.V, editors. Soil organic matter in temperate ecosystems in North America. CRC Press; Boca Raton, FL: 1997. pp. 205–225. [Google Scholar]
- Buyanoski H.A, Wagner G.H. Carbon cycling in cultivated land and its global significance. Global Change Biol. 1998;4:131–141. doi:10.1046/j.1365-2486.1998.00130.x [Google Scholar]
- Campbell C.A, Biederbeck V.O, Zentner R.P, Lafond G.P. Effect of crop rotations and cultural practices on soil organic mater, microbial biomass and respiration in a thin, black Chernozem. Can. J. Soil Sci. 1991;71:363–376. [Google Scholar]
- Caulfield J.A, Adams E.E, Auerbach D.I, Herzog H.J. Impacts of ocean CO2 disposal on marine life: probalistic plume exposure model with a time-varying dose-responsive analysis. Environ. Model. Assess. 1997;2:345–353. doi:10.1023/A:1019081915826 [Google Scholar]
- Chisholm S.W, Falkowski P.G, Cullen J.J. Dis-crediting ocean fertilization. Science. 2001;294:309–310. doi: 10.1126/science.1065349. doi:10.1126/science.1065349 [DOI] [PubMed] [Google Scholar]
- Christensen B.T. The Askov long-term experiments on animal manure and mineral fertilizers. In: Powlson D.S, Smith P, Smith J.U, editors. Evaluation of soil organic matter: models using existing datasets. NATO, ASI 138. Springer; Heidelberg, Germany: 1996. pp. 301–312. [Google Scholar]
- Cramer W.A, et al. Global response of terrestrial ecosystem structure and function to CO2 and climate change: results from six dynamic global vegetation models. Global Change Biol. 2001;7:357–373. doi:10.1046/j.1365-2486.2001.00383.x [Google Scholar]
- Delgado J.A, Sparks R.T, Follet R.F, Sharkoff J.L, Riggenbach R.R. Use of winter cover crops to conserve soil and water quality in the San Luis Valley of south central Colorado. In: Lal R, editor. Erosion impact on soil quality. CRC; Boca Raton, FL: 1998. pp. 125–142. [Google Scholar]
- Dick, W. A., Van Doren Jr, D. M., Triplett Jr, G. B. & Henry, J. E. 1986 Influence of long-term tillage and rotation combinations on crop yields and selected soil parameters: results obtained for a Mollic Ochraqualf soil. OARDC Res. Bull. 1180, Wooster, OH.
- Drinkwater L.E, Wagoner P, Sarrantonio M. Legume based cropping systems have reduced carbon and nitrogen losses. Nature. 1998;396:262–264. doi:10.1038/24376 [Google Scholar]
- Dumanski J, Desjardins R.L, Tarnocai C.G, Monreal D, Gregorich E.G, Kirkwood V, Campbell C.A. Possibilities for future carbon sequestration in Canadian agriculture in relation to land use changes. Clim. Change. 1998;40:81–103. doi:10.1023/A:1005390815340 [Google Scholar]
- Falkowski P.G. Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature. 1997;387:272–275. doi:10.1038/387272a0 [Google Scholar]
- Falkowski P, et al. The global carbon cycle: a test of our knowledge of earth as a system. Science. 2000;290:291–296. doi: 10.1126/science.290.5490.291. doi:10.1126/science.290.5490.291 [DOI] [PubMed] [Google Scholar]
- Fan L.S, Park A. CO2 mineral sequestration in a high pressure, high temperature, 3-phase fluidizied bed reactor. Can. J. Chem. Eng. 2004;81:855–890. [Google Scholar]
- Fan S, Gloor M, Mahlman J, Pacala S, Sarmiento J, Takahashi T, Tan P. A large terrestrial carbon sink in North America implied by atmospheric and oceanic carbon dioxide data and models. Science. 1998;282:442–446. doi: 10.1126/science.282.5388.442. doi:10.1126/science.282.5388.442 [DOI] [PubMed] [Google Scholar]
- Fang J, Chen A, Peng C, Zhao S, Ci L. Changes in forest biomass carbon storage in China between 1949 and 1998. Science. 2001;292:2320–2316. doi: 10.1126/science.1058629. doi:10.1126/science.1058629 [DOI] [PubMed] [Google Scholar]
- Follett R.F. Soil management concepts and carbon sequestration in cropland soils. Soil Till. Res. 2001;61:77–92. doi:10.1016/S0167-1987(01)00180-5 [Google Scholar]
