Abstract
The neural crest (NC) cells have been called the ‘explorers of the embryos’ because they migrate all over the embryo where they differentiate into a variety of diverse kinds of cells. In this work, we analyse the role of different molecules controlling the migration of NC cells. First, we describe the strong similarity between the process of NC migration and metastasis in tumour cells. The epithelial–mesenchymal transition process that both kinds of cells undergo is controlled by the same molecular machinery, including cadherins, connexins, Snail and Twist genes and matrix metalloproteases. Second, we analysed the molecular signals that control the patterned migration of the cephalic and trunk NC cells. Most of the factors described so far, such as Eph/ephrins, semaphorins/neuropilins and Slit/Robo, are negative signals that prohibit the migration of NC cells into target areas of the embryo. Finally, we analyse how the direction of migration is controlled by regulation of cell polarity and how the planar cell polarity or non-canonical Wnt signalling is involved in this process.
Keywords: neural crest, metastasis, tumour cells, planar cell polarity, Wnt signalling, epithelial–mesenchymal transition
1. Introduction
The neural crest (NC) is one of the most pluripotent embryonic tissues. It gives rise to typical ectodermal cells such as neurons and glia, typical mesodermal cells such as cartilage and muscle cells, and typical endodermal cells such as gland cells. As a consequence of this pluripotent status, NC contributes to the formation of many organs such as the face, neck, heart, adrenal gland, peripheral nervous system and skin. This vast range of different kinds of cell seems to be determined by a combination of intrinsic factors, specified in the genetic network activated during NC induction, and by external cues coming from the environment that the cells encounter during their migration or after they have reached their final target. Thus, understanding the mechanisms that control cell migration is essential to comprehend how NC pluripotency is regulated.
The NC is induced at the border of the neural plate by signalling coming from the surrounding epidermis, neural plate and underlying mesoderm (Raven & Kloos 1945; Mayor et al. 1995; Selleck & Bronner-Fraser 1995; Mancilla & Mayor 1996; Bonstein et al. 1998). A complex array of different signals is produced by these tissues that specify the NC at a precise location along the dorsoventral and anterior–posterior axis of the embryo. An initial gradient of bone morphogenic protein (BMP) activity specifies the border of the neural plate, and later a combination of Wnts, fibroblast growth factors (FGFs) and retinoic acid (RA) transforms the border of the neural plate into NC cells (Barembaum & Bronner-Fraser 2005; Steventon et al. 2005). The integration of all these signals initially leads to the activation of a genetic cascade in the entire neural fold which later becomes restricted to the NC (Mayor & Aybar 2001; Meulemans & Bronner-Fraser 2004; Steventon et al. 2005). The initial set of genes induced at the neural plate border has been called cell specification or neural plate border specifier genes. Their expressions are not restricted to the prospective NC as they are expressed in a wider domain that includes prospective epidermal and neural plate cells. They control the expression of the second group of genes, called cell survival or NC specifier genes. The expression of this second group of genes is restricted to the prospective NC cells and most of these genes control the survival of the NC cells. The third group of genes is the last to be expressed in the NC that are starting to migrate and differentiate and they are the target of the two previous groups of genes; they have been called migration/differentiation or NC effector genes. They are involved in the last step of NC migration and differentiation.
One of the most notable characteristics of the NC cells is their ability to migrate. They have been called the ‘explorers of the embryos’ because they are able to migrate extremely long distance, following specific pathways and colonizing almost all the tissues of the embryo. It has been found in recent years that many of the factors that belong to the NC genetic cascade are also involved in controlling tumour progression. Furthermore, cellular and embryological evidence seems to indicate that cancer cells share many characteristics with NC cells. In this work, we will analyse the molecular signals that control NC migration; we will describe the molecules and pathways that are common to NC and cancer cells, the signals that have been identified as inhibitors of NC migration and finally the data that describe how the directionality of NC migration is controlled through the regulation of cell polarity.
2. Migration of NC cells and tumour progression
Molecular and cellular evidences show common mechanisms between NC migration and metastasis in cancer cells. When metastatic human melanoma is transplanted into the neural tube of a chick embryo, the melanoma cells migrate and behave as NC cells, suggesting that melanoma cells are able to respond to embryonic environmental cues similar to the signals that control NC migration (Kulesa et al. 2006).
Migration of NC cells and metastasis of tumour cells is a complex and multi-step process that has been characterized, and specific steps have been defined for them (Thiery 2002; Trainor 2005). Before NC emigrates from the neural tube, they undergo a process called epithelial–mesenchymal transition (EMT) that will confer to them the ability to migrate. A similar EMT process takes place in cancer cells during tumour progression. The EMT involves different cellular machineries and implies deep changes in cell morphology and in the repertoire of cell surface adhesion and recognition molecules. When the EMT is complete, the NC cells delaminate from the neuroepithelia and migrate along specific pathways. EMT is required for the tumour cell to acquire the invasive property characteristic of metastasis. The invasion of tumour cells is one of the first steps of metastasis and it allows the entry into the systemic circulatory system and, from there, into a new host tissue, where the tumour cells proliferate and generate a secondary tumour. Most of the deaths caused by cancer are dependent on this metastatic process and not on the primary tumour.
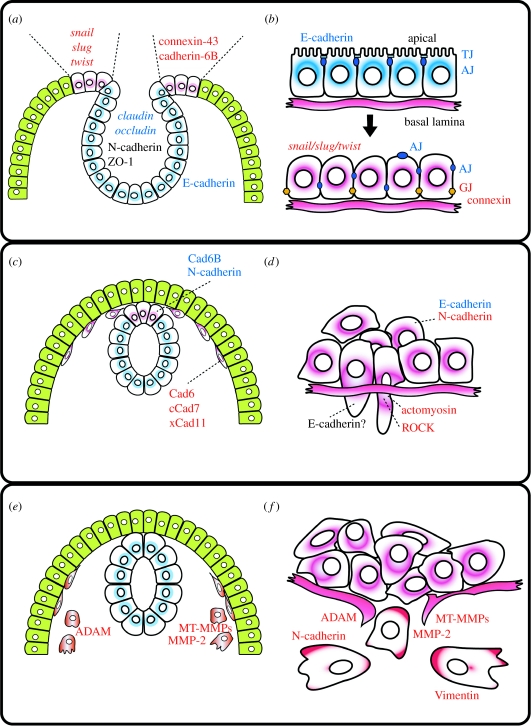
(a) Loss of polarity: from tight to gap junctions
Premigratory NC cells and non-metastatic tumour cells form an epithelium with a typical apical–basal polarity; however, this polarity is lost in order for these cells to migrate (figure 1a,b). In avian NC cells, the apical zones are joined by intercellular junctions at the stage of neural fold fusion, but such junctions disappear progressively approximately 5 hours before the onset of migration (Newgreen & Gibbins 1982; Newgreen et al. 1982). During this time, occludin, which is a major component of tight junctions, is downregulated from the neural tube and a de-epithelization process takes place (Aaku-Saraste et al. 1996). Interestingly, the activation of Raf-1 in tumour cells, which triggers EMT, leads to the downregulation of occludin and claudin (Li & Mrsny 2000; Wang et al. 2007). Thus, there is a progressive replacement of tight/adherence junctions by gap junctions both in NC and tumour cells (figure 1a,b). Connexin is one of the major proteins of gap junctions and it has been shown that they are upregulated during NC migration. Connexin-43 (Cxn-43) is expressed in mouse NC cells and its knockout produces defects in NC derivatives, including heart morphogenesis (Lo et al. 1997). However, NC targeted loss of Cxn-43, using Wnt1-Cre KO mice that failed to recapitulate null-Cxn-43 phenotype. The Cxn-43-deficient mice using Pax3-Cre recombinase, which is targeted to NC and dorsal neural tube or the other neuroepithelial tissues, show abnormal coronary development (Liu et al. 2006b). It seems that Cxn-43 act through non-crest neuroepithelium to regulate the ability to carry out EMT. It has been observed that in tumour cells the expression of some connexins is altered, and often reduced, during tumour progression (Yamasaki 1990; Wilgenbus et al. 1992; Neveu et al. 1994; Sawey et al. 1996). However, it should be mentioned that although in many tumours connexins are upregulated, in many others they are downregulated, especially in more advanced stages of tumour progression (Yamasaki 1990; Wilgenbus et al. 1992; Neveu et al. 1994; Sawey et al. 1996).
Figure 1.
NC migrations versus cancer metastasis. (a,b) Loss of polarity; (c,d) changes in cell adherence and cytoskeleton; (e,f) epithelial mesenchymal transition. (a,c,e) Different steps during NC migration. (b,d,f) Different steps of cell migration in cancer cells. Red letters represent factors upregulated and blue letters represent factors downregulated (purple cells, NC or metastatic cells; green cells, epidermis; blue cells, neural tube; AJ, adherence junction; TJ, tight junction; GJ, gap junction; see text for details).
The replacement of tight by gap junctions during this early step of EMT is linked to changes in cell–cell interactions. A key element in cell interaction during EMT is cadherin-dependent cell adhesion.
(b) Changes in cell adhesion: cadherins
The cadherin family of cell–cell adhesion proteins is a large family that includes classical cadherins, protocadherins and atypical cadherins (Fat, Dachsous, and Flamingo; for a review see Halbleib & Nelson (2006)). The extracellular domain of type I cadherins contains characteristic repeats that regulate homophilic and heterophilic interactions during adhesion and cell sorting (Takeichi 1995). At the beginning of NC migration and tumour progression, a lack of cadherins correlates with cells that start to migrate (Nakagawa & Takeichi 1995; Aaku-Saraste et al. 1996). Loss of cadherin expression has emerged as one of the clearest indicators of EMT (figure 1c,d). Classic cadherins comprise type I cadherins expressed preferentially in stable cell assemblies, whereas type II cadherins are also expressed in less cohesive mesenchymal cells. In particular, a shift from type I classical cadherin to type II cadherin correlates with the acquisition of cell motility (DeLuca et al. 1999).
In cancer, during the transition from adenoma to carcinoma, E-cadherin expression is downregulated, while the expression of N-cadherin is switched on (Frixen et al. 1991; Hsu et al. 1996; Cavallaro et al. 2002). Inhibition of E-cadherin by antibody against E-cadherin or dominant-negative form of E-cadherin can induce mesenchymal phenotypes in epithelial cancer cells (Imhof et al. 1983; Andersen et al. 2005). Conversely, it has been reported that the exogenous addition of N-cadherin can induce cell migration, invasion and metastasis in breast cancer cells (Nieman et al. 1999).
