Abstract
In Xenopus, experiments performed with isolated ectoderm suggest that neural determination is a ‘by default’ mechanism, which occurs when bone morphogenetic proteins (BMPs) are antagonized by extracellular antagonists, BMP being responsible for the determination of epidermis. However, Ca2+ imaging of intact Xenopus embryos reveals patterns of Ca2+ transients which are generated via the activation of dihydropyridine-sensitive Ca2+ channels in the dorsal ectoderm but not in the ventral ectoderm. These increases in the concentration of intracellular Ca2+([Ca2+]i) appear to be necessary and sufficient to orient the ectodermal cells towards a neural fate as increasing the [Ca2+]i artificially results in neuralization of the ectoderm. We constructed a subtractive cDNA library between untreated and caffeine-treated ectoderms (to increase [Ca2+]i) and then identified early Ca2+-sensitive target genes expressed in the neural territories. One of these genes, an arginine methyltransferase, controls the expression of the early proneural gene, Zic3. Here, we discuss the evidence for the existence of an alternative model to the ‘by default’ mechanism, where Ca2+ plays a central regulatory role in the expression of Zic3, an early proneural gene, and in epidermal determination which only occurs when the Ca2+-dependent signalling pathways are inactive.
Keywords: calcium, dihydropyridine-channels, neural determination, Xenopus laevis, gene expression
1. Introduction
In amphibians, the formation of the nervous system occurs during gastrulation, with a process called neural induction. In the past 15 years, it has been suggested that neural induction results from the opposing action of ventralizing signals such as bone morphogenetic proteins (BMPs) from the ectoderm, which are responsible for the determination of the epidermis, and dorsalizing signals, such as noggin, chordin, follistatin, XnR3 and Cerberus, from the dorsal mesoderm (reviewed by Sasai & De Robertis (1997)). However, mounting evidence suggest that antagonizing BMP signalling is not sufficient to explain neural induction and that other signalling components such as fibroblast growth factor (FGF) are also required (Delaune et al. 2005; Stern 2005).
We suggest that neural induction is controlled by a different signalling pathway, in which transient rises in the concentration of intracellular calcium ([Ca2+])i play a role in controlling the binary determination decision (i.e. epidermis versus neural tissue). We present a new model to explain the role of Ca2+ in neural induction and to revaluate the concept of ‘by default’ neural induction.
2. Calcium is involved in the choice between neural and epidermal fate
Ectoderm (i.e. the animal cap), dissected at the blastula stage, exhibits a high level of plasticity. Without inducing factors it develops into atypical epidermis and with appropriate neural inducers such as noggin, the animal cap cells express a variety of neural markers.
Over 40 years ago, Barth & Barth (1964) were the first to suggest that Ca2+ is required to trigger neuralization in Rana pipiens embryos. In addition, the dissociation of animal caps in Ca2+- and Mg2+-free medium propelled the cells towards a neural fate (Grunz & Tacke 1989; Saint-Jeannet et al. 1989, 1990, 1993). Furthermore, we have shown that the dissociation of animal caps in Ca2+-free medium triggers an increase in [Ca2+]i. This increase is due to an efflux of Ca2+ from internal stores, resulting from the inversion of the gradient of concentration in Ca2+ between intra- and extracellular compartments (Leclerc et al. 2001). In addition, neuralization by dissociation is blocked when animal cap cells are loaded with the Ca2+ chelator N,N′-[1,2-ethanediylbis(oxy-2,1-phenylene)]bis[N-[2-[(acetyloxy)methoxy]-2-oxoethyl]]bis[(acetyloxy)methyl]ester (BAPTA); the neural marker neural cell adhesion molecule (NCAM) is not expressed (Leclerc et al. 2001). This shows that in animal caps a Ca2+-dependent signal is necessary both to trigger neuralization of the ectoderm and to inhibit epidermal determination.