- Fowles M. Black carbon sequestration as an alternative to bioenergy. Biomass Bioenergy. 2007;31:426–432. [Google Scholar]
- Freund P, Ormerod W.G. Progress towards storage of carbon dioxide. Energy Convers. Manage. 1997;38:198–205. doi:10.1016/S0196-8904(96)00269-5 [Google Scholar]
- Fullen M.A, Auerswald K. Effect of grass ley set aside on runoff, erosion and organic mater levels in sandy soil in east Shropshire, U.K. Soil Till. Res. 1998;46:41–49. [Google Scholar]
- Fung I. Variable carbon sinks. Science. 2000;290:1313. doi: 10.1126/science.290.5495.1313. doi:10.1126/science.290.5495.1313 [DOI] [PubMed] [Google Scholar]
- Gale J. Why do we need to consider geological storage of CO2? In: Baines S.J, Worden R.H, editors. Geological storage of carbon dioxide. The Geological Society; London, UK: 2004. pp. 7–15. [Google Scholar]
- Garnett M.H, Ineson P, Stevenson A.C, Howard D.C. Terrestrial organic carbon in a British moorland. Global Change Biol. 2001;7:375–388. doi:10.1046/j.1365-2486.2001.00382.x [Google Scholar]
- Gerdemann, S. J., Dahlin, D. C., O'Connor, W. K. & Penner, L. R. 2003 Carbon dioxide sequestration by aqueous mineral carbonation of magnesium silicate miners. In Second Annual Conference on Carbon Sequestration, NETL Proceedings 5–9 May, 2003, Alexandria, VA Pittsburgh, PA: NETL. See www.carbonsq.com/pdf/psoters/BCI2
- Gerdemann, S. J., Dahlinm, D. C., O'Connor, W. K., Penner, L. R. & Rush, G. E. 2004 Factors affecting ex-situ aqueous mineral carbonation using Ca and Mg silicate minerals. In Proc. Clean Water Conf., 30 April 2004, Portland Oregon Portland OR: Environmental Law Education Center.
- Gile L.H. Carbon storage in sand soils of the Leasburg Surface, southern New Mexico. Soil Sci. 1993;156:101–110. doi:10.1097/00010694-199308000-00006 [Google Scholar]
- Goff, F., Guthrie, G., Counce, D., Kluk, E., Bergfeld, D. & Snow, M. 1997 Preliminary investigations on the CO2 sequestering potential of ultrafamic rocks. LA-13328-MS, Los Alamos National Laboratory, Los Alamos, NM, USA.
- Goff, F., Guthrie, G., Lipin, B., Fite, M., Chipera, S., Counce, D., Kluk, E. & Ziock, H. 2000 Evaluation of ultrafamic deposits in the eastern U.S. and Puerto Rico as sources of magnesium for CO2 sequestration. LA-13694-MS, Los Alamos National Laboratory, Los Alamos, NM, USA.
- Goldemberg J. Ethanol for a sustainable energy future. Science. 2007;315:808–810. doi: 10.1126/science.1137013. doi:10.1126/science.1137013 [DOI] [PubMed] [Google Scholar]
- Gorham E. Northern peatlands: role in the carbon cycle and probable responses to climate warming. Ecol. Appl. 1991;1:182–195. doi: 10.2307/1941811. doi:10.2307/1941811 [DOI] [PubMed] [Google Scholar]
- Grace P, Oades J.M. Long-term field trials in Australia. In: Leigh R.A, Johnston A.E, editors. Long-term experiments in agricultural and ecological sciences. CAB International; Wallingford, UK: 1994. pp. 53–81. [Google Scholar]
- Graham R.L, Nelson R, Sheehan J, Perlack R.D, Wright L.L. Current and potential U.S. corn stover supplies. Agron. J. 2007;99:1–11. [Google Scholar]
- Grainger A. Modeling the anthropogenic degradation of drylands and the potential to mitigate global climate change. In: Squires V.R, Glenn E.P, Ayoub T.A, editors. Global climate change by combating land degradation. UNEP; Nairobi, Kenya: 1995. pp. 193–199. [Google Scholar]
- Greene C.H, Pershing A.J. Climate drives sea change. Science. 2007;315:1084–1085. doi: 10.1126/science.1136495. doi:10.1126/science.1136495 [DOI] [PubMed] [Google Scholar]
- Gregorich E.G, Ellert B.H, Dury C.F, Linang B.C. Fertilization effects on soil organic matter turnover and crop residue carbon storage. Soil Sci. Soc. Am. J. 1996;61:1159–1175. [Google Scholar]