Many type II cadherins have been found in migrating cells such as NC cells (Tanihara et al. 1994; Hoffmann & Balling 1995; Nakagawa & Takeichi 1995, 1998; Inoue et al. 1997; Hadeball et al. 1998; Vallin et al. 1998). In avian NC, N-cadherin and cadherin 6B are downregulated during the process of EMT, while the expression of cadherin-7 is increased (Nakagawa & Takeichi 1995). Cadherin-11, a type II cadherin, is specifically expressed in Xenopus migratory NC cells (Hadeball et al. 1998). Hence, the shift of cadherin from type I to II also seems important in NC EMT. It has been reported that the exogenous addition of N-cadherin can induce cell migration, invasion and metastasis in breast cancer cells, even in the presence of E-cadherin (Nieman et al. 1999) and that the expression of a cytoplasmic fragment of N-cadherin can promote NC EMT and delamination working as a transcriptional regulator (Shoval et al. 2007). Interestingly, in some cancer, full exchange from E-cadherin to N-cadherin is fairly observed (Perl et al. 1998; Hazan et al. 2000; Wicki et al. 2006; Wicki & Christofori 2007); and other mechanisms for EMT have been proposed without loss of E-cadherin (Wolf & Friedl 2006; Wicki & Christofori 2007). Although there is an overwhelming accumulation of data that shows the requirement of cadherin downregulation for NC and tumour cells during EMT, the exact mechanism by which cadherins control EMT transition is not known.
An important breakthrough has been the identification of some of the factors that downregulate cadherins during EMT. It has been shown that members of the Snail family of genes bind directly to the E-cadherin promoter and repress its transcription in many tumour cells (Batlle et al. 2000; Cano et al. 2000; Elloul et al. 2005). Interestingly, two members of the Snail gene family, Snail1 (formerly Snail) and Snail2 (formerly Slug) were initially identified as genes expressed in the NC and required for NC specification and migration (Carl et al. 1999; LaBonne & Bronner-Fraser 2000; Mayor et al. 2000; Aybar et al. 2003). Loss- and gain-of-function experiments have shown that Snail2 is a key regulator of EMT in NC (Nieto et al. 1994; del Barrio & Nieto 2002). Another transcription factor, Twist, which is expressed in and required for vertebrate NC (Hopwood et al. 1989; Gitelman 1997; Soo et al. 2002), has also been found to play a key role in cancer EMT and it has been shown to repress E-cadherin expression (Yang et al. 2004). This shift in cadherin expression together with the loss of polarity is one of the first steps of EMT in NC and cancer cells.
(c) Full EMT requires proteases activity
Although switching the expression of cadherins seems to be a key element in many EMT cells, there are many migratory cells in which this change is not essential. There is another enzymatic activity that is present in many tumour cells: digestion of the extracellular matrix (ECM) carried out by proteins called matrix metalloproteases (MMPs; figure 1e,f). The MMPs have been regarded as major critical molecules assisting tumour cells during metastasis (Egeblad & Werb 2002; Lynch & Matrisian 2002; Fingleton 2006). From the earliest work on MMPs in cancer, there has been a clear connection between MMPs, ECM degradation and cancer cell invasion. Numerous studies have linked inhibition of MMPs by synthetic and natural inhibitors (tissue inhibitors of matrix metalloproteinase or TIMPs) with a corresponding inhibition of cell invasion. Conversely, upregulation of MMPs by inducers, transcriptional enhancement or transgene constructs usually led to enhanced tumour cell invasion, monitored in vivo by histology or in model systems by appearance of tumour cells in distant sites and in vitro by Matrigel or ECM invasion. All these studies led to the conclusion that enhanced MMP levels yielded increased tumour cell invasion.
The MMPs are usually expressed as pro-MMP, and then activated by a cleavage of the prodomain by other MMPs. Many of the MMPs involved in this cleavage are expressed in human invasive cancer, MMP2 being one of the most common metalloproteases expressed in various human cancer cells (Stetler-Stevenson et al. 1989; Springman et al. 1990; Nomura et al. 1995; Pulyaeva et al. 1997). Interestingly, Snail also regulates the expression of some MMP, such as MMP2 in melanoma cells (Kuphal et al. 2005).
The MMPs are also expressed during embryo development and they play an important role in NC migration. The MMP2 and its natural inhibitor, TIMP-2, are required for cardiac NC development (Cai et al. 2000; Cantemir et al. 2004; Duong & Erickson 2004). Xenopus NC also expresses several MMPs, including members of the novel metalloproteinase/disintegrin family ADAM, and inhibition of some of them completely block migration of cephalic NC (Alfandari et al. 2001; Gaultier et al. 2002; Smith et al. 2002; Harrison et al. 2004). Recently, many ADAM family members have been identified in cancer cells, suggesting that they might have a role in cancer as well (Karan et al. 2003; Lendeckel et al. 2005; Liu et al. 2006a).
3. NC migratory pathways: inhibitory signals
Once the NC has undergone EMT, they start migration following specific pathways. NC cells migrate in segmentally restricted streams in both the branchial region and the trunk of the vertebrate embryo (figure 2). We will describe here what is known about the molecular bases for such restriction in the cephalic and trunk NCs.
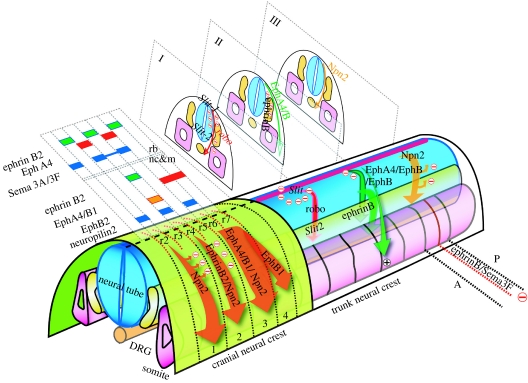
Figure 2.
Migration of cranial and trunk NC. Different factors that control the migration in the cranial and trunk NC are shown. Arrows represents streams of migrating NC. Cranial NC: migration in the head is controlled by Eph/ephrins and semaphorins/neuropilins. The expression of the different factors is shown in the rhombomeres (rb) and in the NC and mesoderm (nc&m). Trunk NC: migration in the trunk: I, ventromedial migration controlled by Slit/Robo; II, dorsolateral migration controlled by Eph/ephrins; III, ventromedial migration controlled by semaphorins/neuropilins. In addition, the anterior–posterior (A–P) patterning of NC migration is controlled by Eph/ephrins and semaphorins/neuropilins. See text for details.
(a) Migration of cephalic NC
In the cephalic region, NC cells migrate into the branchial arches where they differentiate into specific bones, cartilages and cranial ganglia (Kontges & Lumsden 1996). The cephalic NC migrates in three major streams that are related to the segmental organization of the brain. In Xenopus, the NC segregates early during neurulation and remains adjacent to the neural plate for many hours before initiating migration (Sadaghiani & Thiebaud 1987). The premigratory cephalic NC becomes segmented into three groups of cells that are destined to be the mandibular, hyoid and branchial NC (Sadaghiani & Thiebaud 1987; Bradley et al. 1993; Smith et al. 1997). Therefore, mechanisms seem to exist to restrict the intermingling of adjacent groups of NC cells prior to and during migration and to guide them into specific pathways. In the chick embryo, NC cells migrate from the midbrain rhombomeres r1 and r2 into the first branchial arch, from r4 into the second arch, and from r6 and more caudal regions into the third and fourth arches (Lumsden & Guthrie 1991). Recent work has implicated two groups of proteins in the molecular mechanism of this migration, ephrins and semaphorins.
(i) Ephrins
Eph receptors and ephrins are membrane-bound proteins that function as a receptor-ligand pair. Thirteen Eph receptors and eight ephrins have been identified in mammals (Tuzi & Gullick 1994; Orioli & Klein 1997; Pasquale 1997). It was thought until recently that EphA receptors bound preferentially to ephrin-As (with the exception of EphA4) while EphB receptors had a preference for ephrin-Bs. However, new data suggest that interactions across classes occur and are functional in specific contexts (Himanen et al. 2004). The Eph receptors belong to the superfamily of receptor tyrosine kinases and as such they autophosphorylate upon binding to their cognate ephrin ligands and subsequently activate downstream signalling cascades (forward signalling). While neither class of ephrins possesses a catalytic activity, both can activate signal transduction pathways after interaction with Eph receptors (reverse signalling). At the cellular level, both forward and reverse signalling through Eph receptors and ephrins regulate motility (Davy et al. 2004).
In Xenopus embryos, EphA4 and EphB1 are expressed in migrating NC cells and mesoderm of the third arch, and third plus fourth arches (Smith et al. 1997; Helbling et al. 1998), respectively; while the ephrin-B2 ligand, which interacts with these receptors, is expressed in the adjacent second arch NC and mesoderm (Robinson et al. 1997; Smith et al. 1997). Using truncated forms of these proteins, it has been shown that the complementary expression of EphA4/EphB1 receptors and ephrin-B2 is involved in restricting the intermingling of third and second arch NC and in targeting third arch NC to the correct destination (Smith et al. 1997). The cytoplasm domain of ephrin-B2 is able to rescue NC migration in ephrin-B2 knockout mice (Adams et al. 2001). Furthermore, human mutations in ephrin-B1 or ephrin-B4 have been associated with a failure in NC migration (Twigg et al. 2004; Merrill et al. 2006). It has also been shown that gap junction communication (GJC) is inhibited at ectopic ephrin boundaries and that ephrin-B1 interacts with connexin-43 and regulates its distribution. Moreover, genetic evidence indicates that GJC is implicated in cephalic NC defects observed in ephrin-B1 (±) embryos. Thus, Eph/ephrins can regulate GJC during NC migration (Mellitzer et al. 1999).
