Abstract
Imaging studies, using both luminescent and fluorescent Ca2+-sensitive reporters, have revealed that during the first few meroblastic cleavages of the large embryos of teleosts, localized elevations of intracellular Ca2+ accompany positioning, propagation, deepening and apposition of the cleavage furrows. Here, we will review the Ca2+ transients reported during the cleavage period in these embryos, with reference mainly to that of the zebrafish (Danio rerio). We will also present the latest findings that support the proposal that Ca2+ transients are an essential feature of embryonic cytokinesis. In addition, the potential upstream triggers and downstream targets of the different cytokinetic Ca2+ transients will be discussed. Finally, we will present a hypothetical model that summarizes what has been suggested to be the various roles of Ca2+ signalling during cytokinesis in teleost embryos.
Keywords: Ca2+, cleavage period, cytokinesis, zebrafish development
1. Introduction
There is a wealth of literature relating to cytokinesis in animal cells. From the study of a variety of different cell types, many hypotheses and suggestions have been proposed over the years in an attempt to explain this complex process (Swann & Mitchison 1958; Wolpert 1960; Mabuchi 1986; Salmon 1989; Schroeder 1990; Satterwhite & Pollard 1992; Fishkind & Wang 1995; Rappaport 1996; Field et al. 1999; Robinson & Spudich 2000; Wang 2001; Glotzer 2003; Strickland & Burgess 2004; Burgess 2005; Glotzer 2005). A number of these reports have considered the possible roles played by intracellular Ca2+ transients in the cytokinetic process. In general, however, these reports have focused on either ‘small’ embryos (e.g. of echinoderms, with a diameter of approx. 60 μm) or tissue culture cells, and though a lot has been revealed about the cytokinetic process in these cell types, the involvement of Ca2+ signalling is still not well understood. Therefore, in this review, we have focused specifically on the possible roles played by Ca2+ in initiating and orchestrating cytokinesis in the large (i.e. approx. 600 μm in diameter) embryos of teleosts (mainly zebrafish). These systems provide an excellent platform, from both a spatial and temporal viewpoint, as well as good Ca2+ imaging optics, which enable one to explore each consecutive step in the cytokinetic process. Furthermore, they are beginning to provide some of the first clues as to the specific functional roles played by cytokinetic Ca2+ transients, which may turn out to be the common features of cell division in other cell types.
In these large teleost embryos, there are several distinct basic processes that contribute to cytokinesis. These include an initial positioning of the cleavage furrow within the cell cortex, followed by the propagation (without significant deepening) of the furrow across the cell surface. This is then followed by furrow deepening (i.e. furrow ingression), which results in two daughter cells that are separated by a distinct groove, and finally furrow apposition, where the daughter cells ‘zip up’ together. In small embryos (such as echinoderms), the furrow appears almost simultaneously in the equatorial cortex of the late anaphase cell and so furrow positioning and propagation may be indistinguishable as separate entities. In addition, in small somatic and tissue culture cells, and in dividing unicellular organisms, the final stage of cytokinesis may involve the abscission of the last intercellular connection between the prospective daughter cells, which results in complete cell separation (Rappaport 1996; Low et al. 2003; Glotzer 2005; Gromley et al. 2005). However, during embryonic cell division, while the daughter cells must be separated from one another, it is also necessary that the embryonic blastoderm be held together. Thus, following furrow deepening, the respective daughter cell plasma membranes do not separate from one another, but undergo a process of apposition that holds the cells together. Cleavage furrow apposition, therefore, represents the final step in this type of distinct embryonic cytokinesis (Fluck et al. 1991; Lee et al. 2003). Owing to the large size of their embryos, and thus the fact that the different cytokinetic components (positioning, propagation, deepening and apposition) are separated both temporally and spatially, the first few meroblastic cleavages of teleost embryos provide a unique opportunity to explore Ca2+ signalling dynamics and generation mechanisms, as well as their relationship to the cytoskeletal rearrangements specific to each stage in the embryonic cytokinetic process.
2. Early reports describing Ca2+ fluctuations during cytokinesis
Ridgway et al. (1977) were the first to report that the early cleavages in teleost embryos were accompanied by transient elevations in intracellular Ca2+. Using medaka (Oryzias latipes) embryos loaded with the luminescent Ca2+-sensitive reporter, aequorin (Shimomura et al. 1990), in conjunction with a photomultiplier tube (PMT), this group was mainly focusing on the single large Ca2+ transient that is associated with egg activation. However, they reported that one experimental embryo generated small transient increases in intracellular Ca2+, which appeared to correlate with the first two cell divisions.