3. DHP-sensitive Ca2+ channels and neural induction
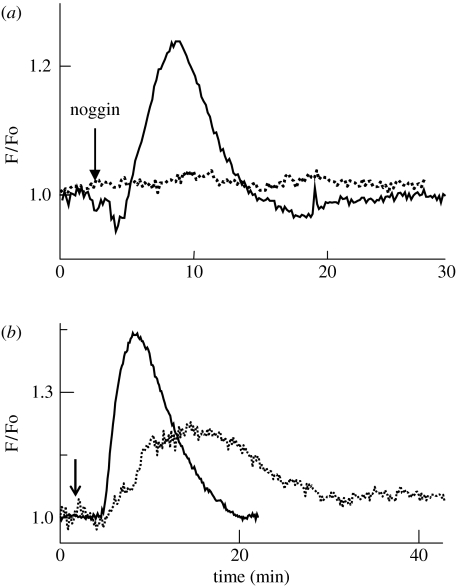
Addition of noggin to animal caps triggers an increase in [Ca2+]i (figure 1a). This increase has a duration of approximately 10–20 min and represents approximately 15% of the resting level of [Ca2+]i (Moreau et al. 1994; Batut et al. 2005). This [Ca2+]i increase is completely inhibited by antagonists of dihydropyridine (DHP)-sensitive Ca2+ channels, such as nifedipine or nimodipine (figure 1a). On the other hand, animal caps that are treated with specific agonists of DHP-sensitive Ca2+ channels, such as S(−)Bay K 8644, generate a transient increase in [Ca2+]i with a duration of approximately 20 min (figure 1b). This increase is sufficient, even in an active BMP context, to trigger not only the expression of neural markers but also the formation of neurons and glial cells (Moreau et al. 1994). Conversely, the inhibition of DHP-sensitive Ca2+ channels inhibits neural induction (Leclerc et al. 1997). In addition, methylxanthines, such as caffeine or theophylline, which are known to stimulate the release of Ca2+ from internal stores, are also potent neural inducers (figure 1b; Moreau et al. 1994; Leclerc et al. 1995). These latter experiments suggest that Ca2+ plays a crucial role, since whatever its provenance is, it triggers neuralization of the ectoderm.
Figure 1.
An increase in intracellular Ca2+ concentration ([Ca2+]i) is necessary and sufficient to trigger neural induction on isolated animal caps. (a) Noggin protein triggers an increase in [Ca2+]i via DHP-sensitive Ca2+ channels (solid line) since its effect is blocked by nimodipine, a specific agonist of DHP-sensitive Ca2+ channels (dotted line). Nimodipine blocks neural induction. (b) An artificial increase in [Ca2+], either by a release from internal stores with caffeine (solid line) or by direct stimulation of DHP-sensitive Ca2+ channels with a specific agonist S(−)BayK 8644 (dotted line), triggers neural induction. These treatments lead to the differentiation of neurons and glial cells. Ca2+ was recorded by loading the animal caps with the fluorescent probe fluo-3.
4. Imaging Ca2+ transients during neural induction in intact amphibian embryos
Using intact embryos during gastrulation, we have confirmed all the results obtained with the ex vivo animal cap system. Using the Ca2+-sensitive photoprotein, aequorin in conjunction with a custom-designed photon imaging microscope (Webb et al. 1997), we have directly visualized the Ca2+ dynamics that occur in Xenopus ectodermal cells. The onset of Ca2+ signalling activity occurs at the blastula stage (i.e. stage 8), long before the start of gastrulation (i.e. before mesoderm invagination) and the Ca2+ transients are localized in the most anterior part of the dorsal ectoderm. These observations indicate that neural induction might be initiated earlier than was previously thought, but this possibility still needs further investigation. Recent studies have demonstrated that neural induction requires the combined activity of the Nieuwkoop centre and the blastula chordin and noggin-expressing (BCNE) centre located in dorsal animal cells (Kuroda et al. 2004). The BCNE centre contains the prospective neuroectoderm and Spemann organizer precursor cells, and is required for brain formation. We suggest that the Ca2+ transients observed in the dorsal ectoderm during the blastula stage might well be localized in the BCNE centre. Thus, these transients appear so far to be the first visualized events linked to neural induction. As gastrulation proceeds, the number and intensity of the Ca2+ transients increasingly reach a peak of activity by mid-gastrulation (i.e. stage 11–11.5). This activity was found to be restricted to the dorsal ectoderm (i.e. the tissue where neural induction takes place) and never occurred in the ventral ectoderm cells (i.e. those which do not receive neural inductive signals).
Intact embryos also yielded results (i.e. neural induction was blocked) similar to the animal caps on treatment with either the calcium chelator BAPTA or specific antagonists of the DHP-sensitive Ca2+ channels. In addition, when treated with DHP-sensitive Ca2+ channel blockers, the embryos lacked anterior brain structures (Moreau et al. 1994; Leclerc et al. 1997, 2001). This phenotype is similar to the one obtained when the BCNE centre is removed (Kuroda et al. 2004).