- Gressel N. From greener production to carbon trading: sustainable energy careers. Science. 2007;315:868–869. doi: 10.1126/science.315.5813.868. doi:10.1126/science.315.5813.868 [DOI] [PubMed] [Google Scholar]
- Halvorson A.D, Reule C.A, Follett R.F. Nitrogen fertilization effects on soil carbon and nitrogen in a dryland cropping system. Soil Sci. Soc. Am. J. 1999;63:912–917. [Google Scholar]
- Halvorson A.D, Wienhold B.J, Black A.L. Tillage, nitrogen and cropping system effects on soil carbon sequestration. Soil Sci. Soc. Am. J. 2002;66:906–912. [Google Scholar]
- Herzog, H., Drake, E. & Adams, E. E. 1997 CO2 capture, reuse and storage technologies for mitigating climate change. A white paper prepared for USDOE by the MIT Energy Lab. See web.mit.edu/energylab/www/
- Herzog H, Eliasson B, Kaarstad O. Capturing greenhouse gases. Sci. Am. 2002;282:72–80. doi: 10.1038/scientificamerican0200-72. [DOI] [PubMed] [Google Scholar]
- Hillman G.R. Effects of engineered drainage on water table and peat subsidence in an Alberta treed fern. In: Trettin C.C, Jurgensen M.F, Grigal D.F, Gale M.R, Jeglum J.K, editors. Northern forested wetlands: ecology and management. CRC Press; Boca Raton, FL: 1997. pp. 253–272. [Google Scholar]
- Himes F.L. Nitrogen, sulfur and phosphorus and the sequestering of carbon. In: Lal R, Kimble J.M, Follett R.F, Stewart B.A, editors. Soil processes and the carbon cycle. CRC Press; Boca Raton, FL: 1998. pp. 315–319. [Google Scholar]
- Himmel M.E, Ding S.-Y, Johnson D.K, Adney W.S, Nimlos M.R, Brady J.W, Foust T.D. Biomass recalcitrance: engineering plants and enzymes for biofuels production. Science. 2007;315:804–807. doi: 10.1126/science.1137016. doi:10.1126/science.1137016 [DOI] [PubMed] [Google Scholar]
- IPCC. Inter-government panel on climate change. Cambridge University Press; Cambridge, UK: 1999. Land use, land use change and forestry. [Google Scholar]
- IPCC. Inter-government panel on climate change. Cambridge University Press; Cambridge, UK: 2001. Climate change 2001: the scientific basis. [Google Scholar]
- IPCC. Working Group II. IPCC; Geneva, Switzerland: 2007. Climate change 2007. Climate change impacts, adaptation and vulnerability. [Google Scholar]
- Jacinthe P.A, Lal R, Kimble J.M. Effects of wheat residue fertilization on accumulation and biochemical attributes of organic carbon in central Ohio Luvisol. Soil Sci. 2002;167:750–758. doi:10.1097/00010694-200211000-00005 [Google Scholar]
- Jackson R.B, et al. Trading water for carbon with biological carbon sequestration. Science. 2005;310:1944–1947. doi: 10.1126/science.1119282. doi:10.1126/science.1119282 [DOI] [PubMed] [Google Scholar]
- Janzen H.H, Campell C.A, Izaurralde R.C, Ellert B.H, Juma N, McGill W, Zentner R.P. Management effects on soil C storage on the Canadian prairies. Soil Till. Res. 1998;47:181–195. doi:10.1016/S0167-1987(98)00105-6 [Google Scholar]
- Jenkinson D.S. The turnover of organic carbon and nitrogen in soil. Phil. Trans. R. Soc. B. 1990;329:361–368. doi:10.1098/rstb.1990.0177 [Google Scholar]
- Johnson E, Heinen R. Carbon trading: time for industry involvement. Environ. Int. 2004;30:279–288. doi: 10.1016/j.envint.2003.09.001. doi:10.1016/j.envint.2003.09.001 [DOI] [PubMed] [Google Scholar]
- Johnson K.S, Karl D.M, Chisholm S.W, Falowski P.G, Cullen J.J. Is ocean fertilization credible and creditable? Science. 2002;296:467–468. doi: 10.1126/science.296.5567.467b. doi:10.1126/science.296.5567.467b [DOI] [PubMed] [Google Scholar]
- Johnston, A. E. 1973 The effects of ley and arable cropping systems on the amount of organic matter in the Rothamstead and Woburn ley-arable experiments. Rothamstead Report for 1972, Part 2, pp. 131–159.