Several years after this first report, Shantz (1985) demonstrated through the use of inserted Ca2+-sensitive microelectrodes that (once again) in a single experimental medaka embryo during two successive cleavages, Ca2+ rose transiently fourfold above the original resting level in synchrony with each cell division. However, in the same year, Yoshimoto et al. (1985) reported that the intracellular concentration of Ca2+ appeared to be lowest at the time of furrowing. Like Ridgway et al. (1977), this group also used a PMT to measure the total luminescent output from a single aequorin-injected medaka embryo. This apparent contradiction in the involvement of Ca2+ during cytokinesis in medaka embryos was only addressed in the early 1990s with the development of techniques that allowed the direct visualization of Ca2+ signals.
3. Direct visualization of the cytokinetic Ca2+ signals
Using aequorin, in conjunction with a custom-built photon imaging microscope, Fluck et al. (1991) confirmed that localized increases in Ca2+ were coincident with cytokinesis in medaka embryos. This group was the first to image slow Ca2+ waves accompanying the progression of furrows across blastomere surfaces in these embryos during the first few meroblastic cell divisions. Two successive Ca2+ waves, travelling at approximately 0.5 μm s−1, were observed: the first, relatively narrow wave, was reported to accompany furrow extension (i.e. propagation) while the second, which was approximately 3–7 times wider than the first, accompanied the subsequent furrow deepening and apposition of the daughter cells.
Following the reports from medaka embryos, the existence of Ca2+ transients during the early embryonic cleavages in zebrafish (Danio rerio) was subsequently identified using both fluorescent and luminescent Ca2+ reporters. Interestingly, as in the case of the medaka embryo, the first reports describing the occurrence of Ca2+ transients during early zebrafish embryonic patterning also yielded conflicting results. Reinhard et al. (1995) reported no Ca2+ activity during the early cleavage cycles of zebrafish, whereas in the same year Chang & Meng observed that localized elevations in free Ca2+ were associated with cytokinesis. Furthermore, Chang & Meng (1995) demonstrated that intracellular Ca2+ was elevated ‘not only in the ‘right’ place, but also at the ‘right’ time’ to play a role in determining the position of the furrowing plane. While both groups used the fluorescent Ca2+ reporter calcium green-1 dextran, the only apparent difference between the two investigations was the size of the dextran to which the reporter was conjugated. The fact that conflicting results were obtained from the same embryonic system using similar Ca2+ reporters and imaging techniques serves well to illustrate the challenges involved in visualizing cytokinetic Ca2+ transients, even in very large cells!
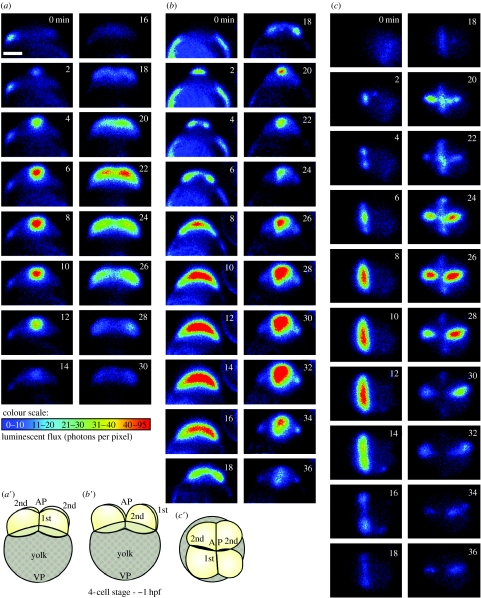
We subsequently addressed the controversy using bioluminescent aequorin-based imaging and confirmed Chang & Meng's (1995) observation, demonstrating that several distinct Ca2+ signals accompany the sequential stages of cytokinesis (Webb et al. 1997). The ‘furrow positioning signal’ was a clear, localized elevation of intracellular Ca2+, which preceded the first appearance of the furrow arc at the blastodisc surface. This was followed by the ‘furrow propagation signal’ that took the form of two subsurface slow Ca2+ waves moving at approximately 0.5 μm s−1 and accompanied the leading edges of the furrow arc as they progressed outward towards the margins of the blastodisc. As these propagation wavefronts approached the margins of the blastodisc, the ‘furrow deepening’ Ca2+ signal then appeared at the apex of the blastodisc, i.e. at the same initiation site as the positioning signal. Like the propagation signal, it extended outward to the margins of the blastodisc at approximately 0.5 μm s−1, but in this case it also moved downward (at approx. 0.1 μm s−1), accompanying the deepening process that separates the daughter cells (Webb et al. 1997). The region of localized elevated Ca2+ then persisted throughout the furrow apposition process and returned to the resting level only when the cleavage furrow was fully apposed (Lee et al. 2003). This sequence of Ca2+ signals was also observed during the second cell division cycle (Webb et al. 1997). More recent reports have also confirmed that such localized increases in Ca2+ occur in zebrafish embryos during the different phases of cytokinesis using both aequorin and fluorescent Ca2+ reporters (Créton et al. 1998; Chang & Lu 2000). As the aequorin method of imaging provides no information in the z-plane, where possible we visualize the signals from different perspectives. This is illustrated in figure 1, which shows the Ca2+ signals observed during the first and second cell division cycles in zebrafish embryos imaged from facial, axial and animal pole (AP) views.