In an attempt to simplify the experimental model, Keller open-face explants (Keller & Danilchik 1988) were used as a two-dimensional system to study neural induction. This model is sufficient to reproduce many aspects of neural induction observed in vivo, such as the expression of neural marker genes, neuronal differentiation and the induction of a regionalized neural plate along the antero-posterior axis. In this model, we observed that Ca2+ transients start from the most anterior part of the open-face explant as was observed in the intact embryo. (Leclerc et al. 2003).
5. What are the Ca2+ target genes?
We have previously shown, with animal caps, that Ca2+ controls the expression of the immediate early gene c-fos (Leclerc et al. 1999) and of two other transcription factors: XlPou2 and Zic3. While Fos is a ubiquitous transcription factor, XlPou2 and Zic3 are involved in neural determination and are primary neural regulators (Witta et al. 1995; Nakata et al. 1997). We demonstrated that specific antagonists of DHP-sensitive Ca2+ channels blocked the expression of XlPou2 in response to noggin in animal caps, and dramatically reduced the expression of Zic3 in the whole embryo (Leclerc et al. 2000). In addition, in planar explants, the accumulated pattern of Ca2+ correlated with the expression of Zic3, and treatment with nifedipine (a DHP-sensitive Ca2+ channel antagonist) blocked the Ca2+ transients and reduced the level of Zic3 expression (Leclerc et al. 2003). These results suggest that the increase in [Ca2+]i occurring during neural induction in the dorsal ectoderm can create compartments of high Ca2+ level, which might activate genes with proneural activity.
To identify new Ca2+ target genes involved in neural induction, we constructed a subtractive cDNA library between untreated (i.e. ectodermal) and short duration (i.e. 15–45 min) caffeine-treated (i.e. neuralized) animal caps. This treatment that triggers neural induction via an increase in [Ca2+]i (Moreau et al. 1994) allows the differential isolation of the earliest Ca2+-dependent genes involved in neural determination (Batut et al. 2003). We selected one gene, xPRMT1b, from the 30 early genes identified that were found to be controlled by Ca2+ and expressed in the presumptive neural territories. xPRMT1b is the Xenopus homologue of the mammalian arginine methyltransferase PRMT1 gene (Batut et al. 2005). On animal caps, the expression of xPRMT1b is an early response to a Ca2+ increase that does not require de novo protein synthesis. Its expression is triggered following the application of noggin or by the inhibition of BMP signalling with tBR (a non-functional form of the BMP4 receptor). These effects are specifically blocked by BAPTA, a calcium chelator. In the whole embryo, xPRMT1b is expressed in neural territories. The early expression of xPRMT1b at the gastrula stage also occurs through a Ca2+-dependent mechanism mediated by the activation of DHP-sensitive Ca2+ channels. Overexpression of xPRMT1b in the neural territories activates the expression of the neural precursor gene Zic3. A morpholino approach, with an oligonucleotide against xPRMT1b, blocks the expression of the neural markers induced by an increase in Ca2+ such as Zic3 in animal caps, and in the whole embryo it impairs anterior neural development (figure 2; Batut et al. 2005). Identical phenotypes are obtained with antagonists of DHP-sensitive Ca2+ channels (Leclerc et al. 2000), or when the BCNE centre is deleted (Kuroda et al. 2004). These results suggest that the xPRMT1b is a direct link between the [Ca2+]i increase and downstream events involved in neural induction.
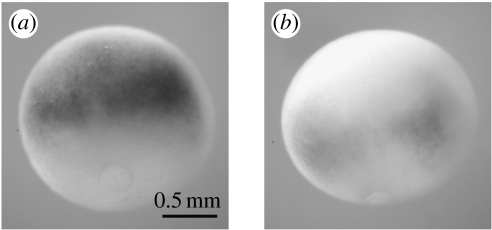
Figure 2.
Loss of function of xPRMT1b decreases Zic3 expression. Late gastrula embryos (stages 12–12.5) were injected at the 2-cell stage with a morpholino (Mo1b) against xPRMT1b and then probed for the expression of Zic3. (a) Control. (b) Mo1b strongly reduces Zic3 expression when compared to the control embryo.