(ii) Semaphorins
Semaphorins are secreted, transmembrane and GPI-linked proteins, defined by cysteine-rich semaphorin protein domains that have important roles in a variety of tissues (for review on semaphorins see Kruger et al. (2005)). Humans have 20 semaphorins; they are grouped into eight classes on the basis of phylogenetic tree analyses and the presence of additional protein motifs. The expression of semaphorins has been described most fully in the nervous system, but they are also present in most other tissues. Functionally, semaphorins were initially characterized for their importance in the development of the nervous system and in the axonal guidance (Kolodkin 1998). More recently, they have been found to be important for the formation and functioning of other cell types including the NC cells (Tamagnone & Comoglio 2004). Semaphorins can alter the cytoskeleton and the organization of actin filaments and the microtubule network. These effects occur primarily through binding of semaphorins to heterophilic receptors, neuropilin and plexin (Yu & Kolodkin 1999). It has been shown that semaphorins are required for the correct migratory patterning of cephalic and cardiac NC cells (Eickholt et al. 1999; Brown et al. 2001; Gitler et al. 2004; Lepore et al. 2006; Sato et al. 2006; figure 2, cranial NC). In zebrafish, genes of the Sema3F and Sema3G class are expressed in the cranial NC-free zones, while neuropilin 2a (Npn2a) and Npn2b are expressed in the migrating NC cells. Overexpression experiments show a restriction in the pattern of cephalic NC migration (Yu & Moens 2005). The interaction between semaphorin 3F (Sema3F) and neuropilin 2 (Npn2) has been described in axon guidance (Giger et al. 2000) and NC guidance (Gammill et al. 2006a). In chick, Sema3A and Sema3F are expressed within rhombomeres at levels adjacent to crest-free mesenchyme, and expression of the receptor components essential for semaphorin activity by NC cells suggests a function in restricting NC cell migration (Osborne et al. 2005; Gammill et al. 2006a). Attenuation of semaphorin signalling by expression of soluble neuropilin–Fc results in NC cells invading adjacent mesenchymal territories that are normally crest free. Thus, Sema3A and Sema3F, expressed and secreted by the hindbrain neuroepithelium contribute to the appropriate positioning of NC cells in proximity to the neural tube (Osborne et al. 2005). Sema3D is also required for NC migration by controlling cell proliferation (Yu et al. 2004). The Sema3D overexpression rescues reduced proliferation caused by ΔTCF expression, suggesting that Sema3D lies downstream of Wnt/TCF signalling in the molecular pathway thought to control cell cycle in NC cells precursors (Berndt & Halloran 2006).
(b) Migration of trunk NC
After NC cells detach from the neural tube, they migrate along stereotypical pathways to their final destinations. In the trunk region of most animals, NC travels two pathways, a medial route through the somitic mesoderm or between the neural tube and somite, and a dorsolateral pathway, between the somites and the overlying ectoderm (Rickmann et al. 1985; Morin-Kensicki & Eisen 1997; Le Douarin & Kalcheim 1999). NC that travels medially contributes to the sensory and sympathetic ganglia, and generates Schwann and chromaffin cells (Le Douarin & Teillet 1974). In contrast, NC that migrates dorsolaterally generates melanocytes. In addition to these differences in the cell fates of NC migrating on these two pathways, the organization of their migration is also distinct. NC that migrates medially travels in a segmental manner through the somitic mesoderm, entering through the rostral, centre or posterior region of each somite in avian, zebrafish or Xenopus embryo, respectively (Krotoski et al. 1988; Keynes et al. 1990; Collazo et al. 1993; Honjo & Eisen 2005). Some of the molecules that control the patterned migration of the cephalic NC, such as ephrins and semaphorins, are also involved in the segmental migration of the trunk NC through the somites.
(i) Ephrins
It is known that in avian embryos NC invades only the anterior but not the posterior portion of each somite, and that this selective migration pattern is imposed by the somite, as the posterior sclerotome seems to express a repulsive guidance cue (Rickmann et al. 1985; Bronner-Fraser 1986; Serbedzija et al. 1990; Bronner-Fraser & Stern 1991). EphB3 localizes to the rostral half-sclerotome, including the NC, and the ligand ephrin-B1 has a complementary pattern of expression in the caudal half-sclerotome. The addition of soluble ephrin-B1 results in a loss of the metameric migratory pattern and a disorganization of NC cell movement. Thus, Eph-family receptors and their ligands are involved in interactions between NC and sclerotomal cells, mediating an inhibitory activity necessary to constrain neural precursors to specific region of the somite (Krull et al. 1997; Wang & Anderson 1997; Koblar et al. 2000; McLennan & Krull 2002; Kasemeier-Kulesa et al. 2006). Surprisingly, the control of the dorsolateral versus the medial pathway is also controlled by ephrins (figure 2, II). NC cells that are specified as neurons and glial cells only migrate ventrally and are prevented from migrating dorsolaterally into the skin, whereas NC cells specified as melanoblasts are directed into the dorsolateral pathway. The RT-PCR analysis, in situ hybridization and cell surface labelling of NC cell cultures demonstrate that melanoblasts express several EphB receptors. When Eph signalling is disrupted in vivo, melanoblasts are prevented from migrating dorsolaterally, suggesting that ephrin-B ligands promote the dorsolateral migration of melanoblasts. Thus, ephrins act as bifunctional guidance cues: they first repel early migratory NC cells from the dorsolateral path and then later stimulate the migration of melanoblasts into this pathway (Santiago & Erickson 2002). The mechanisms by which ephrins regulate repulsion or attraction in NC cells are unknown.
(ii) Semaphorins
A second signal that has been implicated in cephalic and trunk NC migration is semaphorin (Eickholt et al. 1999). Trunk NC cells express the receptor Npn2, while its repulsive ligand Sema3F is restricted to the posterior half-somite. In Npn2 and Sema3F mutant mice, NC cells lose their segmental migration pattern and instead migrate as a uniform sheet, suggesting that Npn2/Sema3F signalling guides the NC migration through the anterior half of each somite (Gammill et al. 2006b). In addition, it has been shown in zebrafish embryos that Sema3D lies downstream of Wnt/TCF signalling in the molecular pathway and is thought to control cell cycle and NC migration (Berndt & Halloran 2006). Cardiac NC originates from the cells that lie in between the cephalic and the trunk NC, and its migration is also dependent on semaphorins (Brown et al. 2001; Lepore et al. 2006; Sato et al. 2006). However, this conclusion is complicated by the discovery that neuropilin receptors control not only NC cell migration but also some aspects of cardiac endothelial cell migration (Gitler et al. 2004).
(iii) Slit/Robo
A third family of molecules has also been involved in trunk NC migration: Slit/Robo. Slit is a secreted protein that binds to the Robo receptor; they were initially identified in Drosophila as playing an important role in axonal guidance (for a review on Slit/Robo see Dickson & Gilestro (2006)). Mammals have at least three Slit genes (Slit1, Slit2 and Slit3) and four Robo genes (Robo1, Robo2, Robo3 and Robo4). All Slit proteins share a common domain structure, consisting of a series of four leucine-rich repeats, seven to nine epidermal growth factor-like domains, a laminin G domain, and a C-terminal cysteine-rich domain. Most Robo proteins consist of an extracellular domain comprising five immunoglobulin-like and three fibronectin type III (FN3) repeats, a single transmembrane segment and a cytoplasmic domain without any obvious catalytic activity.
Slit and Robo seem to be involved in different aspects of trunk NC migration. Slits are expressed in the dermamyotome and early migrating crest cells express Robo1 and Robo2. Furthermore, Slit2 repels migrating crest cells in vitro and in vivo, suggesting that the Slit/Robo interaction is required to repress the entry of the NC into the dorsal pathway (Jia et al. 2005). In addition, Slit/Robo also seems to regulate the migration of vagal cells into the gut, which is not colonized by trunk cells. Slit2 is expressed at the entrance of the gut and only trunk NC cells express Robo receptors. In vivo and in vitro experiments suggest that Slit2 can act bifunctionally, both repulsing and stimulating the motility of trunk NC cells (De Bellard et al. 2003).
4. Directionality during migration of the NC: PCP/non-canonical Wnt signalling
All the molecules and signals that we have described so far seem to restrict NC migration. Once EMT has occurred in the NC cells they start to migrate into the permissive areas and are repelled from the forbidden areas. This produces the characteristic streams of NC migration, with all the cells migrating in specific directions. According to this proposal, there are no positive cues or attractants that direct the migration of the NC cells or control their directionality. This idea is supported by the lack of sound evidence for the existence of a chemoattractant involved in NC migration. Several permissive molecules have been described for NC migration, especially ECM proteins such as fibronectin, laminin or collagen; however, there is no evidence that these molecules can control the directionality of NC migration. More positive evidence has accumulated in favour of glial cell line derived neurotrophic factor (GDNF) and netrin/deleted colon cancer gene (DCC) as possible NC attractants (Young et al. 2001; Natarajan et al. 2002; Iwashita et al. 2003; Jiang et al. 2003). GDNF and DCC are expressed in the regions of the gut where some of the NC cells are fated to migrate. The NC expresses the receptor for these molecules and loss of function experiments lead to failures in NC migration. Furthermore, in vitro analysis of axonal guidance for neurons derived from NC cells show axonal growth towards a source of GDNF (Young et al. 2001; Natarajan et al. 2002). However, no proper chemotaxis assays have been performed for NC cultured in vitro, and some of the results could still be explained as chemokinesis instead of chemotaxis. Chemokinesis is the process by which a factor stimulates the migration of cells but with no directionality, while in chemotaxis, in addition to promoting cell migration the cell is able to migrate along a gradient of chemoattractant in a particular direction. Thus, an assay for chemoattraction requires not only the number of cells to be counted after exposure to the factor but, most importantly, the trajectory to be followed during its migration to analyse if the factor affects the directionality of the cell (Wells & Ridley 2005). A well-established method to analyse chemoattraction is the Dunn chamber in conjunction with time-lapse microscopy, where the migration of each cell can be tracked after being exposed to a gradient of the chemoattractant. Recently, the Dunn chamber was used to analyse the migration of dorsal root ganglia, a NC derivative, exposed to a gradient of stromal derived factor-1 (SDF-1). However, no time-lapse analysis was performed of the migrating NC, and an increase in the number of cells in the well with a higher concentration of SDF-1 could also be interpreted as SDF-1 promoting chemokinesis instead of chemotaxis, as the authors claim (Belmadani et al. 2005). In summary, there is no unequivocal evidence for positive chemotaxis for the NC cells. However, the NC migrates with very persistent directionality, either as a mass of migrating cells or as individual cells. How is this directionality controlled?
Once the NC cells have undergone EMT, it is possible to analyse the morphology of individually migrating cells. They exhibit typical mesenchymal morphology and are highly polarized in the direction of migration. Large lamellipodia and filopodia are observed at the front of the cell and a typical tail is observed at the back (figures 3 and 4). We have analysed some of the molecular mechanisms that control this polarity and we will focus here on planar cell polarity (PCP) or non-canonical Wnt signalling.
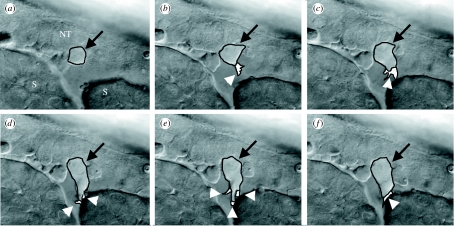
Figure 3.
Polarization of migrating NC in vivo. Differential interference contrast/time-lapse analysis of trunk NC migration in zebrafish embryos. Frames were taken every 3 min. In (a) neural tube (NT) and somites (S) are indicated (arrow, migrating NC; arrowheads, cell protrusions). (a) t=0 min, (b) t=3 min, (c) t=6 min, (d) t=9 min, (e) t=12 min and (f) t=15 min.