Figure 1.
Representative (a) facial, (b) axial and (c) animal pole (AP) views of f-aequorin-loaded zebrafish embryos to show the changes in intracellular free Ca2+ during the first two cell division cycles. The pseudocolour images represent 60 s of accumulated luminescence with a 60 s gap between each image. (a′–c′) Schematics of embryos at the end of furrow deepening of the second cell division cycle from (a′) facial, (b′) axial and (c′) AP views. VP, vegetal pole; hpf, hours postfertilization. Scale bar, 200 μm.
4. Investigating the requirement of elevated Ca2+ for cytokinesis
In addition to direct visualization, there is also good indirect evidence (for example, from injecting Ca2+ chelators such as BAPTA-type buffers; Pethig et al. 1989) at various times during the cell cycle to unambiguously indicate that Ca2+ signalling plays a required and necessary role in the cleavage of both medaka (Fluck et al. 1994) and zebrafish (Chang & Meng 1995; Webb et al. 1997; Créton et al. 1998; Lee et al. 2003, 2006) embryos.
In order to understand more clearly what effect Ca2+ chelators (such as BAPTA-type buffers) have on the generation of a particular transient, and the developmental significance of blocking or modulating that transient, the timing of their introduction as well as their rate of spread within an early embryo is critical. Taking this into account, Webb et al. (1997) focused on the propagation transient and waited until the furrow had been positioned on the blastodisc surface (by observing either the appearance of the furrow on the surface or the Ca2+ transient associated with this event) before introducing the buffer. These experiments clearly indicated a Ca2+ requirement for furrow propagation in cleaving zebrafish embryos. Subsequently, and again by careful timing of the introduction of the Ca2+ buffer, it has more recently been shown that a localized elevation of Ca2+ is also essential for both furrow deepening (Lee et al. 2003) and furrow positioning (Lee et al. 2006) in zebrafish embryos.
5. Investigating the source of the Ca2+ generating the various cytokinetic transients
Zebrafish embryos have also been treated with antagonists of the various Ca2+ release channels in order to investigate the Ca2+ stores responsible for the generation of cytokinetic Ca2+ transients. Chang & Meng (1995) demonstrated that the cytokinetic Ca2+ signal that they observed using calcium green-1 dextran was blocked via the introduction of heparin, an antagonist of IP3 receptors (IP3Rs), but was not affected by ryanodine (a ryanodine receptor antagonist), nifedipine and La3+ (inhibitors of plasma membrane Ca2+ channels) or by the removal of Ca2+ from the external medium. They concluded that the cytokinetic Ca2+ transient arose from internal stores through the release of Ca2+ via IP3Rs most probably located in the endoplasmic reticulum (ER; Chang & Meng 1995). Using aequorin, we subsequently confirmed that zebrafish embryos could generate a regular series of cytokinetic Ca2+ transients and divide normally (for at least the first few cell division cycles) in Ca2+-free medium (Webb et al. 1997). Our findings thus supported Chang & Meng's (1995) observation that extracellular Ca2+ is not involved in generating these transients.
More recently, we explored the source of cytokinetic Ca2+ in further detail by carefully timing the introduction of the various antagonists in order to focus specifically on the positioning and deepening Ca2+ transients (Lee et al. 2003, 2006). We demonstrated that the introduction of heparin or another IP3R antagonist, 2-aminoethoxydiphenylborate (2-APB), at the appropriate time to challenge only the deepening transient blocked the Ca2+ signal and resulted in an inhibition of furrow deepening. Antagonists of the ryanodine receptor and the nicotinic acid–adenine dinucleotide phosphate (NAADP)-sensitive channel, however, had no effect on either furrow deepening or on the deepening Ca2+ transient (Lee et al. 2003). In addition, we demonstrated that both the ER and IP3Rs are localized on either side of the cleavage furrow during furrow deepening, thus providing further evidence for the possible intracellular Ca2+ store and release mechanism for the deepening Ca2+ transient.