6. Discussion
Acquisition of a neural fate has been, until recently, considered as a permissive event, only requiring the inhibition of BMP signalling. While this ‘by default’ model has allowed us to understand part of the process of early neurogenesis and epidermal determination at the molecular level, a number of important questions still remain to be addressed. The ‘by default’ model conflicts in particular with data from chick and ascidian embryos, which indicate that neural induction is initiated by FGF signalling in a partly BMP-independent manner (Bertrand et al. 2003; Stern 2005). The ‘by default’ model also cannot fully explain the inhibition of neuralization triggered by noggin on isolated ectoderm that expresses truncated forms of FGF receptors (Launay et al. 1996). In addition, in intact Xenopus embryos, it has been recently shown that BMP inhibition is required but is not sufficient to trigger neural induction, and that pre-gastrula FGF signalling is required in the ectoderm for the emergence of neural fates (Delaune et al. 2005).
Finally, our results indicate that an increase in [Ca2+]i is a necessary and sufficient event to neuralize the ectoderm. These results suggest a permissive role played by Ca2+. The identification and functional characterization of new Ca2+ target genes, such as xPRMT1b, will help us to make the link between Ca2+ influx and neural determination.
However, several important and as yet unsolved questions have been raised by our data; for example, the mechanism by which the DHP-sensitive Ca2+ channels are activated during gastrulation in the dorsal ectoderm is still unknown, as is how noggin can stimulate an influx of Ca2+ through DHP-sensitive Ca2+ channels. In this respect, it is important for us to further consider the relationship between noggin and the FGF receptor. It has been demonstrated, for example, in chick embryo neurons and endothelial cells that the activation of the FGF receptor stimulates the release of arachidonic acid and its metabolites, which in turn activate a Ca2+ influx probably via transient receptor potential channels (TRP; Distasi et al. 1995; Antoniotti et al. 2003).
Another important question is why the Ca2+ signals are initially generated in the anterior part of the ectoderm, since the inducing signal is supposed to be via the diffusion of molecules secreted by the dorsal mesoderm. The anterior region of the Ca2+ transients in the blastula stage might correspond to the BCNE centre. The presence of neural inducers, such as noggin, in the blastula ectodermal precursor cells (Kuroda et al. 2004) and the evidence that noggin activates DHP-sensitive Ca2+ channels on animal caps (Leclerc et al. 1999; Batut et al. 2005) support this hypothesis.
7. How gene expression during neural induction might be controlled by Ca2+
Control of gene expression by Ca2+ very often involves changes in the transactivating properties of transcription factors after the induction of Ca2+-dependent kinases and phosphatases. However, direct effectors of Ca2+-induced gene expression have also been suggested to exist in the nucleus. An important cis-regulatory element, downstream regulatory element (DRE), has been implicated in what can be called excitation– or stimulation–transcription coupling mechanisms (Carrion et al. 1999). The DRE sequence, which is located downstream from the TATA box, is the target of the DRE antagonist modulator (DREAM), an EF-hand Ca2+-binding protein of the recovering subfamily, which in the absence of Ca2+ binds to the DRE site and represses transcription. To date, DREAM is the only Ca2+ sensor known to bind specifically to DNA and directly regulate transcription in a Ca2+-dependent manner. Some evidence shows that promoters of some neural-specific genes contain putative Ca2+-dependent cis-regulatory elements.
Recent findings suggest an alternative mechanism by which Ca2+ might control gene expression directly. In our model, the DHP-sensitive Ca2+ channel plays a crucial role in triggering neural induction and neural gene expression. The mechanism that links the activity of this Ca2+ channel to the nucleus is not well understood. The DHP channel may be related to an L-type Ca2+ channel. Recently it was shown that the C-terminal fragment of an L-type Ca2+ channel translocates to the nucleus, binds to a nuclear protein, associates with an endogenous promoter and regulates transcription of a wide variety of endogenous genes important for neuronal signalling (Gomez-Ospina et al. 2006). This work suggests that during neural induction, the activity of DHP-sensitive Ca2+ channels might be involved in a similar way to control the expression of neural genes.
