Abstract
Many aspects of animal development including fertilization as well as organ formation and function are dependent upon the dynamic release of calcium (Ca2+) ions. Although the controlled release and/or accumulation of Ca2+ ions has been extensively studied, how the release dynamics produce a specific biological output in embryonic development is less clear. We will briefly summarize Ca2+ sources, highlight data on endogenous Ca2+ release in vertebrate embryos relevant to body plan formation and cell movement, and integrate pharmacological and molecular-genetic studies to lend insight into the signalling pathways involved. Finally, based on in vivo imaging in zebrafish genetic mutants, we will put forward the model that distinct Ca2+ release dynamics lead to antagonism of the developmentally important Wnt/β-catenin signalling pathway, while sustained Ca2+ release modulates cell polarization or directed migration.
Keywords: calcium, Wnt, β-catenin, zebrafish, dorsal–ventral axis, left–right patterning
1. Calcium sources
Although critical for many processes, calcium ions (Ca2+) are not metabolized by the cell. Instead, Ca2+ enters the cell across either the plasma membrane or the membrane of intracellular organelles. Depending on the location of the ion channels and the extent and duration of the channel opening, local or global changes in Ca2+ levels in the cytosol of the cell can result. Intricate crosstalk and feedback between release circuits can stimulate Ca2+-induced Ca2+ release, influencing neighbouring receptors and potentially triggering a regenerative wave (Berridge 1997; Berridge et al. 2003; Roderick et al. 2003). In addition, continued stimulation and/or depletion of endoplasmic reticulum (ER) stores activate a store-operated Ca2+ entry influx pathway located at the plasma membrane (Parekh & Putney 2005).
In non-excitable (non-neuronal) cells, a majority of intracellular Ca2+ release occurs through inositol 1,4,5-trisphosphate (IP3)-sensitive Ca2+ channels present in the ER membrane (reviewed in Berridge et al. 2003). The phosphatidylinositol (PI) cycle is activated in response to many hormones and growth factors that bind to cell surface receptors. Two predominant receptor classes are the G-protein-coupled receptor class and the receptor tyrosine kinase class. Extracellular ligand stimulation of these receptors activates a PI-specific phospholipase C (PLC). Activated PLC converts membrane-bound phosphatidylinositol (4,5)-bisphosphate (PIP2) into IP3 and lipophilic diacylglycerol (DAG). IP3 subsequently binds to receptors (IP3R) located principally on the ER triggering the rapid release of Ca2+ into the cytosol of the cell. At the same time, DAG produced by PIP2 hydrolysis can act as an additional second messenger to further activate downstream targets such as protein kinase C (PKC).
Relevant to this discussion is the fact that Ca2+ release is heterogeneous. Specific cellular responses can be triggered by differences in the amplitude, frequency and duration of intracellular Ca2+ oscillations. Such oscillations can be derived from changes in upstream steps within the PI cycle, such as G-protein activity, PLC activity and IP3 levels (Hirose et al. 1999; Luo et al. 2001; McCarron et al. 2004; Thore et al. 2004; Nomikos et al. 2005; Rey et al. 2005). Oscillatory small molecules such as IP3 may be transmitted to other cells via gap junctions (Lin et al. 2004), a phenomenon that may be of significance in the regulation of axis induction in the zebrafish blastula (see below). Feedback from activated Ca2+-binding proteins adds another layer of complexity to the dynamics of Ca2+ release and removal. For example, IP3R activity integrates signals from small molecules and proteins, including PKC and Ca2+/calmodulin-dependent protein kinase II (CaMKII; Nadif Kasri et al. 2002; Assefa et al. 2004; Patterson et al. 2004).
2. Calcium and the vertebrate body plan
After fertilization, the next major developmental programme involves the establishment of the primary axes, in which regions of the embryo receive signals to determine the cells that will contribute to the dorsal (back) or ventral (belly) tissue as well as anterior (head/top) and posterior (tail/bottom) regions. A number of studies have linked PI-cycle activity with body plan formation. Classical work using lithium, an inhibitor of inositol turnover (Berridge et al. 1989), induced expansion of dorsal structures in Xenopus (Kao et al. 1986; Kao & Elinson 1989, 1998), and similar effects were obtained in the zebrafish (Danio rerio) embryo (Stachel et al. 1993; Aanstad & Whitaker 1999). Lithium-induced embryonic defects are rescued by supplying an intermediate of the PI cycle, myo-inositol (Busa & Gimlich 1989). In further support, zebrafish and Xenopus embryos injected with antibodies that disrupt IP3R function displayed expanded dorsal structures with the loss of ventral structures (Kume et al. 1997; Westfall et al. 2003b). Treatment of zebrafish embryos with inhibitors that target different steps in the PI cycle generated dorsalized or axis-duplication phenotypes (figure 1a, inset). The dorsalization defects generated by the use of pharmacological inhibitors that target inositol turnover in the zebrafish were likewise rescued by myo-inositol (Westfall et al. 2003b). The findings of an involvement for PI-cycle activity in axis induction are consistent with the observed spontaneous increase in IP3 levels in the Xenopus embryo at the blastula stage (Busa & Gimlich 1989; Maslanski et al. 1992). Moreover, in vivo imaging of calcium release dynamics in the zebrafish embryo identified rapid aperiodic Ca2+ release that persists until the midblastula transition stage (Reinhard et al. 1995; Slusarski et al. 1997b; Slusarski & Corces 2000). The idea that increased IP3 levels may trigger Ca2+ release during these stages has been corroborated by drug inhibition studies (Slusarski et al. 1997a). The effects of lithium were most pronounced when exposure occurred on the ventral side of the embryo, suggesting that in the embryo PI-cycle activity is normally high on the ventral side and low on the dorsal side. Consistent with this is the described role of activated CaMKII for ventral fates in Xenopus embryos (Kühl et al. 2000a).
Figure 1.