More recently, we demonstrated that the positioning Ca2+ transient is also generated by Ca2+ release via IP3Rs (Lee et al. 2006). Furthermore, we also started to explore the upstream events that might be involved in generating the furrow positioning Ca2+ transient and presented evidence to demonstrate that a dynamic array of microtubules may be involved. We showed that this array, which we termed the ‘pre-furrowing microtubule array’ (or pf-MTA), originates from the mid-zone of the mitotic spindle and then expands both upward and outward towards the surface of the blastodisc (Lee et al. 2004). In addition, we showed that this dynamic pf-MTA eventually colocalizes with a zone of corticular ER (and associated IP3Rs) in the blastoderm cortex just before the morphological appearance of the cleavage furrow at the blastodisc surface and suggested that the array might be involved in reorganizing the ER (and thus the IP3Rs) required to generate the Ca2+ signals that are essential for cleavage furrow formation in zebrafish embryos (Lee et al. 2004). The reorganized ER assumes a linear architecture and this may explain the generation of linear rather than radial Ca2+ waves (Fluck et al. 1991).
6. Investigating the possible targets of the cytokinetic Ca2+ signals
It was suggested, following studies in the embryos of newt, sand dollar and several sea urchin species, that cleavage involves the contraction of an actomyosin band (or ring) that lies at the base of the cleavage furrow, via a mechanism comparable to the contraction of smooth muscle (Mabuchi et al. 1988; Mabuchi & Takano-Ohmuro 1990). In addition, it was suggested that an increase in Ca2+ in the furrow region might (i) induce the recruitment of microfilaments into the contractile band (Fluck et al. 1991), (ii) regulate the binding of myosin to the actin filaments (Suzuki et al. 1995; Silver 1996), and/or (iii) trigger actomyosin contraction via the activation of the Ca2+-sensitive myosin light chain kinase (MLCK; Yamakita et al. 1994; Murthy & Wadsworth 2005). Indeed, at the 2004 ASCB Summer Symposium on cytokinesis (refer to conference report; Canman & Wells 2004), the possibility that furrow positioning might be closely associated with myosin regulation via the activation of MLCK was discussed at length. Apart from these suggestions, the possible role of Ca2+ on contractile band assembly and function during cytokinesis has not yet been explored fully in the embryos of teleost fish. However, Foe et al. (2000) demonstrated that in Drosophila embryos at the syncytial blastoderm stage, F-actin and myosin II are transported along microtubules and colocalize in areas where pseudocleavage furrows form. These results thus suggest that many of the components of the contractile mechanism required for successful cytokinesis are a conserved feature of many animal cells, as might be the suggested modulatory role played by Ca2+. Furthermore, Field et al. (2005) provided evidence for another possible role of cytokinetic Ca2+ signalling in the formation of the cleavage furrow by demonstrating that PIP2 is required for the adhesion of the contractile ring to the plasma membrane in a variety of tissue culture cells.
In addition to the possible role of Ca2+ in the assembly, positioning and contraction of the contractile band, a suggested downstream function, specifically of the furrow deepening and apposition Ca2+ transients, in zebrafish embryos was the recruitment followed by the exocytosis of vesicles at the ingressing furrow membrane (Lee et al. 2003; Li et al. 2006). Several lines of evidence indicate that membrane trafficking plays a key role in membrane remodelling during cytokinesis in animal embryos (Jesuthasan 1998; Shuster & Burgess 2002; Albertson et al. 2005). Indeed, membrane remodelling has been found to be a common feature of cytokinesis in many species including zebrafish (Jesuthasan 1998; Feng et al. 2002). How the furrow membrane is restructured as well as the identity and precise function of the trafficking molecules involved in this process are areas of current intense interest. Indeed, we recently demonstrated that two cognate SNARE partners, VAMP-2 and SNAP-25, mediate vesicle fusion at the deepening and apposing cleavage furrow membranes in zebrafish embryos and that vesicle fusion is not required for furrow deepening but is essential for apposition. In addition, we demonstrated that extracellular Ca2+ is not required for VAMP-2 vesicle fusion, but confirmed that it is essential for successful daughter cell apposition (Li et al. 2006). This supports an earlier report by Jesuthasan (1998) where he demonstrated that microtubules are required for the apposition of daughter blastomeres. He suggested that they mediate trafficking of intracellular vesicles to the furrow surface, where they are subsequently exocytosed, thus delivering cargos of proteins such as cadherins and catenins that promote Ca2+-sensitive blastomere apposition.