Another way for Ca2+ to control neural gene expression is through the inhibition of BMP signalling, by acting downstream of Smad phosphorylation. The spatial distribution of activated Smad1 (i.e. phosphorylated Smad1) has been reported to change at the onset of gastrulation. Prior to gastrulation, phosphorylated Smad1 (which reflects the activation of the BMP4 signalling pathway) is equally distributed on the dorsal and ventral sides of the embryo. In contrast, at late blastula, Smad1 phosphorylation is enriched on the ventral side, and by early gastrulation most of the activated Smad1 is localized to the ventral side (Faure et al. 2000). This correlates with the pattern of Ca2+ increase, which starts in the dorsal ectoderm at the blastula stage and is maximal at mid gastrulation (Leclerc et al. 2000). One can hypothesize that the dephosphorylation of Smad1 in the dorsal ectoderm during gastrulation is controlled by calcineurin, a Ca2+-/calmodulin-dependent phosphatase 2B. Xenopus calcineurin is expressed throughout early development (Saneyoshi et al. 2000). Furthermore, injection of constitutively active mouse calcineurin into a ventral position produces a double axis (Nishinakamura et al. 1997).
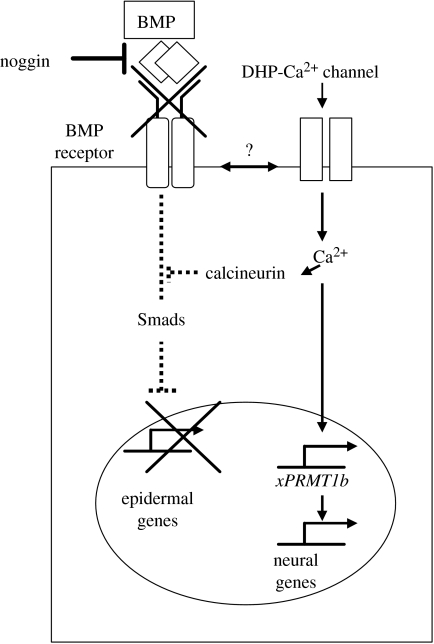
To conclude, we propose a new model of neural induction to modulate the concept of the ‘by default’ mechanism. Our new model integrates the activation of a Ca2+-dependent signalling pathway due to an influx of Ca2+ through DHP-sensitive Ca2+ channels. While Ca2+ is required for the activation of neural-specific genes, epidermal determination occurs when the Ca2+-dependent signalling pathway is inactive (figure 3).
Figure 3.
A new model for neural induction in Xenopus laevis embryos. In this model Ca2+ plays a central role, by directly activating Ca2+ target genes such as xPRMT1b, which in turn control neural gene transcription either directly or via the activation of a Ca2+/calmodulin kinase type II. The Ca2+ signals may also inhibit the BMP signalling pathway by activating a calcineurin, which prevents the phosphorylation of Smads (see text for more details). The activation of the DHP-Ca2+ channels is performed by an as yet unidentified mechanism (indicated by the ‘?’) at the level of the binding of BMP by neural inducers.
Acknowledgments
The work reported here was supported by Centre National de la Recherche Scientifique (CNRS); a joint PICS grant funded by the CNRS; the PROCORE France/Hong Kong Joint Research Scheme (F-HK98/99.SC06) sponsored by the Research Grants Council (RGC) of Hong Kong and the Consulate General of France in Hong Kong; Association pour la Recherche sur le Cancer (ARC); and the following Hong Kong RGC grants: HKUST6214/02M, HKUST6279/03M, HKUST6241/04M and HKUST6416/06M. We also thank Dr Osamu Shimomura for his generous contribution to aequorin-based imaging over the years.
Footnotes
One contribution of 11 to a Discussion Meeting Issue ‘Calcium signals and developmental patterning’.