Signal transduction pathways in vertebrate axis formation. During embryogenesis, the basic body plan generates a dorsal–ventral and anterior–posterior orientation of tissues and organs. (a) Lateral view of a wild-type zebrafish 48 hour larvae with normal axial patterning with anterior to the right and dorsal to the top. Key tissues are noted. Inset: a two-headed embryo as a result of PI-cycle inhibition. (b) A simplified schematic of the Wnt signalling network. β-catenin-independent pathways are encased in green region and β-catenin-dependent components are in the yellow region. β-cat, β-catenin. (c) A single ratiometric image taken from a time course during the cellular blastoderm stage of a fura-2-injected zebrafish embryo. The image is pseudo-coloured to represent high calcium levels as warm colouring (yellow) and low calcium levels as blue. Arrows denote transient fluxes observed in the enveloping layer and the arrowhead designates the yolk syncytial layer region. (d) hecate mutant embryo lacking dorsal–anterior structures such as the eyes and brain reflective of a ventralized phenotype. (e) ppt mutant embryo with a shortened anterior–posterior axis and kinked tail.
3. The Wnt signalling network
The Wnt family of growth factors and components of their signalling pathways have diverse roles in development and disease. Wnt signalling influences many aspects of embryonic patterning, cell proliferation as well as the maintenance and differentiation of stem cells, and is critical in axis formation (figure 1b; reviewed in Moon et al. 2004; Kohn & Moon 2005; Clevers 2006). In the absence of the so-called canonical Wnt signalling (Wnt/β-catenin), β-catenin is rapidly sequestered in a cytoplasmic degradation complex containing axin, the adenomatous polyposis tumour suppressor protein (APC) and the serine threonine kinase GSK-3β. GSK-3 phosphorylation of β-catenin targets the latter for proteasomal degradation (figure 1b). Wnt binding to its co-receptors Frizzled and LRP5/6 (low-density lipoprotein receptor-related protein) activates a cytoplasmic phosphoprotein (Dishevelled, Dsh) which downregulates GSK-3 and inhibits the degradation of β-catenin. Stabilized β-catenin protein interacts with the members of the LEF/TCF transcription factor family in the nucleus to promote the activation of downstream target genes involved in axis specification. Another endogenous target of lithium is the β-catenin degradation complex component GSK-3, which when inhibited promotes dorsal axis induction (Klein & Melton 1996; Stambolic et al. 1996). The fact that exogenous myo-inositol can suppress the effects of GSK-3 inhibition (Hedgepeth et al. 1997) further supports the notion of communication between PI-cycle activity and Wnt/β-catenin signalling to regulate axis induction.
The Wnt network has layers of complexity including the fact that different Wnt ligands can activate distinct cellular outputs. In vertebrate embryos, overexpression of a subset of Wnts induces hyperdorsalization and ectopic axes by virtue of increased Wnt/β-catenin signalling activity (Moon et al. 1993b; Moon & Kimelman 1998). Additional Wnts (including Wnt-5, -4 and -11) appear to act independently of β-catenin function (Dale 1998; Kühl et al. 2000b). Emerging evidence suggests that the ability of Wnt ligands to activate different signalling pathways, β-catenin dependent (canonical) and β-catenin independent (non-canonical), appears to be controlled by the timing of expression and receptor context, not to mention the correct combination of intracellular effectors. In the zebrafish embryo, Wnt-5 overexpression results in an increase in the frequency of intracellular Ca2+ release in a manner that is dependent on G-protein activity and the PI cycle (Slusarski et al. 1997a,b), thus linking Wnt activity to IP3-dependent Ca2+ release and defining the Wnt/Ca2+ signalling pathway.
The concept that Wnt/Ca2+, either in parallel or as part of a complex signalling network, appears to interact with the Wnt/β-catenin pathway in early axis specification was initially suggested by the apparent antagonism of certain pairs of Wnt ligands when expressed in Xenopus and zebrafish embryos (Moon et al. 1993b; Slusarski et al. 1997b). Expression of ligands that activate Wnt/β-catenin signalling in these embryos, such as Wnt-8, results in ectopic axis induction. However, when Wnt-5 is co-expressed with Wnt-8, the Wnt-8 axis induction phenotype is suppressed. Stimulating Ca2+ release, via activated serotonin receptor, also antagonizes Wnt-8-induced expansion of dorsal domains (Slusarski et al. 1997b), supporting that Wnt-5 antagonism of Wnt/β-catenin is mediated by Ca2+ release. On the other hand, pharmacological or genetic reduction of the Wnt/Ca2+ pathway in zebrafish embryos or mouse limb buds generates ectopic accumulation of nuclear β-catenin and activation of β-catenin transcriptional targets (Topol et al. 2003; Westfall et al. 2003a,b). Additionally, inhibition of G-protein function dorsalizes Xenopus embryos (Kume et al. 2000). These observations are consistent with a model in which IP3-dependent Ca2+ release, promoted by Wnt/Ca2+ signalling activity, negatively regulate the Wnt/β-catenin signalling pathway and therefore axis induction (figure 1b).
Various studies have shown that there are common components between the Wnt/Ca2+ and the planar cell polarity pathways (Wnt/PCP), another non-canonical Wnt pathway involved in the polarization of cells in Drosophila and vertebrate species (Kohn & Moon 2005; Solnica-Krezel 2005). During gastrulation, vertebrate embryos undergo a variety of morphogenetic movements to thicken (dorsal convergence) and elongate (axis extension) the embryo, also called convergence extension (CE; Keller 2002). Ca2+ mobilization associated with waves of tissue contraction can be observed in Xenopus explants (Wallingford et al. 2001a). In intact zebrafish embryos, intercellular Ca2+ waves have been observed at the margin during gastrulation (Gilland et al. 1999). The relationship between Ca2+ waves and cell movement is supported by the finding that, in Xenopus embryos, pharmacological inhibition of such waves results in CE defects without altering cell fate (Wallingford et al. 2001a,b). Wnt genes that result in the activation of Ca2+ release in the blastula embryo, such as Wnt-5 (Slusarski et al. 1997b; Westfall et al. 2003a), can also alter morphogenetic movements later during gastrulation when misexpressed (Moon et al. 1993a; Ungar & Moon 1995). Core components involved in the polarization of epithelial cells in the Drosophila cuticle are also necessary for the polarization of migrating cells during vertebrate gastrulation (Solnica-Krezel 2005). Indeed, several core components of Wnt/PCP have been shown to activate Ca2+ release in zebrafish including Frizzled-2, Dsh and the intracellular protein Prickle (Slusarski et al. 1997a; Sheldahl et al. 2003; Veeman et al. 2003). The fact that interference with either Ca2+ release or Wnt/PCP signalling results in CE defects suggests non-canonical Wnt signalling activity can be characterized as a complex network with cellular outputs defined by Ca2+ modulation and polarized cell movement (figure 1b).