7. Conclusions
A close relationship has thus been shown to exist between the different cytokinetic Ca2+ transients and the various morphological events that occur during zebrafish cytokinesis, i.e. furrow positioning, propagation, deepening and apposition. Furthermore, a specific requirement for each transient has been clearly established. Preliminary evidence is now beginning to accumulate to suggest what the functional role of each of these transients might be, although the precise molecular details of the interactions between localized Ca2+ elevations and Ca2+-sensitive cytoskeletal and cytosolic signalling elements still remain unclear. A number of hypothetical models have been proposed that attempt to link these Ca2+ transients to specific cytokinetic events (Fluck et al. 1991; Webb et al. 1997; Lee et al. 2004, 2006). We have attempted to summarize and update these models in figure 2. It is clear, however, that additional work still needs to be done before we can fully understand the specific (and perhaps multiple and overlapping) functions of each of the cytokinetic Ca2+ transients. We suggest that this work will be greatly aided through the development of more sensitive imaging methodologies and intracellular Ca2+ reporters, which may be used in combination with the powerful molecular tools and techniques that are currently available.
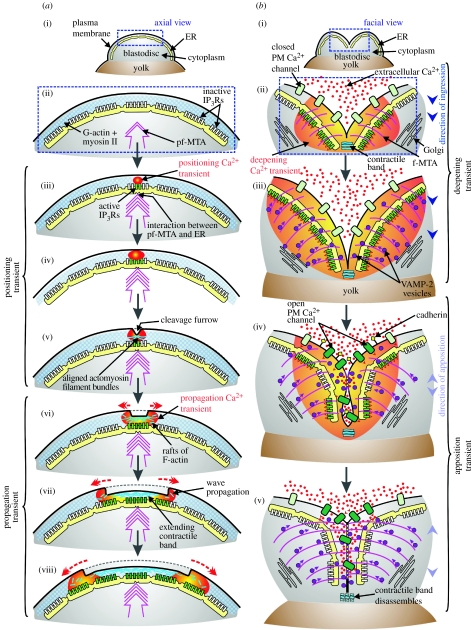
Figure 2.
A hypothetical model that summarizes the possible roles of Ca2+ signalling during cytokinesis in teleost embryos. Axial and facial views of a zebrafish blastodisc to illustrate how Ca2+ released via the activation of IP3Rs in the ER might (a) generate the furrow positioning and propagation of Ca2+ transients (Lee et al. 2006) during the first cell division cycle via a blip/puff/wave Ca2+ signalling cascade and (b) generate the furrow deepening transient and how extracellular Ca2+ might contribute to furrow apposition. (a(i)) Axial view of a zebrafish blastodisc to show the field of view (in blue rectangle) shown in (a(ii)–a(viii)). (a(ii)) At the start of cytokinesis, a pre-furrowing microtubule array (pf-MTA) grows upward and outward towards the blastodisc surface (Lee et al. 2004). (a(iii)) Onset of furrow positioning. The pf-MTA stimulates the release of Ca2+ from the ER via the activation of several IP3Rs. This ‘puff’ of elevated Ca2+ has been termed the ‘positioning transient’ (Webb et al. 1997). (a(iv)) A larger puff of elevated Ca2+ is produced via the activation of more IP3Rs. (a(v)) First morphological appearance of the cleavage furrow at the apex of the blastodisc following the correct positioning of the cytokinetic contractile apparatus. (a(vi)) Onset of furrow propagation. An intracellular wave of Ca2+-induced Ca2+ release (CICR) is initiated. This has been termed the ‘propagation transient’ (Webb et al. 1997). (a(vii),a(viii)) The CICR stimulates the extension of the contractile band, and thus the cleavage furrow, across the blastodisc. (b(i)) Facial view of the blastodisc to show the field of view (in blue rectangle) shown in (b(ii)–b(v)). (b(ii)) Onset of furrow deepening. Ca2+, released from the ER via IP3Rs, contributes to most if not all of the furrow deepening transient (Lee et al. 2003) and stimulates (via calmodulin/MLCK) the contraction of the contractile band. On the other hand, extracellular Ca2+ does not appear to contribute to the furrow deepening transient (or furrow deepening itself) as the transient is generated and the furrows deepen normally in Ca2+-free medium (Webb et al. 1997). (b(iii)) Furrow deepening continues. The FMA plays a role in transporting vesicles responsible for membrane remodelling (Jesuthasan 1998; Li et al. 2006). The vesicles, perhaps coming from the Golgi apparatus, are recruited to and fuse with the ingressing furrow. (b(iv)) Furrow apposition begins. The release of Ca2+ from the ER via IP3Rs continues, possibly promoting the delivery and fusion of vesicles containing cargos such as adhesion proteins to the cell surface. In addition, extracellular Ca2+ is required for successful daughter cell apposition via the stimulation of Ca2+-sensitive adhesion proteins such as cadherins (Jesuthasan 1998; Li et al. 2006). (b(v)) Apposition proceeds with the continuing recruitment and fusion of vesicles. The contractile band disassembles in a region of low Ca2+. PM, plasma membrane.