- Kerr R.A. Bush backs spending for a “global problem”. Science. 2001;292:1978. doi: 10.1126/science.292.5524.1978. doi:10.1126/science.292.5524.1978 [DOI] [PubMed] [Google Scholar]
- Kerr R.A. Scientists tell policy makers we're all warming the world. Science. 2007;315:754–757. doi: 10.1126/science.315.5813.754. doi:10.1126/science.315.5813.754 [DOI] [PubMed] [Google Scholar]
- Kintisch E. Report backs more projects to sequester CO2 from coal. Science. 2007a;315:1481. doi: 10.1126/science.315.5818.1481a. doi:10.1126/science.315.5818.1481a [DOI] [PubMed] [Google Scholar]
- Kintisch E. New congress may be warming up to plans for capping emissions. Science. 2007b;315:444. doi: 10.1126/science.315.5811.444. doi:10.1126/science.315.5811.444 [DOI] [PubMed] [Google Scholar]
- Klara S.M, Srivastava R.D, McElvried H.G. Integrated collaborative technology development program for CO2 sequestration in geologic formations. Energy Convers. Manage. 2003;44:2699–2712. doi:10.1016/S0196-8904(03)00042-6 [Google Scholar]
- Kluger J. Global warming: what now? Our feverish planet badly needs a cure. Time Magazine. 2007;9:50–109. [PubMed] [Google Scholar]
- Klusman R.W. Evaluation of leakage potential CO2 EOR/sequestration project. Energy Convers. Manage. 2003;33:1921–1940. doi:10.1016/S0196-8904(02)00226-1 [Google Scholar]
- Kobak K.I, Knodrasheva N.Y, Turchinovich I.E. Changes in C pools of peatland and forests in northwestern Russia during the Holocene. Global Planet. Change. 1998;16/17:75–84. doi:10.1016/S0921-8181(98)00011-3 [Google Scholar]
- Kohlmaier G.H, Hager C, Ift F, Wurth G, Joos F, Bruno M. Forestry mitigation options under future climate change and socioeconomic pressures. 4.1. Future developments of the carbon cycle; the role of the biota/forests within the IPCC stabilization scenarios. In: Kohlmaier G.H, Weber M, Houghton R.A, editors. Carbon dioxide mitigation in forestry and wood industry. Springer; Berlin, Germany: 1998. pp. 269–291. [Google Scholar]
- Korschens M, Muller A. The static experiment Bad Lauchst dt. Germany. In: Powlson D.S, Smith P, Smith J.U, editors. Evaluation of soil organic matter: models using existing datasets. NATO, ASI 138. Springer; Heidelberg, Germany: 1996. pp. 369–378. [Google Scholar]
- Krishnamurthy R.V, Machavaram M. Is there a stable isotope evidence for the fertilization effect? Proc. Indian Acad. Sci. Earth Planet. Sci. 2000;109:141–144. [Google Scholar]
- Lackner K.S. A guide to CO2 sequestration. Science. 2003;300:1677–1678. doi: 10.1126/science.1079033. doi:10.1126/science.1079033 [DOI] [PubMed] [Google Scholar]
- Lackner, K. S., Butt, D. P., Wendt, C. H. & Sharp, D. H. 1996 Carbon dioxide disposal in solid form. In Proc. 21st Int. Conf. on Coal Utilization and Fuel System Clearwater, FL: Coal Technology Association.
- Lackner, K. S., Butt, D. P. & Wendt, C. H. 1997 Magnesite disposal of carbon dioxide. LANL, LA-UR-97-660, Los Alamos, New Mexico.