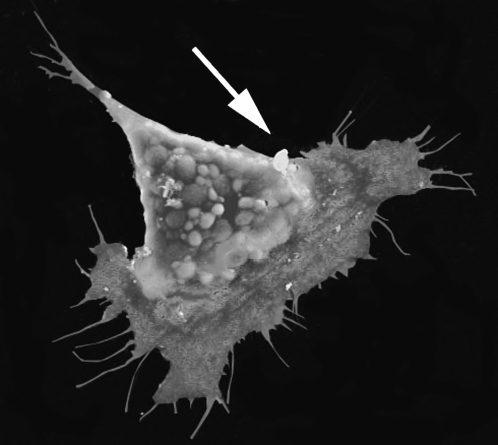
Figure 4.
Polarization of migrating NC in vitro. Scanning electron micrograph of a Xenopus NC cultured in fibronectin. The polarity of the cells can be easily distinguished, with large lamellipodia and several fillopodia at the front of the cells and a retracting protrusion at the back. Arrow indicates the direction of migration.
(a) PCP or non-canonical Wnt signalling
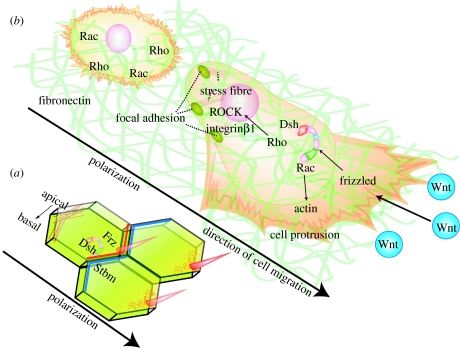
PCP was first recognized in insects. In Drosophila melanogaster, mutations of PCP genes cause disorganization of cuticular structures and/or the compound eye (Gubb & Garcia-Bellido 1982). The cellular hairs, for example, show swirls and wavy patterns instead of the characteristic proximal–distal orientation on the wing blade and show similar abnormalities on the body wall. Similarly in the eye, PCP mutants show defects in the arrangement of photoreceptors. Based on these phenotypes, Drosophila researchers discovered several genes that are required for PCP and uncovered an evolutionarily conserved set of genes that control the establishment of planar polarity not only in flies but also in vertebrates (the core PCP factors). One of the key molecules in this pathway are the Wnt-signalling receptors called frizzled (Fz), which are required for controlling transcriptional activity via β-catenin and Lef/Tcf (Brunner et al. 1997; van de Wetering et al. 1997). However, the Fz receptors also activate a different branch of the Wnt pathway usually known as the non-canonical pathway, which is β-catenin-independent, and that regulates cellular polarity as a consequence of cytoskeletal structure rearrangement (Strutt et al. 1997). An intracellular component of the pathway transduced by Fz is dishevelled (Dsh), which is required for both canonical and non-canonical Wnt pathways (Boutros et al. 1998). The activity of PCP factors polarizes a field of cells along a specific axis, which is reflected by the differential distribution of molecular markers or a gradient of activity in polarized cells. The core PCP molecules, Strabismus (Stbm)/van Gogh, Frz and Dsh become asymmetrically localized during the molecular aspects of PCP signalling (Taylor et al. 1998; Wolff & Rubin 1998; figure 5a). The PCP pathway regulates many aspects of development including convergent extension and neural tube closure, inner ear development, hair orientation in mammals and ciliogenesis (Wang & Nathans 2007).
Figure 5.
PCP control polarity of migrating NC cells. (a) Tissue polarity in Drosophila. Several PCP factors such as Fz (red line), Dsh and Stbm (blue line) are localized in a polarized manner, leading to the polarization of the cytoskeleton (red mesh represent actin) and the formation of the bristle in one end of the cell. (b) A non-polarized NC cell is shown at the back and a polarized cell at the front. Non-canonical Wnts are expressed in the direction of NC migration. The polarized NC cell is migrating on fibronectin and shows cell protrusions at the front of the cell and focal adhesion/stress fibres at the back. Polarized levels of Rho/Rac are expected. PCP signalling is required for the polarized formation of cell protrusions. The cytoskeleton and interaction with the ECM play an important role in this process.
The unifying theme of PCP signalling is cellular polarization which is usually associated with the polarity within an epithelium. However, the NC cells are mesenchymal cells when they are migrating and no obvious role of PCP on individual migratory cells can be expected. Interestingly, inhibition of several of the PCP factors, such as Wnt11, Fz7 and Dsh leads to a complete inhibition of NC migration (De Calisto et al. 2005). Analysis of zebrafish PCP mutants, such as Stbm/trilobite (Jessen et al. 2002), Wnt11/silberblick (Heisenberg et al. 2000) or Wnt5/pipetail (Rauch et al. 1997), also shows a failure in NC migration (L. Marchant & R. Mayor 2007, unpublished results). It should be mentioned that the NC phenotypes in the zebrafish PCP mutants are not as strong as the phenotype produced by the expression of a dominant negative of Dsh. This observation could be explained by some kind of redundancy in the function of the PCP factors, and could explain why no NC phenotype has been described in mouse PCP mutants.
One of the most appealing models of positional information in the PCP signalling is based on the presence of a morphogen-like molecule that would activate Fz in a dose-dependent manner (Adler et al. 2000; Fanto et al. 2003). It has been suggested that such a molecule could belong to the Wnt family, as it binds to the Fz receptor. In addition, it should be produced in a localized fashion in order to generate a gradient of activity. No such molecule has been found so far in the Drosophila PCP pathway, or in any vertebrate system. Interestingly, Wnt11 and Wnt11R (a related member of Wnt11) are expressed adjacent to premigratory NC and just before the onset of crest delamination (De Calisto et al. 2005; Garriock & Krieg 2006). In addition, several observations show that the localized expression of Wnt11 adjacent to the premigratory NC is essential for its function and could be the morphogen that activates the PCP pathway (De Calisto et al. 2005).
However, there is an apparent contradiction between PCP signalling and NC migration. PCP controls the polarity of tissues (epithelia) whereas the NC cells get polarized after they have been released from the neuroepithelia and they are individual mesenchymal cells. How can PCP control the migration of individual cells? Interestingly, recent analysis of NC migration shows that these cells keep close contact during migration, suggesting that even though they might not be as tight as epithelia, they migrate in a more cohesive way than was previously thought (Teddy & Kulesa 2004). Although no functional analysis of these contacts has been performed, it is possible to speculate that these contacts are required to maintain the integrity of the NC tissue to coordinate the orientation of cell protrusion through PCP signalling.
PCP signalling controls the polarity of an epithelium and the migration of neural cells. How are these two processes related? The epithelial polarity is dependent on the localization of specific PCP factors at each end of the cell, which will organize cell properties such as the cytoskeleton and cell adhesion in a polarized manner (figure 5a). The polarized localization of the PCP factors has been hard to visualize in vertebrates and even more difficult in mesenchymal cells, such as NC. However, in these mesenchymal cells, the effect of these PCP factors seems to be on the organization of the cytoskeleton and the formation of cell protrusions required for a directional cell migration (figure 5b). Interestingly, analysis of migrating crest cells in vitro shows that PCP signalling is required to stabilize the cell protrusions. Inhibition of the PCP pathway increases the number of cell protrusions in each cell but in a non-polarized manner (De Calisto et al. 2005). Two important players in the formation of cell protrusions during cell migration are the small GTPases, Rho and Rac, which are controlled by the Wnt-PCP pathway (Hall 1998). It is known that Rac activity promotes actin polymerization and drives lamellipodial extension of growth cones, while Rho activity promotes stress fibre formation and stabilization of focal adhesion (Hall 1998; Kuhn et al. 1999). Rac1 is known to control actin cytoskeletal rearrangement through membrane trafficking during axon guidance (Jurney et al. 2002). In zebrafish gastrulation, cytoplasmic endoreticulum docking protein Rab5c is involved in endocytosis and membrane trafficking, and is one of the effectors of Wnt11 signalling (Ulrich et al. 2005) and Rab11, a different type of Rab protein, is a component of the PCP pathway to drive cellular polarity of Drosophila wing (Classen et al. 2005). Thus, it is possible that the Wnt-PCP pathway is required to coordinate the activities of small GTPases, cytoskeleton (actin) polymerization, cell adhesion and membrane trafficking, to produce an efficient directional migration.
Acknowledgments
The authors would like to thank Carlos Carmona-Fontaine, Helen Matthews and Ben Steventon for their comments on the manuscript. This investigation was supported by an International Research Scholar Award from the Howard Hughes Medical Institute to R.M., and by grants from MRC and BBSRC. S.K. was supported by the Uehara memorial foundation.
Footnotes
One contribution of 11 to a Discussion Meeting Issue ‘Calcium signals and developmental patterning’.