Acknowledgments
We acknowledge the financial support from Hong Kong RGC grants HKUST6214/02M, HKUST6279/03M, HKUST6241/04M and HKUST6416/06M. A.L.M. is the recipient of a Croucher Senior Research Fellowship. Special thanks to Dr Osamu Shimomura for his generous support of aequorin-based imaging over the years.
Footnotes
One contribution of 11 to a Discussion Meeting Issue ‘Calcium signals and developmental patterning’.
References
- Albertson R, Riggs B, Sullivan W. Membrane traffic: a driving force in cytokinesis. Trends Cell Biol. 2005;15:92–101. doi: 10.1016/j.tcb.2004.12.008. doi:10.1016/j.tcb.2004.12.008 [DOI] [PubMed] [Google Scholar]
- Burgess D. Cytokinesis: new roles for myosin. Curr. Biol. 2005;15:310–311. doi: 10.1016/j.cub.2005.04.008. doi:10.1016/j.cub.2005.04.008 [DOI] [PubMed] [Google Scholar]
- Canman J.C, Wells W.A. Rappaport furrows on our minds: the ASCB Cytokinesis meeting, Burlington, VT, July 22–25, 2004. J. Cell Biol. 2004;166:943–948. doi: 10.1083/jcb.200409019. doi:10.1083/jcb.200409019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D.C, Meng C. A localized elevation of cytosolic free calcium is associated with cytokinesis in the zebrafish embryo. J. Cell Biol. 1995;131:1539–1545. doi: 10.1083/jcb.131.6.1539. doi:10.1083/jcb.131.6.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang D.C, Lu P. Multiple types of calcium signals are associated with cell division in zebrafish embryo. Microsc. Res. Tech. 2000;49:111–122. doi: 10.1002/(SICI)1097-0029(20000415)49:2<111::AID-JEMT2>3.0.CO;2-Z. doi:10.1002/(SICI)1097-0029(20000415)49:2<111::AID-JEMT2>3.0.CO;2-Z [DOI] [PubMed] [Google Scholar]
- Créton R, Speksnijder J.E, Jaffe L.F. Patterns of free calcium in zebrafish embryos. J. Cell Sci. 1998;111:1613–1622. doi: 10.1242/jcs.111.12.1613. [DOI] [PubMed] [Google Scholar]
- Feng B, Schwarz H, Jesuthasan S. Furrow-specific endocytosis during cytokinesis of zebrafish blastomeres. Exp. Cell Res. 2002;279:14–20. doi: 10.1006/excr.2002.5579. doi:10.1006/excr.2002.5579 [DOI] [PubMed] [Google Scholar]
- Field C, Li R, Oegema K. Cytokinesis in eukaryotes: a mechanistic comparison. Curr. Opin. Cell Biol. 1999;11:68–80. doi: 10.1016/s0955-0674(99)80009-x. doi:10.1016/S0955-0674(99)80009-X [DOI] [PubMed] [Google Scholar]
- Field S.J, Madson N, Kerr M.L, Galbraith K.A, Kennedy C.E, Tahiliani M, Wilkins A, Cantley L.C. PtdIns(4,5)P2 functions at the cleavage furrow during cytokinesis. Curr. Biol. 2005;15:1407–1412. doi: 10.1016/j.cub.2005.06.059. doi:10.1016/j.cub.2005.06.059 [DOI] [PubMed] [Google Scholar]
- Fishkind D.J, Wang Y.L. New horizons for cytokinesis. Curr. Opin. Cell Biol. 1995;7:23–31. doi: 10.1016/0955-0674(95)80041-7. doi:10.1016/0955-0674(95)80041-7 [DOI] [PubMed] [Google Scholar]
- Fluck R.A, Miller A.L, Jaffe L.F. Slow calcium waves accompany cytokinesis in medaka fish eggs. J. Cell Biol. 1991;115:1259–1265. doi: 10.1083/jcb.115.5.1259. doi:10.1083/jcb.115.5.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluck R.A, Miller A.L, Abraham V.C, Jaffe L.F. Calcium buffer injections inhibit ooplasmic segregation in medaka eggs. Biol. Bull. 1994;186:254–262. doi: 10.2307/1542271. doi:10.2307/1542271 [DOI] [PubMed] [Google Scholar]
- Foe V.E, Field C.M, Odell G.M. Microtubules and mitotic cycle phase modulate spatiotemporal distributions of F-actin and myosin II in Drosophila syncytial blastoderm embryos. Development. 2000;127:1767–1787. doi: 10.1242/dev.127.9.1767. [DOI] [PubMed] [Google Scholar]