- Lal R. World cropland soils as a source or sink for atmospheric carbon. Adv. Agron. 2001;71:145–191. [Google Scholar]
- Lal R. Agricultural activities and the global carbon cycle. Nutr. Cycl. Agroecosyst. 2004a;70:103–116. doi:10.1023/B:FRES.0000048480.24274.0f [Google Scholar]
- Lal R. Soil carbon sequestration impacts on global climate change and food security. Science. 2004b;304:1623–1627. doi: 10.1126/science.1097396. doi:10.1126/science.1097396 [DOI] [PubMed] [Google Scholar]
- Lal R. Forest soils and carbon sequestration. Forest Ecol. Manage. 2005a;220:242–258. doi:10.1016/j.foreco.2005.08.015 [Google Scholar]
- Lal R. Soil carbon sequestration in natural and managed tropical forest ecosystems. J. Sustain. Forestry. 2005b;21:1–30. [Google Scholar]
- Lal R. World crop residues production and implications of its use as a biofuel. Environ. Int. 2005c;31:575–584. doi: 10.1016/j.envint.2004.09.005. doi:10.1016/j.envint.2004.09.005 [DOI] [PubMed] [Google Scholar]
- Lal R. Enhancing crop yields through restoration of soil organic carbon pool in agricultural lands. Land Degrad. Dev. 2006;17:197–206. doi:10.1002/ldr.696 [Google Scholar]
- Lal R, Kimble J.M, Follett R.F, Cole C.V. Ann Arbor Press; Chelsea, MI: 1998. The potential of U.S. cropland to sequester carbon and mitigate the greenhouse effect. [Google Scholar]
- Lal R, Follett R.F, Kimble J.M. Achieving soil carbon sequestration in the United States: a challenge to the policy makers. Soil Sci. 2003;168:827–845. doi:10.1097/01.ss.0000106407.84926.6b [Google Scholar]
- Lamb D, Erskine P.D, Parrotta J.A. Restoration of degraded tropical forest landscape. Science. 2005;310:1628–1632. doi: 10.1126/science.1111773. doi:10.1126/science.1111773 [DOI] [PubMed] [Google Scholar]
- Landi, A. 2002 Carbon balance in the major soil zones of Saskatchewan. PhD dissertation, University of Saskatchewan, SK, Canada.
- Liebig M.A, Varvel G.E, Doran J.W, Wienhold B.J. Crop sequence and nitrogen fertilization effects on soil properties in the western corn belt. Soil Sci. Soc. Am. J. 2002;66:596–601. [Google Scholar]
- Machette, M. N. 1985 Calcic soils of the southwestern United States. In Soils and quarternary geomorphology of southwestern United States, vol. 203 (D. L. Weide), pp. 1–21. Boulder, CO: Geological Society of America Special Paper.
- Malhi S.S, Nyborg M, Harpiak J.T, Heier K, Flore N.A. Increasing organic carbon and nitrogen under bromegrass with long-term N fertilization. Nutr. Cycl. Agroecosyst. 1997;49:255–260. doi:10.1023/A:1009727530325 [Google Scholar]
- Marion G.M, Schlesinger W.H, Fonteyn P.J. CALDEP: a regional model for soil CaCO3 (caliche) deposition in southwestern deserts. Soil Sci. 1985;139:468–481. doi:10.1097/00010694-198505000-00014 [Google Scholar]
- Martin J.H, Fitzwater S.E. Iron deficiency limits of phytoplankton growth in the north-east Pacific sub-arctic. Nature. 1988;331:341–343. doi:10.1038/331341a0 [Google Scholar]
- Martin J.H.K.H, et al. Testing the iron hypothesis in ecosystems of the equatorial Pacific Ocean. Nature. 2002;371:123–129. doi:10.1038/371123a0 [Google Scholar]
- McCarl B.A, Schneider U.A. Greenhouse gas mitigation in U.S. agriculture and forestry. Science. 2001;294:2481–2482. doi: 10.1126/science.1064193. doi:10.1126/science.1064193 [DOI] [PubMed] [Google Scholar]
- McGowan, E. 2007 WM joins carbon market model discussion. Waste News 22 January 2007, 23.
- Mermut A.R, Landi A. Secondary/pedogenic carbonates. In: Lal R, editor. Encyclopedia of soil science. Taylor and Francis; Boca Raton, FL: 2006. pp. 1551–1554. [Google Scholar]
- Milne R, Brown T.A. Carbon in the vegetation and soils of Great Britain. J. Environ. Manage. 1997;49:413–433. doi:10.1006/jema.1995.0118 [Google Scholar]
- Monger, H. C. 2002 Pedogenic carbonate: links between biotic and abiotic CaCO3 Presented at the 17th World Cong. Soil Science, 14–21 August 2002, Bangkok, Thailand.