References
- Aaku-Saraste E, Hellwig A, Huttner W.B. Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure–remodeling of the neuroepithelium prior to neurogenesis. Dev. Biol. 1996;180:664–679. doi: 10.1006/dbio.1996.0336. doi:10.1006/dbio.1996.0336 [DOI] [PubMed] [Google Scholar]
- Adams R.H, Diella F, Hennig S, Helmbacher F, Deutsch U, Klein R. The cytoplasmic domain of the ligand ephrinB2 is required for vascular morphogenesis but not cranial neural crest migration. Cell. 2001;104:57–69. doi: 10.1016/s0092-8674(01)00191-x. doi:10.1016/S0092-8674(01)00191-X [DOI] [PubMed] [Google Scholar]
- Adler P.N, Taylor J, Charlton J. The domineering non-autonomy of frizzled and van Gogh clones in the Drosophila wing is a consequence of a disruption in local signaling. Mech. Dev. 2000;96:197–207. doi: 10.1016/s0925-4773(00)00392-0. doi:10.1016/S0925-4773(00)00392-0 [DOI] [PubMed] [Google Scholar]
- Alfandari D, Cousin H, Gaultier A, Smith K, White J.M, Darribere T, DeSimone D.W. Xenopus ADAM 13 is a metalloprotease required for cranial neural crest-cell migration. Curr. Biol. 2001;11:918–930. doi: 10.1016/s0960-9822(01)00263-9. doi:10.1016/S0960-9822(01)00263-9 [DOI] [PubMed] [Google Scholar]
- Andersen H, Mejlvang J, Mahmood S, Gromova I, Gromov P, Lukanidin E, Kriajevska M, Mellon J.K, Tulchinsky E. Immediate and delayed effects of E-cadherin inhibition on gene regulation and cell motility in human epidermoid carcinoma cells. Mol. Cell Biol. 2005;25:9138–9150. doi: 10.1128/MCB.25.20.9138-9150.2005. doi:10.1128/MCB.25.20.9138-9150.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aybar M.J, Nieto M.A, Mayor R. Snail precedes slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development. 2003;130:483–494. doi: 10.1242/dev.00238. doi:10.1242/dev.00238 [DOI] [PubMed] [Google Scholar]
- Barembaum M, Bronner-Fraser M. Early steps in neural crest specification. Semin. Cell Dev. Biol. 2005;16:642–646. doi: 10.1016/j.semcdb.2005.06.006. doi:10.1016/j.semcdb.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. doi:10.1038/35000034 [DOI] [PubMed] [Google Scholar]
- Belmadani A, Tran P.B, Ren D, Assimacopoulos S, Grove E.A, Miller R.J. The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J. Neurosci. 2005;25:3995–4003. doi: 10.1523/JNEUROSCI.4631-04.2005. doi:10.1523/JNEUROSCI.4631-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt J.D, Halloran M.C. Semaphorin 3d promotes cell proliferation and neural crest cell development downstream of TCF in the zebrafish hindbrain. Development. 2006;133:3983–3992. doi: 10.1242/dev.02583. doi:10.1242/dev.02583 [DOI] [PubMed] [Google Scholar]
- Bonstein L, Elias S, Frank D. Paraxial-fated mesoderm is required for neural crest induction in Xenopus embryos. Dev. Biol. 1998;193:156–168. doi: 10.1006/dbio.1997.8795. doi:10.1006/dbio.1997.8795 [DOI] [PubMed] [Google Scholar]
- Boutros M, Paricio N, Strutt D.I, Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. doi: 10.1016/s0092-8674(00)81226-x. doi:10.1016/S0092-8674(00)81226-X [DOI] [PubMed] [Google Scholar]
- Bradley L.C, Snape A, Bhatt S, Wilkinson D.G. The structure and expression of the Xenopus Krox-20 gene: conserved and divergent patterns of expression in rhombomeres and neural crest. Mech. Dev. 1993;40:73–84. doi: 10.1016/0925-4773(93)90089-g. doi:10.1016/0925-4773(93)90089-G [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Guidance of neural crest migration. Latex beads as probes of surface–substratum interactions. Dev. Biol. (N Y 1985) 1986;3:301–337. [PubMed] [Google Scholar]
- Bronner-Fraser M, Stern C. Effects of mesodermal tissues on avian neural crest cell migration. Dev. Biol. 1991;143:213–217. doi: 10.1016/0012-1606(91)90071-a. doi:10.1016/0012-1606(91)90071-A [DOI] [PubMed] [Google Scholar]
- Brown C.B, Feiner L, Lu M.M, Li J, Ma X, Webber A.L, Jia L, Raper J.A, Epstein J.A. PlexinA2 and semaphorin signaling during cardiac neural crest development. Development. 2001;128:3071–3080. doi: 10.1242/dev.128.16.3071. [DOI] [PubMed] [Google Scholar]
- Brunner E, Peter O, Schweizer L, Basler K. Pangolin encodes a Lef-1 homologue that acts downstream of Armadillo to transduce the Wingless signal in Drosophila. Nature. 1997;385:829–833. doi: 10.1038/385829a0. doi:10.1038/385829a0 [DOI] [PubMed] [Google Scholar]
- Cai D.H, Vollberg T.M, Sr, Hahn-Dantona E, Quigley J.P, Brauer P.R. MMP-2 expression during early avian cardiac and neural crest morphogenesis. Anat. Rec. 2000;259:168–179. doi: 10.1002/(SICI)1097-0185(20000601)259:2<168::AID-AR7>3.0.CO;2-U. doi:10.1002/(SICI)1097-0185(20000601)259:2<168::AID-AR7>3.0.CO;2-U [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno M.A, Rodrigo I, Locascio A, Blanco M.J, del Barrio M.G, Portillo F, Nieto M.A. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat. Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. doi:10.1038/35000025 [DOI] [PubMed] [Google Scholar]
- Cantemir V, Cai D.H, Reedy M.V, Brauer P.R. Tissue inhibitor of metalloproteinase-2 (TIMP-2) expression during cardiac neural crest cell migration and its role in proMMP-2 activation. Dev. Dyn. 2004;231:709–719. doi: 10.1002/dvdy.20171. doi:10.1002/dvdy.20171 [DOI] [PubMed] [Google Scholar]
- Carl T.F, Dufton C, Hanken J, Klymkowsky M.W. Inhibition of neural crest migration in Xenopus using antisense slug RNA. Dev. Biol. 1999;213:101–115. doi: 10.1006/dbio.1999.9320. doi:10.1006/dbio.1999.9320 [DOI] [PubMed] [Google Scholar]
- Cavallaro U, Schaffhauser B, Christofori G. Cadherins and the tumour progression: is it all in a switch? Cancer Lett. 2002;176:123–128. doi: 10.1016/s0304-3835(01)00759-5. doi:10.1016/S0304-3835(01)00759-5 [DOI] [PubMed] [Google Scholar]
- Classen A.K, Anderson K.I, Marois E, Eaton S. Hexagonal packing of Drosophila wing epithelial cells by the planar cell polarity pathway. Dev. Cell. 2005;9:805–817. doi: 10.1016/j.devcel.2005.10.016. doi:10.1016/j.devcel.2005.10.016 [DOI] [PubMed] [Google Scholar]
- Collazo A, Bronner-Fraser M, Fraser S.E. Vital dye labelling of Xenopus laevis trunk neural crest reveals multipotency and novel pathways of migration. Development. 1993;118:363–376. doi: 10.1242/dev.118.2.363. [DOI] [PubMed] [Google Scholar]
- Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes Dev. 2004;18:572–583. doi: 10.1101/gad.1171704. doi:10.1101/gad.1171704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellard M.E, Rao Y, Bronner-Fraser M. Dual function of Slit2 in repulsion and enhanced migration of trunk, but not vagal, neural crest cells. J. Cell Biol. 2003;162:269–279. doi: 10.1083/jcb.200301041. doi:10.1083/jcb.200301041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Calisto J, Araya C, Marchant L, Riaz C.F, Mayor R. Essential role of non-canonical Wnt signalling in neural crest migration. Development. 2005;132:2587–2597. doi: 10.1242/dev.01857. doi:10.1242/dev.01857 [DOI] [PubMed] [Google Scholar]
- del Barrio M.G, Nieto M.A. Overexpression of snail family members highlights their ability to promote chick neural crest formation. Development. 2002;129:1583–1593. doi: 10.1242/dev.129.7.1583. [DOI] [PubMed] [Google Scholar]
- DeLuca S.M, Gerhart J, Cochran E, Simak E, Blitz J, Mattiacci-Paessler M, Knudsen K, George-Weinstein M. Hepatocyte growth factor/scatter factor promotes a switch from E- to N-cadherin in chick embryo epiblast cells. Exp. Cell Res. 1999;251:3–15. doi: 10.1006/excr.1999.4577. doi:10.1006/excr.1999.4577 [DOI] [PubMed] [Google Scholar]
- Dickson B.J, Gilestro G.F. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu. Rev. Cell Dev. Biol. 2006;22:651–675. doi: 10.1146/annurev.cellbio.21.090704.151234. doi:10.1146/annurev.cellbio.21.090704.151234 [DOI] [PubMed] [Google Scholar]
- Duong T.D, Erickson C.A. MMP-2 plays an essential role in producing epithelial–mesenchymal transformations in the avian embryo. Dev. Dyn. 2004;229:42–53. doi: 10.1002/dvdy.10465. doi:10.1002/dvdy.10465 [DOI] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer. 2002;2:161–174. doi: 10.1038/nrc745. doi:10.1038/nrc745 [DOI] [PubMed] [Google Scholar]
- Eickholt B.J, Mackenzie S.L, Graham A, Walsh F.S, Doherty P. Evidence for collapsin-1 functioning in the control of neural crest migration in both trunk and hindbrain regions. Development. 1999;126:2181–2189. doi: 10.1242/dev.126.10.2181. [DOI] [PubMed] [Google Scholar]
- Elloul S, Elstrand M.B, Nesland J.M, Trope C.G, Kvalheim G, Goldberg I, Reich R, Davidson B. Snail, Slug, and Smad-interacting protein 1 as novel parameters of disease aggressiveness in metastatic ovarian and breast carcinoma. Cancer. 2005;103:1631–1643. doi: 10.1002/cncr.20946. doi:10.1002/cncr.20946 [DOI] [PubMed] [Google Scholar]
- Fanto M, Clayton L, Meredith J, Hardiman K, Charroux B, Kerridge S, McNeill H. The tumor-suppressor and cell adhesion molecule fat controls planar polarity via physical interactions with Atrophin, a transcriptional co-repressor. Development. 2003;130:763–774. doi: 10.1242/dev.00304. doi:10.1242/dev.00304 [DOI] [PubMed] [Google Scholar]
- Fingleton B. Matrix metalloproteinases: roles in cancer and metastasis. Front. Biosci. 2006;11:479–491. doi: 10.2741/1811. doi:10.2741/1811 [DOI] [PubMed] [Google Scholar]
- Frixen U.H, Behrens J, Sachs M, Eberle G, Voss B, Warda A, Lochner D, Birchmeier W. E-cadherin-mediated cell–cell adhesion prevents invasiveness of human carcinoma cells. J. Cell Biol. 1991;113:173–185. doi: 10.1083/jcb.113.1.173. doi:10.1083/jcb.113.1.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammill L.S, Gonzalez C, Bronner-Fraser M. Neuropilin 2/semaphorin 3F signaling is essential for cranial neural crest migration and trigeminal ganglion condensation. J. Neurobiol. 2006a;67:47–56. doi: 10.1002/dneu.20326. doi:10.1002/neu.20326 [DOI] [PubMed] [Google Scholar]