- Glotzer M. Cytokinesis: progress on all fronts. Curr. Opin. Cell Biol. 2003;15:684–690. doi: 10.1016/j.ceb.2003.10.003. doi:10.1016/j.ceb.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. doi:10.1126/science.1096896 [DOI] [PubMed] [Google Scholar]
- Gromley A, Yeaman C, Rosa J, Redick S, Chen C.T, Mirabelle S, Guha M, Sillibourne J, Doxsey S.J. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. doi:10.1016/j.cell.2005.07.027 [DOI] [PubMed] [Google Scholar]
- Jesuthasan S. Furrow-associated microtubule arrays are required for the cohesion of zebrafish blastomeres following cytokinesis. J. Cell Sci. 1998;111:3695–3703. doi: 10.1242/jcs.111.24.3695. [DOI] [PubMed] [Google Scholar]
- Lee K.W, Webb S.E, Miller A.L. Ca2+ released via IP3 receptors is required for furrow deepening during cytokinesis in zebrafish embryos. Int. J. Dev. Biol. 2003;47:411–421. [PubMed] [Google Scholar]
- Lee K.W, Ho S.M, Wong C.H, Webb S.E, Miller A.L. Characterization of mid-spindle microtubules during furrow positioning in early cleavage period zebrafish embryos. Zygote. 2004;12:221–230. doi: 10.1017/s0967199404002886. doi:10.1017/S0967199404002886 [DOI] [PubMed] [Google Scholar]
- Lee K.W, Webb S.E, Miller A.L. Requirement for a localized, IP3R-generated Ca2+ transient during the furrow positioning process in zebrafish zygotes. Zygote. 2006;14:143–155. doi: 10.1017/S0967199406003637. doi:10.1017/S0967199406003637 [DOI] [PubMed] [Google Scholar]
- Li W.M, Webb S.E, Lee K.W, Miller A.L. Recruitment and SNARE-mediated fusion of vesicles in furrow membrane remodeling during cytokinesis in zebrafish embryos. Exp. Cell Res. 2006;312:3260–3275. doi: 10.1016/j.yexcr.2006.06.028. doi:10.1016/j.yexcr.2006.06.028 [DOI] [PubMed] [Google Scholar]
- Low S.H, Li X, Miura M, Kudo N, Quinones B, Weimbs T. Syntaxin 2 and endobrevin are required for the terminal step of cytokinesis in mammalian cells. Dev. Cell. 2003;4:753–759. doi: 10.1016/s1534-5807(03)00122-9. doi:10.1016/S1534-5807(03)00122-9 [DOI] [PubMed] [Google Scholar]
- Mabuchi I. Biochemical aspects of cytokinesis. Int. Rev. Cytol. 1986;101:175–213. doi: 10.1016/s0074-7696(08)60249-1. [DOI] [PubMed] [Google Scholar]
- Mabuchi I, Takano-Ohmuro H. Effects of inhibitors of myosin light chain kinase and other protein kinases on the first cell division of sea urchin eggs. Dev. Growth Differ. 1990;32:549–556. doi: 10.1111/j.1440-169X.1990.00549.x. doi:10.1111/j.1440-169X.1990.00549.x [DOI] [PubMed] [Google Scholar]
- Mabuchi I, Tsukita S, Tsukita S, Sawai T. Cleavage furrow isolated from newt eggs: contraction, organization of the actin filaments, and protein components of the furrow. Proc. Natl Acad. Sci. USA. 1988;85:5966–5970. doi: 10.1073/pnas.85.16.5966. doi:10.1073/pnas.85.16.5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy K, Wadsworth P. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr. Biol. 2005;15:724–731. doi: 10.1016/j.cub.2005.02.055. doi:10.1016/j.cub.2005.02.055 [DOI] [PubMed] [Google Scholar]
- Pethig R, Kuhn M, Payne R, Alder E, Chen T.-H, Jaffe L.F. On the dissociation constants of BAPTA-type calcium buffers. Cell Calcium. 1989;10:491–498. doi: 10.1016/0143-4160(89)90026-2. doi:10.1016/0143-4160(89)90026-2 [DOI] [PubMed] [Google Scholar]
- Rappaport R. Cambridge University Press; Cambridge, UK: 1996. Cytokinesis in animal cells. [Google Scholar]
- Reinhard E, Yokoe H, Niebling K.R, Allbritton N.L, Kuhn M.A, Meyer T. Localized calcium signals in early zebrafish development. Dev. Biol. 1995;170:50–61. doi: 10.1006/dbio.1995.1194. doi:10.1006/dbio.1995.1194 [DOI] [PubMed] [Google Scholar]