- Monger H.C, Gallegos R.A. Biotic and abiotic processes and rates of pedogeniccarbonate accumulation in the southwestern U.S., relationship to atmospheric CO2 sequestration. In: Lal R, Kimble J.M, Stewart B.A, editors. Global climate change and pedogenic carbonates. CRC Lewis Publishers; Boca Raton, FL: 2000. pp. 273–290. [Google Scholar]
- Morris E. Putting the carbon back: black is the new green. Nature. 2006;442:624–626. doi: 10.1038/442624a. doi:10.1038/442624a [DOI] [PubMed] [Google Scholar]
- Nilsson L.G. Data of yield and soil analysis in the long-term soil fertility experiments. J. R. Swed. Acad. Agr. Forestery. Suppl. 1986;18:32–70. [Google Scholar]
- Nordt L.C, Wilding L.P, Drees L.R. Pedogenic carbonate transformations in leaching soil systems. In: Lal R, Kimble J.M, Stewart B.A, editors. Global climate change and pedogenic carbonate. CRC Lewis Publishers; Boca Raton, FL: 2000. pp. 43–64. [Google Scholar]
- O'Connor W.K, Dahlin D.C, Turner P.C, Walters R.P. Carbon dioxide sequestration by ex-situ mineral carbonation. Technology. 2000;75:115–123. [Google Scholar]
- O'Connor, W. K., Dahlin, D. C., Nilsen, D. N., Rush, G. E., Walters, R. P. & Turner, P. C. 2001 Carbon dioxide sequestration by direct mineral carbonation: results from recent studies and current status. See. www.netl.doe.gov/publications/proceedings/o1/carbon_seq/bC2.pdf
- Orr J.C, et al. Estimates of anthropogenic carbon uptake from four three-dimensional global ocean models. Global Biogeochem. Cycles. 2001;15:43–60. doi:10.1029/2000GB001273 [Google Scholar]
- Paavilainen E, Paivanen J. Springer; New York, NY: 1995. Peatland forestry: ecology and principles. [Google Scholar]
- Pacala S.W. Consistent land and atmosphere-based U.S. carbon sink estimates. Science. 2001;292:2316–2320. doi: 10.1126/science.1057320. doi:10.1126/science.1057320 [DOI] [PubMed] [Google Scholar]
- Pacala S, Socolow R. Stabilization wedges: solving the climate problem for the next 50 years with current technologies. Science. 2004;305:968–972. doi: 10.1126/science.1100103. doi:10.1126/science.1100103 [DOI] [PubMed] [Google Scholar]
- Pacala S.W, et al. Consistent land- and atmosphere- based U.S. carbon sink estimates. Science. 2001;292:2316–2319. doi: 10.1126/science.1057320. doi:10.1126/science.1057320 [DOI] [PubMed] [Google Scholar]
- Paustian K, Collins H.P, Paul E.A. Management controls on soil carbon. In: Paul E.A, Paustian K, Elliott T, Cole C.V, editors. Soil organic matter in temperate agroecosystems: long-term experiments in North America. CRC Press; Boca Raton, FL: 1997. pp. 15–49. [Google Scholar]
- Prather, M. et al 2001 Atmospheric chemistry and greenhouse gases. In Climate change 2001: the scientific basis, (eds J. T. Houghton, Y. Ding, D. J. Griggs, M. Noguer, P. J. van der Linden & D. Xiaosu), pp. 239–287. Cambridge, UK: IPCC, Cambridge University Press.
- Rabenhorst M.C, Wilding L.P. Pedogenesis on the Edwards Plateau, Tesas. III. A new model for the formation of Petrocalcic horizon. Soil Sci. Soc. Am. J. 1986;50:693–699. [Google Scholar]
- Ramaswamy, V., Boucher, O., Haigh, J., Hauglustaine, D., Haywood, J., Myhre, G., Nakajima, T., Shi, G. Y. & Solomon, S. 2001 Radiative forcing of climate change. In Climate change 2001: the scientific basis, pp. 349–416. Cambridge, UK: IPCC, Cambridge University Press.