- Gammill L.S, Gonzalez C, Gu C, Bronner-Fraser M. Guidance of trunk neural crest migration requires neuropilin 2/semaphorin 3F signaling. Development. 2006b;133:99–106. doi: 10.1242/dev.02187. doi:10.1242/dev.02187 [DOI] [PubMed] [Google Scholar]
- Garriock R.J, Krieg P.A. Wnt11-R signaling regulates a calcium sensitive EMT event essential for dorsal fin development of Xenopus. Dev. Biol. 2006;304:127–140. doi: 10.1016/j.ydbio.2006.12.020. doi:10.1016/j.ydbio.2006.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaultier A, Cousin H, Darribere T, Alfandari D. ADAM13 disintegrin and cysteine-rich domains bind to the second heparin-binding domain of fibronectin. J. Biol. Chem. 2002;277:23 336–23 344. doi: 10.1074/jbc.M201792200. doi:10.1074/jbc.M201792200 [DOI] [PubMed] [Google Scholar]
- Giger R.J, et al. Neuropilin-2 is required in vivo for selective axon guidance responses to secreted semaphorins. Neuron. 2000;25:29–41. doi: 10.1016/s0896-6273(00)80869-7. doi:10.1016/S0896-6273(00)80869-7 [DOI] [PubMed] [Google Scholar]
- Gitelman I. Twist protein in mouse embryogenesis. Dev. Biol. 1997;189:205–214. doi: 10.1006/dbio.1997.8614. doi:10.1006/dbio.1997.8614 [DOI] [PubMed] [Google Scholar]
- Gitler A.D, Lu M.M, Epstein J.A. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev. Cell. 2004;7:107–116. doi: 10.1016/j.devcel.2004.06.002. doi:10.1016/j.devcel.2004.06.002 [DOI] [PubMed] [Google Scholar]
- Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J. Embryol. Exp. Morphol. 1982;68:37–57. [PubMed] [Google Scholar]
- Hadeball B, Borchers A, Wedlich D. Xenopus cadherin-11 (Xcadherin-11) expression requires the Wg/Wnt signal. Mech. Dev. 1998;72:101–113. doi: 10.1016/s0925-4773(98)00022-7. doi:10.1016/S0925-4773(98)00022-7 [DOI] [PubMed] [Google Scholar]
- Halbleib J.M, Nelson W.J. Cadherins in development: cell adhesion, sorting, and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. doi:10.1101/gad.1486806 [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. doi:10.1126/science.279.5350.509 [DOI] [PubMed] [Google Scholar]
- Harrison M, Abu-Elmagd M, Grocott T, Yates C, Gavrilovic J, Wheeler G.N. Matrix metalloproteinase genes in Xenopus development. Dev. Dyn. 2004;231:214–220. doi: 10.1002/dvdy.20113. doi:10.1002/dvdy.20113 [DOI] [PubMed] [Google Scholar]
- Hazan R.B, Phillips G.R, Qiao R.F, Norton L, Aaronson S.A. Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J. Cell Biol. 2000;148:779–790. doi: 10.1083/jcb.148.4.779. doi:10.1083/jcb.148.4.779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisenberg C.P, Tada M, Rauch G.J, Saude L, Concha M.L, Geisler R, Stemple D.L, Smith J.C, Wilson S.W. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. doi:10.1038/35011068 [DOI] [PubMed] [Google Scholar]
- Helbling P.M, Tran C.T, Brandli A.W. Requirement for EphA receptor signaling in the segregation of Xenopus third and fourth arch neural crest cells. Mech. Dev. 1998;78:63–79. doi: 10.1016/s0925-4773(98)00148-8. doi:10.1016/S0925-4773(98)00148-8 [DOI] [PubMed] [Google Scholar]
- Himanen J.P, et al. Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat. Neurosci. 2004;7:501–509. doi: 10.1038/nn1237. doi:10.1038/nn1237 [DOI] [PubMed] [Google Scholar]
- Hoffmann I, Balling R. Cloning and expression analysis of a novel mesodermally expressed cadherin. Dev. Biol. 1995;169:337–346. doi: 10.1006/dbio.1995.1148. doi:10.1006/dbio.1995.1148 [DOI] [PubMed] [Google Scholar]
- Honjo Y, Eisen J.S. Slow muscle regulates the pattern of trunk neural crest migration in zebrafish. Development. 2005;132:4461–4470. doi: 10.1242/dev.02026. doi:10.1242/dev.02026 [DOI] [PubMed] [Google Scholar]
- Hopwood N.D, Pluck A, Gurdon J.B. A Xenopus mRNA related to Drosophila twist is expressed in response to induction in the mesoderm and the neural crest. Cell. 1989;59:893–903. doi: 10.1016/0092-8674(89)90612-0. doi:10.1016/0092-8674(89)90612-0 [DOI] [PubMed] [Google Scholar]
- Hsu M.Y, Wheelock M.J, Johnson K.R, Herlyn M. Shifts in cadherin profiles between human normal melanocytes and melanomas. J. Investig. Dermatol. Symp. Proc. 1996;1:188–194. [PubMed] [Google Scholar]
- Imhof B.A, Vollmers H.P, Goodman S.L, Birchmeier W. Cell–cell interaction and polarity of epithelial cells: specific perturbation using a monoclonal antibody. Cell. 1983;35:667–675. doi: 10.1016/0092-8674(83)90099-5. doi:10.1016/0092-8674(83)90099-5 [DOI] [PubMed] [Google Scholar]
- Inoue T, Chisaka O, Matsunami H, Takeichi M. Cadherin-6 expression transiently delineates specific rhombomeres, other neural tube subdivisions, and neural crest subpopulations in mouse embryos. Dev. Biol. 1997;183:183–194. doi: 10.1006/dbio.1996.8501. doi:10.1006/dbio.1996.8501 [DOI] [PubMed] [Google Scholar]
- Iwashita T, Kruger G.M, Pardal R, Kiel M.J, Morrison S.J. Hirschsprung disease is linked to defects in neural crest stem cell function. Science. 2003;301:972–976. doi: 10.1126/science.1085649. doi:10.1126/science.1085649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen J.R, Topczewski J, Bingham S, Sepich D.S, Marlow F, Chandrasekhar A, Solnica-Krezel L. Zebrafish trilobite identifies new roles for Strabismus in gastrulation and neuronal movements. Nat. Cell Biol. 2002;4:610–615. doi: 10.1038/ncb828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, Cheng L, Raper J. Slit/Robo signaling is necessary to confine early neural crest cells to the ventral migratory pathway in the trunk. Dev. Biol. 2005;282:411–421. doi: 10.1016/j.ydbio.2005.03.021. doi:10.1016/j.ydbio.2005.03.021 [DOI] [PubMed] [Google Scholar]
- Jiang Y, Liu M.T, Gershon M.D. Netrins and DCC in the guidance of migrating neural crest-derived cells in the developing bowel and pancreas. Dev. Biol. 2003;258:364–384. doi: 10.1016/s0012-1606(03)00136-2. doi:10.1016/S0012-1606(03)00136-2 [DOI] [PubMed] [Google Scholar]
- Jurney W.M, Gallo G, Letourneau P.C, McLoon S.C. Rac1-mediated endocytosis during ephrin-A2- and semaphorin 3A-induced growth cone collapse. J. Neurosci. 2002;22:6019–6028. doi: 10.1523/JNEUROSCI.22-14-06019.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karan D, Lin F.C, Bryan M, Ringel J, Moniaux N, Lin M.F, Batra S.K. Expression of ADAMs (a disintegrin and metalloproteases) and TIMP-3 (tissue inhibitor of metalloproteinase-3) in human prostatic adenocarcinomas. Int. J. Oncol. 2003;23:1365–1371. [PubMed] [Google Scholar]
- Kasemeier-Kulesa J.C, Bradley R, Pasquale E.B, Lefcort F, Kulesa P.M. Eph/ephrins and N-cadherin coordinate to control the pattern of sympathetic ganglia. Development. 2006;133:4839–4847. doi: 10.1242/dev.02662. doi:10.1242/dev.02662 [DOI] [PubMed] [Google Scholar]
- Keynes R, Cook G, Davies J, Lumsden A, Norris W, Stern C. Segmentation and the development of the vertebrate nervous system. J. Physiol. (Paris) 1990;84:27–32. [PubMed] [Google Scholar]
- Koblar S.A, Krull C.E, Pasquale E.B, McLennan R, Peale F.D, Cerretti D.P, Bothwell M. Spinal motor axons and neural crest cells use different molecular guides for segmental migration through the rostral half-somite. J. Neurobiol. 2000;42:437–447. doi:10.1002/(SICI)1097-4695(200003)42:4<437::AID-NEU5>3.0.CO;2-O [PubMed] [Google Scholar]
- Kolodkin A.L. Semaphorin-mediated neuronal growth cone guidance. Prog. Brain Res. 1998;117:115–132. doi: 10.1016/s0079-6123(08)64012-1. [DOI] [PubMed] [Google Scholar]
- Kontges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- Krotoski D.M, Fraser S.E, Bronner-Fraser M. Mapping of neural crest pathways in Xenopus laevis using inter- and intra-specific cell markers. Dev. Biol. 1988;127:119–132. doi: 10.1016/0012-1606(88)90194-7. doi:10.1016/0012-1606(88)90194-7 [DOI] [PubMed] [Google Scholar]
- Kruger R.P, Aurandt J, Guan K.-L. Semaphorins command cells to move. Nat. Rev. Mol. Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. doi:10.1038/nrm1740 [DOI] [PubMed] [Google Scholar]
- Krull C.E, Lansford R, Gale N.W, Collazo A, Marcelle C, Yancopoulos G.D, Fraser S.E, Bronner-Fraser M. Interactions of Eph-related receptors and ligands confer rostrocaudal pattern to trunk neural crest migration. Curr. Biol. 1997;7:571–580. doi: 10.1016/s0960-9822(06)00256-9. doi:10.1016/S0960-9822(06)00256-9 [DOI] [PubMed] [Google Scholar]
- Kuhn T.B, Brown M.D, Wilcox C.L, Raper J.A, Bamburg J.R. Myelin and collapsin-1 induce motor neuron growth cone collapse through different pathways: inhibition of collapse by opposing mutants of rac1. J. Neurosci. 1999;19:1965–1975. doi: 10.1523/JNEUROSCI.19-06-01965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesa P.M, Kasemeier-Kulesa J.C, Teddy J.M, Margaryan N.V, Seftor E.A, Seftor R.E, Hendrix M.J. Reprogramming metastatic melanoma cells to assume a neural crest cell-like phenotype in an embryonic microenvironment. Proc. Natl Acad. Sci. USA. 2006;103:3752–3757. doi: 10.1073/pnas.0506977103. doi:10.1073/pnas.0506977103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuphal S, Bauer R, Bosserhoff A.K. Integrin signaling in malignant melanoma. Cancer Metastasis Rev. 2005;24:195–222. doi: 10.1007/s10555-005-1572-1. doi:10.1007/s10555-005-1572-1 [DOI] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Snail-related transcriptional repressors are required in Xenopus for both the induction of the neural crest and its subsequent migration. Dev. Biol. 2000;221:195–205. doi: 10.1006/dbio.2000.9609. doi:10.1006/dbio.2000.9609 [DOI] [PubMed] [Google Scholar]
- Le Douarin, N.M. & Kalcheim, C. 1999 The neural crest, p. 445. Cambridge University Press Cambridge, UK.