4. Calcium dynamics and biological outputs
The Ca2+ activity in the zebrafish blastula is observed in the enveloping layer (EVL) and yolk syncytial layer (YSL; figure 1c; Reinhard et al. 1995; Slusarski et al. 1997b; Slusarski & Corces 2000). Although present in overlapping stages, the EVL-specific Ca2+ fluxes are present in a cell or small cluster of cells lasting for short intervals. The YSL-specific Ca2+, on the other hand, displays sustained elevation in a population of cells. The distinct dynamics of Ca2+ increases and the bimodal role of Wnt/Ca2+, in β-catenin antagonism and polarized cell movement, led us to hypothesize that the rapid aperiodic Ca2+ release is coupled to Wnt/β-catenin antagonism and the sustained Ca2+ levels integrates into polarizing cells or their directed migration.
Support for this theory comes from the analysis of Ca2+ release dynamics in zebrafish mutants. A mutation in the zebrafish maternal gene hecate produces ventralized embryos (figure 1d) that lack nuclear β-catenin (Lyman Gingerich et al. 2005). Consistent with our theory, these mutant embryos display increased Ca2+ release frequency in the EVL (Lyman Gingerich et al. 2005). Suppression of the Ca2+ dynamics with pharmacological reagents was sufficient to rescue the defects in dorsal cell fate specification observed in these mutants (Lyman Gingerich et al. 2005), highlighting the relationship between Ca2+ release and β-catenin antagonism in a genetic context.
In the zebrafish, Wnt-5 has been shown to correspond to the genetic mutation pipetail (ppt; Rauch et al. 1997). The ppt zygotic mutants have axis extension defects, reflected in a shorter anterior–posterior length and kinks in the tail, resembling a pipe (figure 1e; Hammerschmidt et al. 1996; Kilian et al. 2003). Analysis of zygotic ppt mutant embryos revealed reduced Ca2+ levels in the YSL region (Westfall et al. 2003a). Suggestive that reduced Ca2+ levels are central to the ppt defects is the ability to rescue the mutant phenotype with the expression of activated CaMKII (Westfall et al. 2003a). These data raise the possibility that the YSL-specific Ca2+ dynamics contributes to polarized cell movements during vertebrate gastrulation.
5. Calcium and left–right asymmetry
There are many cases in development where a signalling cassette is used in multiple processes. Thus, it would be predicted that transient Ca2+ release will correlate with β-catenin antagonism or sustained Ca2+ signalling with cell polarization at additional stages or in different tissues. Section 6 describes the orientation of organs relative to the body axis and the implications of both transient and sustained Ca2+ modulations in this process. Although vertebrates appear bilaterally symmetrical from the outside, the heart, lungs, liver and gut are carefully positioned across the left–right (LR) axis. The development of this asymmetry is highly conserved across species and most likely will use conserved signalling molecules.
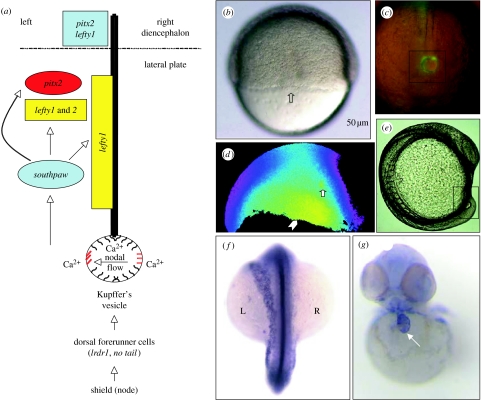
Embryonic organ laterality is preceded by molecular and physiological asymmetries. Shown in figure 2a is a schematic of developmental structures and gene products implicated in LR patterning with a focus on zebrafish. At the morphological level, non-involuting dorsal mesoderm cells, the dorsal forerunner cells (DFCs), migrate ahead of the dorsal blastoderm during gastrulation (figure 2b, arrow; Cooper & D'Amico 1996; Melby et al. 1996). At the start of gastrulation, these cells express key signalling molecules such as nodal-related squint, a Brachyury homologue no tail and left–right dynein (lrdr1; Schulte-Merker et al. 1994; Feldman et al. 1998; Essner et al. 2002). The DFCs are highly endocytotic and readily take up vital dyes (Cooper & D'Amico 1996) allowing for the visualization of their migration to the tailbud region where they undergo morphogenesis and form a ciliated structure, the Kupffer's vesicle (KV), during early somite stages (figure 2c; Cooper & D'Amico 1996; Melby et al. 1996). Ablation of the DFCs or mechanical disruption of the KV disrupts LR patterning (Amack & Yost 2004; Essner et al. 2005). Endogenous Ca2+ release activity in and around the DFC region during epiboly suggests striking similarities to the transient activity both in a cell or a few cells (figure 2d, arrow) and in a region of sustained high Ca2+ (figure 2d, arrowhead) previously described during the cellular blastoderm stages (figure 1c; Schneider et al. 2008). Whether this activity correlates with β-catenin antagonism and polarized cell movement bears further study.
Figure 2.