- Rasmussen P.E, Albrecht S.L, Smiley R.W. Soil C and N changes under tillage and cropping systems in semiarid Pacific Northwest agriculture. Soil Till. Res. 1998;47:197–205. doi:10.1016/S0167-1987(98)00106-8 [Google Scholar]
- Raven J.A, Falkowski P.G. Oceanic sinks for atmospheric CO2. Plant Cell Environ. 1999;22:741–755. doi:10.1046/j.1365-3040.1999.00419.x [Google Scholar]
- Ridley A.M, Slattery W.J, Helyer K.R, Cowling A. The importance of the carbon cycle to acidification of a grazed pasture. Aust. J. Exp. Agr. 1990;30:539–544. doi:10.1071/EA9900539 [Google Scholar]
- Rivkin R.B, Legendre L. Biogenic carbon cycling in the upper ocean: effects of microbial respiration. Science. 2001;291:2398–2400. doi: 10.1126/science.291.5512.2398. doi:10.1126/science.291.5512.2398 [DOI] [PubMed] [Google Scholar]
- Rojstaczer S, Deverel S.J. Land subsidence in drained Histosols and highly organic mineral soils of California. Soil Sci. Soc. Am. J. 1995;59:11 162–11 167. [Google Scholar]
- Running S.M. Is global warming causing more large wildfires? Science. 2006;313:927–928. doi: 10.1126/science.1130370. doi:10.1126/science.1130370 [DOI] [PubMed] [Google Scholar]
- Sauerbeck D.R. CO2 emissions and C sequestration by agriculture—perspectives and limitations. Nutr. Cycl. Agroecosyst. 2001;60:253–266. doi:10.1023/A:1012617516477 [Google Scholar]
- Schjonning P, Christensen B.T, Christensen B. Physical and chemical properties of a sandy loam receiving animal manure, mineral fertilizer of no fertilizer for 90 years. Eur. J. Soil Sci. 1994;45:257–268. doi:10.1111/j.1365-2389.1994.tb00508.x [Google Scholar]
- Schlesinger W.H. The formation of caliche in soils of Mojave Desert, California. Geeocheem. Cosmochim. ACTA. 1985;49:57–66. doi:10.1016/0016-7037(85)90191-7 [Google Scholar]
- Schlesinger W.H. Carbon sequestration in soils. Science. 1999;284:2095. doi:10.1126/science.284.5423.2095 [Google Scholar]
- Schlesinger W.H. Carbon trading. Science. 2006;314:1217. doi: 10.1126/science.1137177. doi:10.1126/science.1137177 [DOI] [PubMed] [Google Scholar]
- Scholes R.J, Noble I.R. Storing carbon on land. Science. 2001;294:1012–1013. doi: 10.1126/science.1065307. doi:10.1126/science.1065307 [DOI] [PubMed] [Google Scholar]
- Schnitzer M. Soil organic mater—the next 75 years. Soil Sci. 1991;151:41–58. doi:10.1097/00010694-199101000-00008 [Google Scholar]
- Schrag D.P. Preparing to capture carbon. Science. 2007;315:812–813. doi: 10.1126/science.1137632. doi:10.1126/science.1137632 [DOI] [PubMed] [Google Scholar]
- Seibel B.A, Walsh P.J. Potential impacts of CO2 injection on deep-sea biota. Science. 2001;294:319–320. doi: 10.1126/science.1065301. doi:10.1126/science.1065301 [DOI] [PubMed] [Google Scholar]
- Service R.F. Cellulosic ethanol: biofuel researchers prepare to reap a new harvest. Science. 2007;315:1488–1491. doi: 10.1126/science.315.5818.1488. doi:10.1126/science.315.5818.1488 [DOI] [PubMed] [Google Scholar]
- Six J.T, Conant R.T, Paul E.A, Paustian K. Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil. 2002;241:155–176. doi:10.1023/A:1016125726789 [Google Scholar]
- Smith P, Powlson S.D.S, Glendining M.J, Smith J.U. Potential for carbon sequestration in European soils: preliminary estimates for five scenarios using results from long-term experiments. Global Change Biol. 1997;3:67–79. doi:10.1046/j.1365-2486.1997.00055.x [Google Scholar]
- Sobecki T.M, Wilding L.P. Formation of calcic and argillic horizons in selected soils of the Texas coast prairie. Soil Sci. Soc. Am. J. 1983;47:707–715. [Google Scholar]
- Somerville C. The billion ton biofuel vision. Science. 2006;312:1277. doi: 10.1126/science.1130034. doi:10.1126/science.1130034 [DOI] [PubMed] [Google Scholar]
- Squires, V., Glenn, E. P. & Ayoub A. T. (eds) 1995 Combating global climate change by combating land degradation. In Proc. Workshop held in Nairobi, Kenya 4–8 Sept. 1995 Nairobi, Kenya: UNEP.