- Le Douarin N.M, Teillet M.A. Experimental analysis of the migration and differentiation of neuroblasts of the autonomic nervous system and of neurectodermal mesenchymal derivatives, using a biological cell marking technique. Dev. Biol. 1974;41:162–184. doi: 10.1016/0012-1606(74)90291-7. doi:10.1016/0012-1606(74)90291-7 [DOI] [PubMed] [Google Scholar]
- Lendeckel U, Kohl J, Arndt M, Carl-McGrath S, Donat H, Rocken C. Increased expression of ADAM family members in human breast cancer and breast cancer cell lines. J. Cancer Res. Clin. Oncol. 2005;131:41–48. doi: 10.1007/s00432-004-0619-y. doi:10.1007/s00432-004-0619-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepore J.J, Mericko P.A, Cheng L, Lu M.M, Morrisey E.E, Parmacek M.S. GATA-6 regulates semaphorin 3C and is required in cardiac neural crest for cardiovascular morphogenesis. J. Clin. Invest. 2006;116:929–939. doi: 10.1172/JCI27363. doi:10.1172/JCI27363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Mrsny R.J. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J. Cell Biol. 2000;148:791–800. doi: 10.1083/jcb.148.4.791. doi:10.1083/jcb.148.4.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.C, et al. Identification of ADAM10 as a major source of HER2 ectodomain sheddase activity in HER2 overexpressing breast cancer cells. Cancer Biol. Ther. 2006a;5:657–664. doi: 10.4161/cbt.5.6.2708. [DOI] [PubMed] [Google Scholar]
- Liu S, Liu F, Schneider A.E, St Amand T, Epstein J.A, Gutstein D.E. Distinct cardiac malformations caused by absence of connexin 43 in the neural crest and in the non-crest neural tube. Development. 2006b;133:2063–2073. doi: 10.1242/dev.02374. doi:10.1242/dev.02374 [DOI] [PubMed] [Google Scholar]
- Lo C.W, Cohen M.F, Huang G.Y, Lazatin B.O, Patel N, Sullivan R, Pauken C, Park S.M. Cx43 gap junction gene expression and gap junctional communication in mouse neural crest cells. Dev. Genet. 1997;20:119–132. doi: 10.1002/(SICI)1520-6408(1997)20:2<119::AID-DVG5>3.0.CO;2-A. doi:10.1002/(SICI)1520-6408(1997)20:2<119::AID-DVG5>3.0.CO;2-A [DOI] [PubMed] [Google Scholar]
- Lumsden, A. & Guthrie, S. 1991 Alternating patterns of cell surface properties and neural crest cell migration during segmentation of the chick hindbrain. Development (Suppl. 2), 9–15. [PubMed]
- Lynch C.C, Matrisian L.M. Matrix metalloproteinases in tumor–host cell communication. Differentiation. 2002;70:561–573. doi: 10.1046/j.1432-0436.2002.700909.x. doi:10.1046/j.1432-0436.2002.700909.x [DOI] [PubMed] [Google Scholar]
- Mancilla A, Mayor R. Neural crest formation in Xenopus laevis: mechanisms of Xslug induction. Dev. Biol. 1996;177:580–589. doi: 10.1006/dbio.1996.0187. doi:10.1006/dbio.1996.0187 [DOI] [PubMed] [Google Scholar]
- Mayor R, Aybar M.J. Induction and development of neural crest in Xenopus laevis. Cell Tissue Res. 2001;305:203–209. doi: 10.1007/s004410100369. doi:10.1007/s004410100369 [DOI] [PubMed] [Google Scholar]
- Mayor R, Morgan R, Sargent M.G. Induction of the prospective neural crest of Xenopus. Development. 1995;121:767–777. doi: 10.1242/dev.121.3.767. [DOI] [PubMed] [Google Scholar]
- Mayor R, Guerrero N, Young R.M, Gomez-Skarmeta J.L, Cuellar C. A novel function for the Xslug gene: control of dorsal mesendoderm development by repressing BMP-4. Mech. Dev. 2000;97:47–56. doi: 10.1016/s0925-4773(00)00412-3. doi:10.1016/S0925-4773(00)00412-3 [DOI] [PubMed] [Google Scholar]
- McLennan R, Krull C.E. Ephrin-as cooperate with EphA4 to promote trunk neural crest migration. Gene. Expr. 2002;10:295–305. doi: 10.3727/000000002783992389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellitzer G, Xu Q, Wilkinson D.G. Eph receptors and ephrins restrict cell intermingling and communication. Nature. 1999;400:77–81. doi: 10.1038/21907. doi:10.1038/21907 [DOI] [PubMed] [Google Scholar]
- Merrill A.E, Bochukova E.G, Brugger S.M, Ishii M, Pilz D.T, Wall S.A, Lyons K.M, Wilkie A.O, Maxson R.E., Jr Cell mixing at a neural crest–mesoderm boundary and deficient ephrin-Eph signaling in the pathogenesis of craniosynostosis. Hum. Mol. Genet. 2006;15:1319–1328. doi: 10.1093/hmg/ddl052. doi:10.1093/hmg/ddl052 [DOI] [PubMed] [Google Scholar]
- Meulemans D, Bronner-Fraser M. Gene-regulatory interactions in neural crest evolution and development. Dev. Cell. 2004;7:291–299. doi: 10.1016/j.devcel.2004.08.007. doi:10.1016/j.devcel.2004.08.007 [DOI] [PubMed] [Google Scholar]
- Morin-Kensicki E.M, Eisen J.S. Sclerotome development and peripheral nervous system segmentation in embryonic zebrafish. Development. 1997;124:159–167. doi: 10.1242/dev.124.1.159. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. Neural crest cell–cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development. 1995;121:1321–1332. doi: 10.1242/dev.121.5.1321. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 1998;125:2963–2971. doi: 10.1242/dev.125.15.2963. [DOI] [PubMed] [Google Scholar]
- Natarajan D, Marcos-Gutierrez C, Pachnis V, de Graaff E. Requirement of signalling by receptor tyrosine kinase RET for the directed migration of enteric nervous system progenitor cells during mammalian embryogenesis. Development. 2002;129:5151–5160. doi: 10.1242/dev.129.22.5151. [DOI] [PubMed] [Google Scholar]
- Neveu M.J, Babcock K.L, Hertzberg E.L, Paul D.L, Nicholson B.J, Pitot H.C. Colocalized alterations in connexin32 and cytochrome P450IIB1/2 by phenobarbital and related liver tumor promoters. Cancer Res. 1994;54:3145–3152. [PubMed] [Google Scholar]
- Newgreen D, Gibbins I. Factors controlling the time of onset of the migration of neural crest cells in the fowl embryo. Cell Tissue Res. 1982;224:145–160. doi: 10.1007/BF00217274. doi:10.1007/BF00217274 [DOI] [PubMed] [Google Scholar]
- Newgreen D.F, Gibbins I.L, Sauter J, Wallenfels B, Wutz R. Ultrastructural and tissue-culture studies on the role of fibronectin, collagen and glycosaminoglycans in the migration of neural crest cells in the fowl embryo. Cell Tissue Res. 1982;221:521–549. doi: 10.1007/BF00215700. doi:10.1007/BF00215700 [DOI] [PubMed] [Google Scholar]
- Nieman M.T, Prudoff R.S, Johnson K.R, Wheelock M.J. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J. Cell Biol. 1999;147:631–644. doi: 10.1083/jcb.147.3.631. doi:10.1083/jcb.147.3.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto M.A, Sargent M.G, Wilkinson D.G, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. doi:10.1126/science.7513443 [DOI] [PubMed] [Google Scholar]
- Nomura H, Sato H, Seiki M, Mai M, Okada Y. Expression of membrane-type matrix metalloproteinase in human gastric carcinomas. Cancer Res. 1995;55:3263–3266. [PubMed] [Google Scholar]
- Orioli D, Klein R. The Eph receptor family: axonal guidance by contact repulsion. Trends Genet. 1997;13:354–359. doi: 10.1016/s0168-9525(97)01220-1. doi:10.1016/S0168-9525(97)01220-1 [DOI] [PubMed] [Google Scholar]
- Osborne N.J, Begbie J, Chilton J.K, Schmidt H, Eickholt B.J. Semaphorin/neuropilin signaling influences the positioning of migratory neural crest cells within the hindbrain region of the chick. Dev. Dyn. 2005;232:939–949. doi: 10.1002/dvdy.20258. doi:10.1002/dvdy.20258 [DOI] [PubMed] [Google Scholar]
- Pasquale E.B. The Eph family of receptors. Curr. Opin. Cell Biol. 1997;9:608–615. doi: 10.1016/s0955-0674(97)80113-5. doi:10.1016/S0955-0674(97)80113-5 [DOI] [PubMed] [Google Scholar]
- Perl A.K, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. doi:10.1038/32433 [DOI] [PubMed] [Google Scholar]
- Pulyaeva H, Bueno J, Polette M, Birembaut P, Sato H, Seiki M, Thompson E.W. MT1-MMP correlates with MMP-2 activation potential seen after epithelial to mesenchymal transition in human breast carcinoma cells. Clin. Exp. Metastasis. 1997;15:111–120. doi: 10.1023/a:1018444609098. doi:10.1023/A:1018444609098 [DOI] [PubMed] [Google Scholar]
- Rauch G.J, Hammerschmidt M, Blader P, Schauerte H.E, Strahle U, Ingham P.W, McMahon A.P, Haffter P. Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harb. Symp. Quant. Biol. 1997;62:227–234. [PubMed] [Google Scholar]
- Raven C.P, Kloos J. Induction by medial and lateral pieces of the archenteron roof with special reference to the determination of the neural crest. Acta Neerl. Morphol. 1945;4:348–362. [Google Scholar]
- Rickmann M, Fawcett J.W, Keynes R.J. The migration of neural crest cells and the growth of motor axons through the rostral half of the chick somite. J. Embryol. Exp. Morphol. 1985;90:437–455. [PubMed] [Google Scholar]
- Robinson V, Smith A, Flenniken A.M, Wilkinson D.G. Roles of Eph receptors and ephrins in neural crest pathfinding. Cell Tissue Res. 1997;290:265–274. doi: 10.1007/s004410050931. doi:10.1007/s004410050931 [DOI] [PubMed] [Google Scholar]
- Sadaghiani B, Thiebaud C. Neural crest development in the Xenopus laevis embryo, studied by interspecific transplantation and scanning electron microscopy. Dev. Biol. 1987;124:91–110. doi: 10.1016/0012-1606(87)90463-5. doi:10.1016/0012-1606(87)90463-5 [DOI] [PubMed] [Google Scholar]