Left–right pattern formation in the zebrafish. (a) Schematic noting key structures and gene products implicated in LR patterning. (b) Epiboly-staged embryo oriented with the animal pole towards the top, yolk region at the bottom and the dorsal shield region in the centre. The arrow notes the DFCs migrating ahead of the shield region. (c) Vital-dye-labelled DFCs (with Syto-11) at the early somite stages. The combined bright field and fluorescent image focused on the tailbud showing the DFCs forming the KV in the black box. The midline of the embryo can be distinguished by the compact notochord cells above the KV. (d) A ratiometric image taken from a time course of an epiboly-staged fura-2-injected embryo. Arrow notes a transient flux while the arrowhead notes a region of high sustained Ca2+. (e) Lateral view of a somite-stage embryo at a time molecular asymmetric markers begin to be expressed. The KV is morphologically visible in the tail (black box). (f) Whole-mount in situ hybridization at approximately 22 somite stage with the southpaw and no tail probes. A dorsal view, anterior to the top, shows left-sided expression of southpaw and midline expression of no tail. (g) Whole-mount in situ hybridization of a 48 hours post-fertilization larva with cardiac myosin light chain to illustrate heart morphogenesis. The arrow points to the heart tube.
The LR signals have been proposed to be modulated by gap junctional communication and/or asymmetrical H+/K+-ATPase expression during Xenopus early cleavage (reviewed in Levin 2005). However, conserved molecular asymmetries in vertebrates become apparent only during later somite stages after the KV/node has formed (figure 2e). Of note is the conserved left-sided expression of the secreted transforming growth factor-β (TGF-β) related factor nodal (reviewed in Ahmad et al. 2004). In zebrafish, the nodal-related gene southpaw (spaw; Long et al. 2003) is the earliest asymmetric marker expressed in the left lateral plate mesoderm (figure 2f). Downstream targets of southpaw, including lefty1 and pitx2, also have conserved asymmetric expression in vertebrates (reviewed in Hamada et al. 2002; Wright & Halpern 2002). lefty1 contributes to a barrier at the midline of the embryo, preventing the left-sided signal from propagating to the right, and pitx2 contributes to the subsequent morphogenetic changes in the organs, such as looping to form the chambers of the heart (figure 2g).
Asymmetric Ca2+ levels across the mouse and chick node have been implicated in LR axis determination (McGrath et al. 2003; Raya et al. 2004). Elevated Ca2+ is thought to act via an unknown mechanism to induce left-sided gene expression in concert with or independent of nodal activity (Brennan et al. 2002; Hashimoto et al. 2004; Marques et al. 2004). Rotation of monocilia generates a leftward fluid flow in the node (Nonaka et al. 1998; Okada et al. 1999) and has been proposed to stimulate mechanosensory cilia and trigger elevated intracellular Ca2+ levels at the left edge of the mouse node (McGrath et al. 2003). The zebrafish KV contains monociliated cells similar to those found in the mouse node (Supp et al. 1997, 1999; Brueckner 2001; Hashimoto et al. 2004; Essner et al. 2005) and these cilia have been shown to beat in the same direction, possibly establishing Ca2+ asymmetries (Kramer-Zucker et al. 2005). Indeed, an intracellular Ca2+ flux with a left-sided bias near the zebrafish KV has been detected and is proposed to be required for normal LR patterning (Sarmah et al. 2005). In an alternate model, leftward flow of vesicular particles containing sonic hedgehog (shh) acts in a distinct atypical signalling pathway to activate Ca2+on the left side of the node (Tanaka et al. 2005).
In contrast to intracellular Ca2+ release, a role for left-sided elevation of extracellular Ca2+ has also been proposed. In the chick node, extracellular Ca2+ levels appear to be higher transiently on the left side, although it is unclear whether intracellular Ca2+ is also increased. This asymmetry was abolished after a treatment with ompremazole, an inhibitor of H+/K+ ATPase, which also caused LR defects. This led to the proposal that differential H+/K+ ATPase activity during gastrulation sets up a spatial gradient of extracellular Ca2+, which is subsequently transduced through Notch to activate asymmetric gene expression (Raya et al. 2004). In zebrafish, pharmacological disruption of H+/K+ ATPase activity leads to LR asymmetry defects (Kawakami et al. 2005) and early H+/K+ ATPase inhibition disrupts cilia number and length in the KV (Adams et al. 2006). However, immunodetection of H+/K+ ATPase did not display obvious asymmetries in zebrafish (Kawakami et al. 2005). Moreover, the H+/K+ ATPase inhibitor used, ompremazole, also induces ectopic shh expression in chick (Raya et al. 2004). Although it is unclear whether extracellular Ca2+ regulation is conserved in other organisms, mouse embryos that are homozygous for mutations in the Polycystin-2 (Pkd2) gene, a Ca2+-permeable cation-selective channel, exhibit loss of asymmetric Ca2+ levels across the node and LR defects (McGrath et al. 2003). Thus, several vertebrate models support a role for Ca2+ signalling in the establishment of LR asymmetry, but many questions and issues remain to be addressed, such as the Ca2+ sources, the Ca2+-dependent responders and the mechanism linking the Ca2+ flux in and around the node to asymmetry in the lateral plate mesoderm. Also, it has not been investigated whether there are multiple stages of Ca2+-dependent events involving the polarized migration and coalescence of the DFCs into a KV, initiation of asymmetric expression and the subsequent maintenance of laterality signals.
6. Conclusions and future directions
In conclusion, progression from egg to embryo requires a dynamic interaction of signal transduction networks. The continuous exchange of information between cells, through both direct contact and diffusible molecules, influences gene expression and cell behaviour. In vivo imaging studies are a critical step in the comprehensive analysis of Ca2+ signalling in development. Coupling in vivo imaging with molecular, genetic or pharmacological tools will determine the mechanism by which Ca2+ signalling is modulated and interpreted in the embryo. Future studies will reconstruct the spatial and temporal dynamics of Ca2+ release and incorporate this activity into known signalling pathways, thus providing the capacity to discern the true nature of the cellular basis of pattern formation. Knowledge about how intricate Ca2+ signals are integrated into developmental pathways, in particular the Wnt growth factors, raises the possibility to address the pathophysiology of diseases that result from misregulation of this network.