References
- Antoniotti S, Fiorio Pla A, Pregnolato S, Mottola A, Lovisolo D, Munaron L. Control of endothelial cell proliferation by calcium influx and arachidonic acid metabolism: a pharmacological approach. J. Cell Physiol. 2003;197:370–378. doi: 10.1002/jcp.10359. doi:10.1002/jcp.10359 [DOI] [PubMed] [Google Scholar]
- Barth L.G, Barth L.J. Sequential induction of the presumptive epidermis of the Rana pipiens gastrula. Biol. Bull. 1964;127:413–427. doi: 10.2307/1539912. doi:10.2307/1539245 [DOI] [PubMed] [Google Scholar]
- Batut J, Neant I, Leclerc C, Moreau M. xMLP is an early response calcium target gene in neural determination in Xenopus laevis. J. Soc. Biol. 2003;197:283–289. [PubMed] [Google Scholar]
- Batut J, Vandel L, Leclerc C, Daguzan C, Moreau M, Neant I. The Ca2+-induced methyltransferase xPRMT1b controls neural fate in amphibian embryo. Proc. Natl Acad. Sci. USA. 2005;102:15 128–15 133. doi: 10.1073/pnas.0502483102. doi:10.1073/pnas.0502483102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand V, Hudson C, Caillol D, Popovici C, Lemaire P. Neural tissue in ascidian embryos is induced by FGF9/16/20, acting via a combination of maternal GATA and Ets transcription factors. Cell. 2003;115:615–627. doi: 10.1016/s0092-8674(03)00928-0. doi:10.1016/S0092-8674(03)00928-0 [DOI] [PubMed] [Google Scholar]
- Carrion A.M, Link W.A, Ledo F, Mellstrom B, Naranjo J.R. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999;398:80–84. doi: 10.1038/18044. doi:10.1038/18044 [DOI] [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. doi:10.1242/dev.01582 [DOI] [PubMed] [Google Scholar]
- Distasi C, Munaron L, Laezza F, Lovisolo D. Basic fibroblast growth factor opens calcium-permeable channels in quail mesencephalic neural crest neurons. Eur. J. Neurosci. 1995;7:516–520. doi: 10.1111/j.1460-9568.1995.tb00348.x. doi:10.1111/j.1460-9568.1995.tb00348.x [DOI] [PubMed] [Google Scholar]
- Faure S, Lee M.A, Keller T, ten Dijke P, Whitman M. Endogenous patterns of TGFbeta superfamily signaling during early Xenopus development. Development. 2000;127:2917–2931. doi: 10.1242/dev.127.13.2917. [DOI] [PubMed] [Google Scholar]
- Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. doi:10.1016/j.cell.2006.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunz H, Tacke L. Neural differentiation of Xenopus laevis ectoderm takes place after disaggregation and delayed reaggregation without inducer. Cell Differ. Dev. 1989;28:211–217. doi: 10.1016/0922-3371(89)90006-3. doi:10.1016/0922-3371(89)90006-3 [DOI] [PubMed] [Google Scholar]
- Keller R, Danilchik M. Regional expression, pattern and timing of convergence and extension during gastrulation of Xenopus laevis. Development. 1988;103:193–209. doi: 10.1242/dev.103.1.193. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Wessely O, De Robertis E.M. Neural induction in Xenopus: requirement for ectodermal and endomesodermal signals via Chordin, Noggin, beta-Catenin, and Cerberus. PLoS Biol. 2004;2:e92. doi: 10.1371/journal.pbio.0020092. doi:10.1371/journal.pbio.0020092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launay C, Fromentoux V, Shi D.L, Boucaut J.C. A truncated FGF receptor blocks neural induction by endogenous Xenopus inducers. Development. 1996;122:869–880. doi: 10.1242/dev.122.3.869. [DOI] [PubMed] [Google Scholar]
- Leclerc C, Moreau M, Gualandris-Parisot L, Drean G, Canaux S, Duprat A.M. An elevation of internal calcium occuring via L-type channels mediate neural induction in the amphibian embryo. In: Zagris N, Duprat A.M, Durston J, editors. Organisation of the early vertebrate. Plenum Press; New York, NY: 1995. pp. 209–226. [Google Scholar]
- Leclerc C, Daguzan C, Nicolas M.T, Chabret C, Duprat A.M, Moreau M. L-type calcium channel activation controls the in vivo transduction of the neuralizing signal in the amphibian embryos. Mech. Dev. 1997;64:105–110. doi: 10.1016/s0925-4773(97)00054-3. doi:10.1016/S0925-4773(97)00054-3 [DOI] [PubMed] [Google Scholar]