- Stephanopoulos G. Challes in engineering microbes for biofuels production. Science. 2007;315:801–804. doi: 10.1126/science.1139612. doi:10.1126/science.1139612 [DOI] [PubMed] [Google Scholar]
- Studdert G.A, Echeverria H.E. Crop rotations and nitrogen fertilization to manage soil organic carbon dynamics. Soil Sci. Soc. Am. J. 2000;64:1496–1503. [Google Scholar]
- Tans P.P, Fung I.Y, Takahashi T. Observational constraints on the global atmospheric CO2 budget. Science. 1990;247:1431–1438. doi: 10.1126/science.247.4949.1431. doi:10.1126/science.247.4949.1431 [DOI] [PubMed] [Google Scholar]
- Tilman D, Hill J, Lehman C. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science. 2006;314:1598–1606. doi: 10.1126/science.1133306. doi:10.1126/science.1133306 [DOI] [PubMed] [Google Scholar]
- Townsend A.R, Asner G.P, White J.W.C, Tans P.P. Land use effects on atmospheric 13C imply a sizeable terrestrial CO2 sink in tropical latitudes. Geophys. Res. Lett. 2002;29:68/1–68/4. doi:10.1029/2001GL013454 [Google Scholar]
- Tsang C.F, Benson S.M, Kogelski B, Smith R.E. Scientific considerations related to regulation development for CO2 sequestration in brine formations. Environ. Geol. 2002;42:275–281. doi:10.1007/s00254-001-0497-4 [Google Scholar]
- Uhlen G. Long-term effects of fertilizers, manures, straw and crop rotation on total C in soil. Acta Agr. Scand. 1991;41:119–127. [Google Scholar]
- Uhlen G, Tveitnes S. Effects of long-term crop rotation, fertilizers, farm manure and straw on soil productivity. Nor. J. Agr. Sci. 1995;9:143–161. [Google Scholar]
- Van Dijk H. Survey of Dutch soil organic research with regard to humification and degradation rates in arable land. In: Boels D.D, Davis B, Johnston A.E, editors. Use seminar on land degradation. Balkema; Rotterdam, The Netherlands: 1982. pp. 133–143. [Google Scholar]
- Wald, M. L. 2007 Is ethanol for the long haul? Sci. Am January 2007, pp. 42–49. [DOI] [PubMed]
- Walsh, B. 2007 Greenhouse airlines: traveling by jet is a dirty business. As passenger load increases, enviros look for ways to cut back the carbon. Time, 12 Feb. 2007, 57.
- Warner B.G, Clymo R.S, Tolonen K. Applications of peat accumulation at Point Escuminac, New Brunswick. Q. Res. 1993;39:245–248. doi:10.1006/qres.1993.1028 [Google Scholar]
- West T.O, Post W.M. Soil organic carbon sequestration rates by tillage and crop rotation: a global data analysis. Soil Sci. Soc. Am. J. 2002;66:1930–1946. [Google Scholar]
- Westerling A.L, Hidalgo H.G, Cayan D.R, Swetnam T.W. Warming and earlier spring increase western U.S. forest wildfire activity. Science. 2006;313:940–943. doi: 10.1126/science.1128834. doi:10.1126/science.1128834 [DOI] [PubMed] [Google Scholar]
- Wilding L.P. Comments on paper by Lal. In: Rosenberg N, Izaurralde R.C, Malone E.L, editors. Carbon sequestration in soils: science, monitoring and beyond. Battelle Press; Columbus, OH: 1999. pp. 146–149. [Google Scholar]
- Wilhelm W.V, Johnson J.M.F, Hatfield J.L, Vorhees W.B, Linden D.R. Crop and soil productivity response to crop residue management: a literature review. Agron. J. 2004;96:1–17. [Google Scholar]
- Witter E, Mortensson A.M, Garcia F.V. Size of the microbial mass in a long-term field experiment as affected by different N fertilizers. Soil Biol. Biochem. 1993;28:659–669. doi:10.1016/0038-0717(93)90105-K [Google Scholar]
- WMO. World Meteorological Organization; Geneva, Switzerland: 2006. Greenhouse gas bulletin: the state of greenhouse gases in the atmosphere using global observations up to December 2004. [Google Scholar]
- Wofsy S.C. Where has all the carbon gone? Science. 2001;292:2261–2263. doi: 10.1126/science.1061077. doi:10.1126/science.1061077 [DOI] [PubMed] [Google Scholar]
- Wosten J.H, Ismail A.B, Van Wijic A.L. Peat subsidence and its practical implications: a case study in Malaysia. Geoderma. 1997;78:25–36. doi:10.1016/S0016-7061(97)00013-X [Google Scholar]