Acknowledgments
Animal care and experiments were performed in accordance with institutional guidelines for animal experiment ethics.We are grateful to our collaborators who have contributed to the research reviewed here. We thank Dr Pelegri and Dr Rebagliati as well as members of the Slusarski lab for their insightful discussions. This work was supported by the National Institutes of Health to D.C.S. and American Heart Association predoctoral fellowships to C.M.F. and I.S.
Footnotes
One contribution of 11 to a Discussion Meeting Issue ‘Calcium signals and developmental patterning’.
References
- Aanstad P, Whitaker M. Predictability of dorso-ventral asymmetry in the cleavage stage zebrafish embryo: an analysis using lithium sensitivity as a dorso-ventral marker. Mech. Dev. 1999;88:33–41. doi: 10.1016/s0925-4773(99)00171-9. doi:10.1016/S0925-4773(99)00171-9 [DOI] [PubMed] [Google Scholar]
- Adams D.S, Robinson K.R, Fukumoto T, Yuan S, Albertson R.C, Yelick P, Kuo L, McSweeney M, Levin M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left–right patterning of non-mammalian vertebrates. Development. 2006;133:1657–1671. doi: 10.1242/dev.02341. doi:10.1242/dev.02341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N, Long S, Rebagliati M. A southpaw joins the roster: the role of the zebrafish nodal-related gene southpaw in cardiac LR asymmetry. Trends Cardiovasc. Med. 2004;14:43–49. doi: 10.1016/j.tcm.2003.11.001. doi:10.1016/j.tcm.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Amack J.D, Yost H.J. The T box transcription factor no tail in ciliated cells controls zebrafish left–right asymmetry. Curr. Biol. 2004;14:685–690. doi: 10.1016/j.cub.2004.04.002. doi:10.1016/j.cub.2004.04.002 [DOI] [PubMed] [Google Scholar]
- Assefa Z, et al. Caspase-3-induced truncation of type 1 inositol trisphosphate receptor accelerates apoptotic cell death and induces inositol trisphosphate-independent calcium release during apoptosis. J. Biol. Chem. 2004;279:43 227–43 236. doi: 10.1074/jbc.M403872200. doi:10.1074/jbc.M403872200 [DOI] [PubMed] [Google Scholar]
- Berridge M.J. The AM and FM of calcium signalling. Nature. 1997;386:759–760. doi: 10.1038/386759a0. doi:10.1038/386759a0 [DOI] [PubMed] [Google Scholar]
- Berridge M, Downes C, Hanley M. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59:411–419. doi: 10.1016/0092-8674(89)90026-3. doi:10.1016/0092-8674(89)90026-3 [DOI] [PubMed] [Google Scholar]
- Berridge M.J, Bootman M.D, Roderick H.L. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. doi:10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- Brennan J, Norris D.P, Robertson E.J. Nodal activity in the node governs left–right asymmetry. Genes Dev. 2002;16:2339–2344. doi: 10.1101/gad.1016202. doi:10.1101/gad.1016202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueckner M. Cilia propel the embryo in the right direction. Am. J. Med. Genet. 2001;101:339–344. doi: 10.1002/1096-8628(20010715)101:4<339::aid-ajmg1442>3.0.co;2-p. doi:10.1002/1096-8628(20010715)101:4<339::AID-AJMG1442>3.0.CO;2-P [DOI] [PubMed] [Google Scholar]
- Busa W, Gimlich R. Lithium-induced teratogenesis in frog embryos prevented by a polyphosphoinositide cycle intermediate or a diacylglycerol analog. Dev. Biol. 1989;132:315–324. doi: 10.1016/0012-1606(89)90228-5. doi:10.1016/0012-1606(89)90228-5 [DOI] [PubMed] [Google Scholar]
- Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. doi:10.1016/j.cell.2006.10.018 [DOI] [PubMed] [Google Scholar]
- Cooper M.S, D'Amico L.A. A cluster of noninvoluting endocytic cells at the margin of the zebrafish blastoderm marks the site of embryonic shield formation. Dev. Biol. 1996;180:184–198. doi: 10.1006/dbio.1996.0294. doi:10.1006/dbio.1996.0294 [DOI] [PubMed] [Google Scholar]
- Dale T. Signal transduction by the Wnt family of ligands. Biochem. J. 1998;329:209–223. doi: 10.1042/bj3290209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essner J.J, Vogan K.J, Wagner M.K, Tabin C.J, Yost H.J, Brueckner M. Conserved function for embryonic nodal cilia. Nature. 2002;418:37–38. doi: 10.1038/418037a. doi:10.1038/418037a [DOI] [PubMed] [Google Scholar]
- Essner J.J, Amack J.D, Nyholm M.K, Harris E.B, Yost H.J. Kupffer's vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left–right development of the brain, heart and gut. Development. 2005;132:1247–1260. doi: 10.1242/dev.01663. doi:10.1242/dev.01663 [DOI] [PubMed] [Google Scholar]
- Feldman B, Gates M.A, Egan E.S, Dougan S.T, Rennebeck G, Sirotkin H.I, Schier A.F, Talbot W.S. Zebrafish organizer development and germ-layer formation require nodal-related signals [see comments] Nature. 1998;395:181–185. doi: 10.1038/26013. doi:10.1038/26013 [DOI] [PubMed] [Google Scholar]