- Leclerc C, Duprat A.M, Moreau M. Noggin upregulates Fos expression by a calcium-mediated pathway in amphibian embryos. Dev. Growth Differ. 1999;41:227–238. doi: 10.1046/j.1440-169x.1999.00421.x. doi:10.1046/j.1440-169x.1999.00421.x [DOI] [PubMed] [Google Scholar]
- Leclerc C, Webb S.E, Daguzan C, Moreau M, Miller A.L. Imaging patterns of calcium transients during neural induction in Xenopus laevis embryos. J. Cell Sci. 2000;113:3519–3529. doi: 10.1242/jcs.113.19.3519. [DOI] [PubMed] [Google Scholar]
- Leclerc C, Rizzo C, Daguzan C, Neant I, Batut J, Auge B, Moreau M. Neural determination in Xenopus laevis embryos: control of early neural gene expression by calcium. J. Soc. Biol. 2001;195:327–337. [PubMed] [Google Scholar]
- Leclerc C, Lee M, Webb S.E, Moreau M, Miller A.L. Calcium transients triggered by planar signals induce the expression of ZIC3 gene during neural induction in Xenopus. Dev. Biol. 2003;261:381–390. doi: 10.1016/s0012-1606(03)00298-7. doi:10.1016/S0012-1606(03)00298-7 [DOI] [PubMed] [Google Scholar]
- Moreau M, Leclerc C, Gualandris-Parisot L, Duprat A.M. Increased internal Ca2+ mediates neural induction in the amphibian embryo. Proc. Natl Acad. Sci. USA. 1994;91:12 639–12 643. doi: 10.1073/pnas.91.26.12639. doi:10.1073/pnas.91.26.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Nagai T, Aruga J, Mikoshiba K. Xenopus Zic3, a primary regulator both in neural and neural crest developement. Proc. Natl Acad. Sci. USA. 1997;94:11 980–11 985. doi: 10.1073/pnas.94.22.11980. doi:10.1073/pnas.94.22.11980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishinakamura R, Matsumoto Y, Uochi T, Asashima M, Yokota T. Xenopus FK 506-binding protein homolog induces a secondary axis in frog embryos, which is inhibited by coexisting BMP 4 signaling. Biochem. Biophys. Res. Commun. 1997;239:585–591. doi: 10.1006/bbrc.1997.7491. doi:10.1006/bbrc.1997.7491 [DOI] [PubMed] [Google Scholar]
- Saint-Jeannet J.P, Foulquier F, Goridis C, Duprat A.M. Expression of N-CAM precedes neural induction in Pleurodeles waltl (urodele, amphibian) Development. 1989;106:675–683. doi: 10.1242/dev.106.4.675. [DOI] [PubMed] [Google Scholar]
- Saint-Jeannet J.P, Huang S, Duprat A.M. Modulation of neural commitment by changes in target cell contacts in Pleurodeles waltl. Dev. Biol. 1990;141:93–103. doi: 10.1016/0012-1606(90)90104-q. doi:10.1016/0012-1606(90)90104-Q [DOI] [PubMed] [Google Scholar]
- Saint-Jeannet J.P, Pituello F, Huang S, Foulquier F, Duprat A.M. Experimentally provoked neural induction results in an incomplete expression of neuronal traits. Exp. Cell Res. 1993;207:383–387. doi: 10.1006/excr.1993.1205. doi:10.1006/excr.1993.1205 [DOI] [PubMed] [Google Scholar]
- Saneyoshi T, Kume S, Natsume T, Mikoshiba K. Molecular cloning and expression profile of Xenopus calcineurin A subunit(1) Biochim. Biophys. Acta. 2000;1499:164–170. doi: 10.1016/s0167-4889(00)00083-5. doi:10.1016/S0167-4889(00)00083-5 [DOI] [PubMed] [Google Scholar]
- Sasai Y, De Robertis E.M. Ectodermal patterning in vertebrate embryos. Dev. Biol. 1997;182:5–20. doi: 10.1006/dbio.1996.8445. doi:10.1006/dbio.1996.8445 [DOI] [PubMed] [Google Scholar]
- Stern C.D. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. doi:10.1242/dev.01794 [DOI] [PubMed] [Google Scholar]
- Webb S.E, Lee K.W, Karplus E, Miller A.L. Localized calcium transients accompany furrow positioning, propagation, and deepening during the early cleavage period of zebrafish embryos. Dev. Biol. 1997;192:78–92. doi: 10.1006/dbio.1997.8724. doi:10.1006/dbio.1997.8724 [DOI] [PubMed] [Google Scholar]
- Witta S.E, Agarwal V.R, Sato S.M. XIPOU 2, a noggin-inducible gene, has direct neuralizing activity. Development. 1995;121:721–730. doi: 10.1242/dev.121.3.721. [DOI] [PubMed] [Google Scholar]