- Ridgway E.B, Gilkey J.G, Jaffe L.F. Free calcium increases explosively in activating medaka eggs. Proc. Natl Acad. Sci. USA. 1977;74:623–627. doi: 10.1073/pnas.74.2.623. doi:10.1073/pnas.74.2.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.N, Spudich J.A. Towards a molecular understanding of cytokinesis. Trends Cell Biol. 2000;10:228–237. doi: 10.1016/s0962-8924(00)01747-5. doi:10.1016/S0962-8924(00)01747-5 [DOI] [PubMed] [Google Scholar]
- Salmon E.D. Cytokinesis in animal cells. Curr. Opin. Cell Biol. 1989;1:541–547. doi: 10.1016/0955-0674(89)90018-5. doi:10.1016/0955-0674(89)90018-5 [DOI] [PubMed] [Google Scholar]
- Satterwhite L.L, Pollard T.D. Cytokinesis. Curr. Opin. Cell Biol. 1992;4:43–52. doi: 10.1016/0955-0674(92)90057-j. doi:10.1016/0955-0674(92)90057-J [DOI] [PubMed] [Google Scholar]
- Schroeder T.E. The contractile ring and furrowing in dividing cells. Ann. N Y Acad. Sci. 1990;582:78–87. doi: 10.1111/j.1749-6632.1990.tb21669.x. doi:10.1111/j.1749-6632.1990.tb21669.x [DOI] [PubMed] [Google Scholar]
- Shantz A.R. Cytosolic free calcium-ion concentration in cleaving embryonic cells of Oryzias latipes measured with calcium-sensitive microelectrodes. J. Cell Biol. 1985;100:947–954. doi: 10.1083/jcb.100.3.947. doi:10.1083/jcb.100.3.947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura O, Inouye S, Musicki B, Kishi Y. Recombinant aequorins and semisynthetic recombinant aequorins. Biochem. J. 1990;270:309–312. doi: 10.1042/bj2700309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster C.B, Burgess D.R. Targeted new membrane addition in the cleavage furrow is a late, separate event in cytokinesis. Proc. Natl Acad. Sci. USA. 2002;99:3633–3638. doi: 10.1073/pnas.052342699. doi:10.1073/pnas.052342699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R.B. Calcium, BOBs, QEDs, microdomains and a cellular decision: control of mitotic cell division in sand dollar blastomeres. Cell Calcium. 1996;20:161–179. doi: 10.1016/s0143-4160(96)90105-0. doi:10.1016/S0143-4160(96)90105-0 [DOI] [PubMed] [Google Scholar]
- Strickland L.I, Burgess D.R. Pathways for membrane trafficking during cytokinesis. Trends Cell Biol. 2004;14:115–118. doi: 10.1016/j.tcb.2004.01.006. doi:10.1016/j.tcb.2004.01.006 [DOI] [PubMed] [Google Scholar]
- Suzuki K, Roegiers F, Tran P, Inoué S. Reversible regression of cytokinesis induced by Ca2+ ionophore. Biol. Bull. 1995;189:201–202. doi: 10.1086/BBLv189n2p201a. [DOI] [PubMed] [Google Scholar]
- Swann M.M, Mitchison J.M. The mechanism of cleavage in animal cells. Biol. Rev. Camb. Phil. Soc. 1958;33:103–135. [Google Scholar]
- Wang Y.-L. The mechanism of cytokinesis: reconsideration and reconciliation. Cell Struct. Funct. 2001;26:633–638. doi: 10.1247/csf.26.633. doi:10.1247/csf.26.633 [DOI] [PubMed] [Google Scholar]
- Webb S.E, Lee K.W, Karplus E, Miller A.L. Localized calcium transients accompany furrow positioning, propagation, and deepening during the early cleavage period of zebrafish embryos. Dev. Biol. 1997;192:78–92. doi: 10.1006/dbio.1997.8724. doi:10.1006/dbio.1997.8724 [DOI] [PubMed] [Google Scholar]
- Wolpert L. The mechanics and mechanism of cleavage. Int. Rev. Cytol. 1960;10:163–216. [Google Scholar]
- Yamakita Y, Yamashiro S, Matsumura F. In vivo phosphorylation of regulatory light chain of myosin II during mitosis of cultured cells. J. Cell Biol. 1994;124:129–137. doi: 10.1083/jcb.124.1.129. doi:10.1083/jcb.124.1.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto Y, Iwamatsu T, Hiramoto Y. Cyclic changes in intracellular free calcium levels associated with cleavage cycles in echinoderm and medaka eggs. Biomed. Res. 1985;6:387–394. [Google Scholar]