- Gilland E, Miller A.L, Karplus E, Baker R, Webb S.E. Imaging of multicellular large-scale rhythmic calcium waves during zebrafish gastrulation. Proc. Natl Acad. Sci. USA. 1999;96:157–161. doi: 10.1073/pnas.96.1.157. doi:10.1073/pnas.96.1.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada H, Meno C, Watanabe D, Saijoh Y. Establishment of vertebrate left–right asymmetry. Nat. Rev. Genet. 2002;3:103–113. doi: 10.1038/nrg732. doi:10.1038/nrg732 [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, et al. Mutations affecting morphogenesis during gastrulation and tail formation in the zebrafish, Danio rerio. Development. 1996;123:143–151. doi: 10.1242/dev.123.1.143. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Rebagliati M, Ahmad N, Muraoka O, Kurokawa T, Hibi M, Suzuki T. The Cerberus/Dan-family protein Charon is a negative regulator of Nodal signaling during left–right patterning in zebrafish. Development. 2004;131:1741–1753. doi: 10.1242/dev.01070. doi:10.1242/dev.01070 [DOI] [PubMed] [Google Scholar]
- Hedgepeth C.M, Conrad L.J, Zhang J, Huang H.-C, Lee V.M, Klein P.S. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev. Biol. 1997;185:82–91. doi: 10.1006/dbio.1997.8552. doi:10.1006/dbio.1997.8552 [DOI] [PubMed] [Google Scholar]
- Hirose K, Kadowaki S, Tanabe M, Takeshima H, Iino M. Spatiotemporal dynamics of inositol 1,4,5-trisphosphate that underlies complex Ca2+ mobilization patterns. Science. 1999;284:1527–1530. doi: 10.1126/science.284.5419.1527. doi:10.1126/science.284.5419.1527 [DOI] [PubMed] [Google Scholar]
- Kao K.R, Elinson R.P. Dorsalization of mesoderm induction by lithium. Dev. Biol. (Orl.) 1989;132:81–90. doi: 10.1016/0012-1606(89)90207-8. doi:10.1016/0012-1606(89)90207-8 [DOI] [PubMed] [Google Scholar]
- Kao K.R, Elinson R.P. The legacy of lithium effects on development. Biol. Cell. 1998;90:585–590. doi:10.1016/S0248-4900(99)80016-1 [PubMed] [Google Scholar]
- Kao K, Masui Y, Elinson R. Lithium-induced respecification of pattern in Xenopus laevis embryos. Nature. 1986;322:371–373. doi: 10.1038/322371a0. doi:10.1038/322371a0 [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Raya A, Raya R.M, Rodriguez-Esteban C, Belmonte J.C. Retinoic acid signalling links left–right asymmetric patterning and bilaterally symmetric somitogenesis in the zebrafish embryo. Nature. 2005;435:165–171. doi: 10.1038/nature03512. doi:10.1038/nature03512 [DOI] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. doi:10.1126/science.1079478 [DOI] [PubMed] [Google Scholar]
- Kilian B, Mansukoski H, Barbosa F.C, Ulrich F, Tada M, Heisenberg C.P. The role of Ppt/Wnt5 in regulating cell shape and movement during zebrafish gastrulation. Mech. Dev. 2003;120:467–476. doi: 10.1016/s0925-4773(03)00004-2. doi:10.1016/S0925-4773(03)00004-2 [DOI] [PubMed] [Google Scholar]
- Klein P.S, Melton D.A. A molecular mechanism for the effect of lithium on development. Proc. Natl Acad. Sci. USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. doi:10.1073/pnas.93.16.8455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn A.D, Moon R.T. Wnt and calcium signaling: β-catenin-independent pathways. Cell Calcium. 2005;38:439–446. doi: 10.1016/j.ceca.2005.06.022. doi:10.1016/j.ceca.2005.06.022 [DOI] [PubMed] [Google Scholar]
- Kramer-Zucker A.G, Olale F, Haycraft C.J, Yoder B.K, Schier A.F, Drummond I.A. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. doi:10.1242/dev.01772 [DOI] [PubMed] [Google Scholar]
- Kühl M, Sheldahl L.C, Malbon C.C, Moon R.T. Ca2+/calmodulin-dependent protein kinase II is stimulated by Wnt and frizzled homologs and promotes ventral cell fates in Xenopus. J. Biol. Chem. 2000a;275:12 701–12 711. doi: 10.1074/jbc.275.17.12701. doi:10.1074/jbc.275.17.12701 [DOI] [PubMed] [Google Scholar]
- Kühl M, Sheldahl L.C, Park M, Miller J.R, Moon R.T. The Wnt/Ca2+ pathway—a new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000b;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. doi:10.1016/S0168-9525(00)02028-X [DOI] [PubMed] [Google Scholar]
- Kume S, Muto A, Inoue T, Suga K, Okano H, Mikoshiba K. Role of inositol 1,4,5-trisphosphate receptor in ventral signaling in Xenopus embryos. Science. 1997;278:1940–1943. doi: 10.1126/science.278.5345.1940. doi:10.1126/science.278.5345.1940 [DOI] [PubMed] [Google Scholar]
- Kume S, Saneyoshi T, Mikoshiba K. Desensitization of IP3-induced Ca2+ release by overexpression of a constitutively active Gqalpha protein converts ventral to dorsal fate in Xenopus early embryos. Dev. Growth Differ. 2000;42:327–335. doi: 10.1046/j.1440-169x.2000.00519.x. doi:10.1046/j.1440-169x.2000.00519.x [DOI] [PubMed] [Google Scholar]
- Levin M. Left–right asymmetry in embryonic development: a comprehensive review. Mech. Dev. 2005;122:3–25. doi: 10.1016/j.mod.2004.08.006. doi:10.1016/j.mod.2004.08.006 [DOI] [PubMed] [Google Scholar]
- Lin G.C, Rurangirwa J.K, Koval M, Steinberg T.H. Gap junctional communication modulates agonist-induced calcium oscillations in transfected HeLa cells. J. Cell Sci. 2004;117:881–887. doi: 10.1242/jcs.00942. doi:10.1242/jcs.00942 [DOI] [PubMed] [Google Scholar]
- Long S, Ahmad N, Rebagliati M. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left–right asymmetry. Development. 2003;130:2303–2316. doi: 10.1242/dev.00436. doi:10.1242/dev.00436 [DOI] [PubMed] [Google Scholar]