- Santiago A, Erickson C.A. Ephrin-B ligands play a dual role in the control of neural crest cell migration. Development. 2002;129:3621–3632. doi: 10.1242/dev.129.15.3621. [DOI] [PubMed] [Google Scholar]
- Sato M, Tsai H.J, Yost H.J. Semaphorin3D regulates invasion of cardiac neural crest cells into the primary heart field. Dev. Biol. 2006;298:12–21. doi: 10.1016/j.ydbio.2006.05.033. doi:10.1016/j.ydbio.2006.05.033 [DOI] [PubMed] [Google Scholar]
- Sawey M.J, Goldschmidt M.H, Risek B, Gilula N.B, Lo C.W. Perturbation in connexin 43 and connexin 26 gap-junction expression in mouse skin hyperplasia and neoplasia. Mol. Carcinog. 1996;17:49–61. doi: 10.1002/(SICI)1098-2744(199610)17:2<49::AID-MC1>3.0.CO;2-O. doi:10.1002/(SICI)1098-2744(199610)17:2<49::AID-MC1>3.0.CO;2-O [DOI] [PubMed] [Google Scholar]
- Selleck M.A, Bronner-Fraser M. Origins of the avian neural crest: the role of neural plate–epidermal interactions. Development. 1995;121:525–538. doi: 10.1242/dev.121.2.525. [DOI] [PubMed] [Google Scholar]
- Serbedzija G.N, Fraser S.E, Bronner-Fraser M. Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labelling. Development. 1990;108:605–612. doi: 10.1242/dev.108.4.605. [DOI] [PubMed] [Google Scholar]
- Shoval I, Ludwig A, Kalcheim C. Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development. 2007;134:491–501. doi: 10.1242/dev.02742. doi:10.1242/dev.02742 [DOI] [PubMed] [Google Scholar]
- Smith A, Robinson V, Patel K, Wilkinson D.G. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr. Biol. 1997;7:561–570. doi: 10.1016/s0960-9822(06)00255-7. doi:10.1016/S0960-9822(06)00255-7 [DOI] [PubMed] [Google Scholar]
- Smith K.M, Gaultier A, Cousin H, Alfandari D, White J.M, DeSimone D.W. The cysteine-rich domain regulates ADAM protease function in vivo. J. Cell Biol. 2002;159:893–902. doi: 10.1083/jcb.200206023. doi:10.1083/jcb.200206023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo K, O'Rourke M.P, Khoo P.L, Steiner K.A, Wong N, Behringer R.R, Tam P.P. Twist function is required for the morphogenesis of the cephalic neural tube and the differentiation of the cranial neural crest cells in the mouse embryo. Dev. Biol. 2002;247:251–270. doi: 10.1006/dbio.2002.0699. doi:10.1006/dbio.2002.0699 [DOI] [PubMed] [Google Scholar]
- Springman E.B, Angleton E.L, Birkedal-Hansen H, Van Wart H.E. Multiple modes of activation of latent human fibroblast collagenase: evidence for the role of a Cys73 active-site zinc complex in latency and a “cysteine switch” mechanism for activation. Proc. Natl Acad. Sci. USA. 1990;87:364–368. doi: 10.1073/pnas.87.1.364. doi:10.1073/pnas.87.1.364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetler-Stevenson W.G, Krutzsch H.C, Liotta L.A. Tissue inhibitor of metalloproteinase (TIMP-2). A new member of the metalloproteinase inhibitor family. J. Biol. Chem. 1989;264:17 374–17 378. [PubMed] [Google Scholar]
- Steventon B, Carmona-Fontaine C, Mayor R. Genetic network during neural crest induction: from cell specification to cell survival. Semin. Cell Dev. Biol. 2005;16:647–654. doi: 10.1016/j.semcdb.2005.06.001. doi:10.1016/j.semcdb.2005.06.001 [DOI] [PubMed] [Google Scholar]
- Strutt D.I, Weber U, Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–295. doi: 10.1038/387292a0. doi:10.1038/387292a0 [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr. Opin. Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. doi:10.1016/0955-0674(95)80102-2 [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Comoglio P.M. To move or not to move? Semaphorin signalling in cell migration. EMBO Rep. 2004;5:356–361. doi: 10.1038/sj.embor.7400114. doi:10.1038/sj.embor.7400114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanihara H, Sano K, Heimark R.L, St John T, Suzuki S. Cloning of five human cadherins clarifies characteristic features of cadherin extracellular domain and provides further evidence for two structurally different types of cadherin. Cell Adhes. Commun. 1994;2:15–26. doi: 10.3109/15419069409014199. doi:10.3109/15419069409014199 [DOI] [PubMed] [Google Scholar]
- Taylor J, Abramova N, Charlton J, Adler P.N. Van Gogh: a new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teddy J.M, Kulesa P.M. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development. 2004;131:6141–6151. doi: 10.1242/dev.01534. doi:10.1242/dev.01534 [DOI] [PubMed] [Google Scholar]
- Thiery J.P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer. 2002;2:442–454. doi: 10.1038/nrc822. doi:10.1038/nrc822 [DOI] [PubMed] [Google Scholar]
- Trainor P.A. Specification of neural crest cell formation and migration in mouse embryos. Semin. Cell Dev. Biol. 2005;16:683–693. doi: 10.1016/j.semcdb.2005.06.007. doi:10.1016/j.semcdb.2005.06.007 [DOI] [PubMed] [Google Scholar]
- Tuzi N.L, Gullick W.J. Eph, the largest known family of putative growth factor receptors. Br. J. Cancer. 1994;69:417–421. doi: 10.1038/bjc.1994.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twigg S.R, Kan R, Babbs C, Bochukova E.G, Robertson S.P, Wall S.A, Morriss-Kay G.M, Wilkie A.O. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proc. Natl Acad. Sci. USA. 2004;101:8652–8657. doi: 10.1073/pnas.0402819101. doi:10.1073/pnas.0402819101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich F, et al. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev. Cell. 2005;9:555–564. doi: 10.1016/j.devcel.2005.08.011. doi:10.1016/j.devcel.2005.08.011 [DOI] [PubMed] [Google Scholar]
- Vallin J, Girault J.M, Thiery J.P, Broders F. Xenopus cadherin-11 is expressed in different populations of migrating neural crest cells. Mech. Dev. 1998;75:171–174. doi: 10.1016/s0925-4773(98)00099-9. doi:10.1016/S0925-4773(98)00099-9 [DOI] [PubMed] [Google Scholar]
- van de Wetering M, et al. Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell. 1997;88:789–799. doi: 10.1016/s0092-8674(00)81925-x. doi:10.1016/S0092-8674(00)81925-X [DOI] [PubMed] [Google Scholar]
- Wang H.U, Anderson D.J. Eph family transmembrane ligands can mediate repulsive guidance of trunk neural crest migration and motor axon outgrowth. Neuron. 1997;18:383–396. doi: 10.1016/s0896-6273(00)81240-4. doi:10.1016/S0896-6273(00)81240-4 [DOI] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. doi:10.1242/dev.02772 [DOI] [PubMed] [Google Scholar]
- Wang Z, Wade P, Mandell K.J, Akyildiz A, Parkos C.A, Mrsny R.J, Nusrat A. Raf 1 represses expression of the tight junction protein occludin via activation of the zinc-finger transcription factor slug. Oncogene. 2006;26:1222–1230. doi: 10.1038/sj.onc.1209902. doi:10.1038/sj.onc.1209902 [DOI] [PubMed] [Google Scholar]
- Wells C.M, Ridley A.J. Analysis of cell migration using the Dunn chemotaxis chamber and time-lapse microscopy. Methods Mol. Biol. 2005;294:31–41. doi: 10.1385/1-59259-860-9:031. [DOI] [PubMed] [Google Scholar]
- Wicki A, Christofori G. The potential role of podoplanin in tumour invasion. Br. J. Cancer. 2007;96:1–5. doi: 10.1038/sj.bjc.6603518. doi:10.1038/sj.bjc.6603518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial–mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–272. doi: 10.1016/j.ccr.2006.03.010. doi:10.1016/j.ccr.2006.03.010 [DOI] [PubMed] [Google Scholar]
- Wilgenbus K.K, Kirkpatrick C.J, Knuechel R, Willecke K, Traub O. Expression of Cx26, Cx32 and Cx43 gap junction proteins in normal and neoplastic human tissues. Int. J. Cancer. 1992;51:522–529. doi: 10.1002/ijc.2910510404. doi:10.1002/ijc.2910510404 [DOI] [PubMed] [Google Scholar]
- Wolf K, Friedl P. Molecular mechanisms of cancer cell invasion and plasticity. Br. J. Dermatol. 2006;154(Suppl. 1):11–15. doi: 10.1111/j.1365-2133.2006.07231.x. doi:10.1111/j.1365-2133.2006.07231.x [DOI] [PubMed] [Google Scholar]
- Wolff T, Rubin G.M. Strabismus, a novel gene that regulates tissue polarity and cell fate decisions in Drosophila. Development. 1998;125:1149–1159. doi: 10.1242/dev.125.6.1149. [DOI] [PubMed] [Google Scholar]
- Yamasaki H. Gap junctional intercellular communication and carcinogenesis. Carcinogenesis. 1990;11:1051–1058. doi: 10.1093/carcin/11.7.1051. doi:10.1093/carcin/11.7.1051 [DOI] [PubMed] [Google Scholar]
- Yang J. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. doi:10.1016/j.cell.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Young H.M, Hearn C.J, Farlie P.G, Canty A.J, Thomas P.Q, Newgreen D.F. GDNF is a chemoattractant for enteric neural cells. Dev. Biol. 2001;229:503–516. doi: 10.1006/dbio.2000.0100. doi:10.1006/dbio.2000.0100 [DOI] [PubMed] [Google Scholar]
- Yu H.H, Kolodkin A.L. Semaphorin signaling: a little less per-plexin. Neuron. 1999;22:11–14. doi: 10.1016/s0896-6273(00)80672-8. doi:10.1016/S0896-6273(00)80672-8 [DOI] [PubMed] [Google Scholar]
- Yu H.H, Moens C.B. Semaphorin signaling guides cranial neural crest cell migration in zebrafish. Dev. Biol. 2005;280:373–385. doi: 10.1016/j.ydbio.2005.01.029. doi:10.1016/j.ydbio.2005.01.029 [DOI] [PubMed] [Google Scholar]
- Yu H.H, Houart C, Moens C.B. Cloning and embryonic expression of zebrafish neuropilin genes. Gene Expr. Patterns. 2004;4:371–378. doi: 10.1016/j.modgep.2004.01.011. doi:10.1016/j.modgep.2004.01.011 [DOI] [PubMed] [Google Scholar]