- Luo X, Popov S, Bera A.K, Wilkie T.M, Muallem S. RGS proteins provide biochemical control of agonist-evoked [Ca2+]i oscillations. Mol. Cell. 2001;7:651–660. doi: 10.1016/s1097-2765(01)00211-8. doi:10.1016/S1097-2765(01)00211-8 [DOI] [PubMed] [Google Scholar]
- Lyman Gingerich J, Westfall T.A, Slusarski D.C, Pelegri F. hecate, a zebrafish maternal effect gene, affects dorsal organizer induction and intracellular calcium transient frequency. Dev. Biol. 2005;286:427–439. doi: 10.1016/j.ydbio.2005.07.031. doi:10.1016/j.ydbio.2005.07.031 [DOI] [PubMed] [Google Scholar]
- Marques S, Borges A.C, Silva A.C, Freitas S, Cordenonsi M, Belo J.A. The activity of the Nodal antagonist Cerl-2 in the mouse node is required for correct L/R body axis. Genes Dev. 2004;18:2342–2347. doi: 10.1101/gad.306504. doi:10.1101/gad.306504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslanski J, Leshko L, Busa W. Lithium-sensitive production of inositol phosphates during amphibian embryonic mesoderm induction. Science. 1992;256:243–245. doi: 10.1126/science.1314424. doi:10.1126/science.1314424 [DOI] [PubMed] [Google Scholar]
- McCarron J.G, MacMillan D, Bradley K.N, Chalmers S, Muir T.C. Origin and mechanisms of Ca2+ waves in smooth muscle as revealed by localized photolysis of caged inositol 1,4,5-trisphosphate. J. Biol. Chem. 2004;279:8417–8427. doi: 10.1074/jbc.M311797200. doi:10.1074/jbc.M311797200 [DOI] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left–right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. doi:10.1016/S0092-8674(03)00511-7 [DOI] [PubMed] [Google Scholar]
- Melby A.E, Warga R.M, Kimmel C.B. Specification of cell fates at the dorsal margin of the zebrafish gastrula. Development. 1996;122:2225–2237. doi: 10.1242/dev.122.7.2225. [DOI] [PubMed] [Google Scholar]
- Moon R.T, Kimelman D. From cortical rotation to organizer gene expression: toward a molecular explanation of axis specification in Xenopus. Bioessays. 1998;20:536–545. doi: 10.1002/(SICI)1521-1878(199807)20:7<536::AID-BIES4>3.0.CO;2-I. doi:10.1002/(SICI)1521-1878(199807)20:7<536::AID-BIES4>3.0.CO;2-I [DOI] [PubMed] [Google Scholar]
- Moon R.T, Campbell R.M, Christian J.L, McGrew L.L, Shih J, Fraser S. Xwnt-5A: a maternal Wnt that affects morphogenetic movements after overexpression in embryos of Xenopus laevis. Development. 1993a;119:97–111. doi: 10.1242/dev.119.1.97. [DOI] [PubMed] [Google Scholar]
- Moon R.T, Christian J.L, Campbell R.M, McGrew L.L, DeMarais A.A, Torres M, Lai C.-J, Olson D.J, Kelly G.M. Dissecting Wnt signalling pathways and Wnt-sensitive developmental processes through transient misexpression analyses in embryos of Xenopus laevis. Dev. Suppl. 1993b:85–94. [PubMed] [Google Scholar]
- Moon R.T, Kohn A.D, De Ferrari G.V, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat. Rev. Genet. 2004;5:691–701. doi: 10.1038/nrg1427. doi:10.1038/nrg1427 [DOI] [PubMed] [Google Scholar]
- Nadif Kasri N, Bultynck G, Sienaert I, Callewaert G, Erneux C, Missiaen L, Parys J.B, De Smedt H. The role of calmodulin for inositol 1,4,5-trisphosphate receptor function. Biochim. Biophys. Acta. 2002;1600:19–31. doi: 10.1016/s1570-9639(02)00440-5. [DOI] [PubMed] [Google Scholar]
- Nomikos M, Blayney L.M, Larman M.G, Campbell K, Rossbach A, Saunders C.M, Swann K, Lai F.A. Role of phospholipase C-zeta domains in Ca2+-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and cytoplasmic Ca2+ oscillations. J. Biol. Chem. 2005;280:31 011–31 018. doi: 10.1074/jbc.M500629200. doi:10.1074/jbc.M500629200 [DOI] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left–right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. doi:10.1016/S0092-8674(00)81705-5 [DOI] [PubMed] [Google Scholar]
- Okada Y, Nonaka S, Tanaka Y, Saijoh Y, Hamada H, Hirokawa N. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol. Cell. 1999;4:459–468. doi: 10.1016/s1097-2765(00)80197-5. doi:10.1016/S1097-2765(00)80197-5 [DOI] [PubMed] [Google Scholar]
- Parekh A.B, Putney J.W., Jr Store-operated calcium channels. Physiol. Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. doi:10.1152/physrev.00057.2003 [DOI] [PubMed] [Google Scholar]
- Patterson R.L, Boehning D, Snyder S.H. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu. Rev. Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. doi:10.1146/annurev.biochem.73.071403.161303 [DOI] [PubMed] [Google Scholar]
- Rauch G.J, Hammerschmidt M, Blader P, Schauerte H.E, Strahle U, Ingham P.W, McMahon A.P, Haffter P. Wnt5 is required for tail formation in the zebrafish embryo. Cold Spring Harb. Symp. Quant. Biol. 1997;62:227–234. [PubMed] [Google Scholar]
- Raya A, Kawakami Y, Rodriguez-Esteban C, Ibanes M, Rasskin-Gutman D, Rodriguez-Leon J, Buscher D, Feijo J.A, Izpisua Belmonte J.C. Notch activity acts as a sensor for extracellular calcium during vertebrate left–right determination. Nature. 2004;427:121–128. doi: 10.1038/nature02190. doi:10.1038/nature02190 [DOI] [PubMed] [Google Scholar]
- Reinhard E, Yokoe H, Niebling K.R, Allbritton N.L, Kuhn M.A, Meyer T. Localized calcium signals in early zebrafish development. Dev. Biol. (Orl.) 1995;170:50–61. doi: 10.1006/dbio.1995.1194. doi:10.1006/dbio.1995.1194 [DOI] [PubMed] [Google Scholar]
- Rey O, Young S.H, Yuan J, Slice L, Rozengurt E. Amino acid-stimulated Ca2+ oscillations produced by the Ca2+-sensing receptor are mediated by a phospholipase C/inositol 1,4,5-trisphosphate-independent pathway that requires G12, Rho, filamin-A, and the actin cytoskeleton. J. Biol. Chem. 2005;280:22 875–22 882. doi: 10.1074/jbc.M503455200. doi:10.1074/jbc.M503455200 [DOI] [PubMed] [Google Scholar]
- Roderick H.L, Berridge M.J, Bootman M.D. Calcium-induced calcium release. Curr. Biol. 2003;13:R425. doi: 10.1016/s0960-9822(03)00358-0. doi:10.1016/S0960-9822(03)00358-0 [DOI] [PubMed] [Google Scholar]
- Sarmah B, Latimer A.J, Appel B, Wente S.R. Inositol polyphosphates regulate zebrafish left–right asymmetry. Dev. Cell. 2005;9:133–145. doi: 10.1016/j.devcel.2005.05.002. doi:10.1016/j.devcel.2005.05.002 [DOI] [PubMed] [Google Scholar]
- Schneider I, Houston D.W, Rebagliati M.R, Slusarski D.C. Calcium fluxes in dorsal forerunner cells antagonize β-catenin and alter left-right patterning. Development. 2008;135:75–84. doi: 10.1242/dev.004713. doi:10.1242/dev.004713 [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, van Eeden F.J, Halpern M.E, Kimmel C.B, Nusslein-Volhard C. No tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development. 1994;120:1009–1015. doi: 10.1242/dev.120.4.1009. [DOI] [PubMed] [Google Scholar]
- Sheldahl L.C, Slusarski D.C, Pandur P, Miller J.R, Kühl M, Moon R.T. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J. Cell Biol. 2003;161:769–777. doi: 10.1083/jcb.200211094. doi:10.1083/jcb.200211094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slusarski D.C, Corces V.G. Calcium imaging in cell–cell signaling. In: Tuan R.S, Lo C.W, editors. Developmental biology protocols. vol. 1. Humana Press; Totowa, NJ: 2000. pp. 253–261. [DOI] [PubMed] [Google Scholar]
- Slusarski D.C, Corces V.G, Moon R.T. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997a;390:410–413. doi: 10.1038/37138. doi:10.1038/37138 [DOI] [PubMed] [Google Scholar]
- Slusarski D.C, Yang-Snyder J, Busa W.B, Moon R.T. Modulation of embryonic intracellular Ca2+ signaling by Wnt-5A. Dev. Biol. 1997b;185:114–120. doi: 10.1006/dbio.1996.8463. doi:10.1006/dbio.1996.8463 [DOI] [PubMed] [Google Scholar]
- Solnica-Krezel L. Conserved patterns of cell movements during vertebrate gastrulation. Curr. Biol. 2005;15:R213–R228. doi: 10.1016/j.cub.2005.03.016. doi:10.1016/j.cub.2005.03.016 [DOI] [PubMed] [Google Scholar]
- Stachel S, Grunwald D, Meyers P. Lithium perturbation and goosecoid expression identify a dorsal specification pathway in the pregastrula zebrafish. Development. 1993;117:1261–1274. doi: 10.1242/dev.117.4.1261. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Ruel L, Woodgett J.R. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells [published erratum appears in Curr. Biol. 1997 Mar 1;7(3):196] Curr. Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. doi:10.1016/S0960-9822(02)70790-2 [DOI] [PubMed] [Google Scholar]
- Supp D.M, Witte D.P, Potter S.S, Brueckner M. Mutation of an axonemal dynein affects left–right asymmetry in inversus viscerum mice. Nature. 1997;389:963–966. doi: 10.1038/40140. doi:10.1038/40140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supp D.M, Brueckner M, Kuehn M.R, Witte D.P, Lowe L.A, McGrath J, Corrales J, Potter S.S. Targeted deletion of the ATP binding domain of left–right dynein confirms its role in specifying development of left–right asymmetries. Development. 1999;126:5495–5504. doi: 10.1242/dev.126.23.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left–right determination. Nature. 2005;435:172. doi: 10.1038/nature03494. doi:10.1038/nature03494 [DOI] [PubMed] [Google Scholar]
- Thore S, Dyachok O, Tengholm A. Oscillations of phospholipase C activity triggered by depolarization and Ca2+ influx in insulin-secreting cells. J. Biol. Chem. 2004;279:19 396–19 400. doi: 10.1074/jbc.C400088200. doi:10.1074/jbc.C400088200 [DOI] [PubMed] [Google Scholar]
- Topol L, Jiang X, Choi H, Garrett-Beal L, Carolan P.J, Yang Y. Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent β-catenin degradation. J. Cell Biol. 2003;162:899–908. doi: 10.1083/jcb.200303158. doi:10.1083/jcb.200303158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar A.R, Moon R.T. Wnt4 affects morphogenesis when misexpressed in the zebrafish embryo. Mech. Dev. 1995;52:153–164. doi: 10.1016/0925-4773(95)00386-f. doi:10.1016/0925-4773(95)00386-F [DOI] [PubMed] [Google Scholar]
- Veeman M.T, Slusarski D.C, Kaykas A, Louie S.H, Moon R.T. Zebrafish prickle, a modulator of noncanonical Wnt/fz signaling, regulates gastrulation movements. Curr. Biol. 2003;13:680–685. doi: 10.1016/s0960-9822(03)00240-9. doi:10.1016/S0960-9822(03)00240-9 [DOI] [PubMed] [Google Scholar]
- Wallingford J.B, Ewald A.J, Harland R.M, Fraser S.E. Calcium signaling during convergent extension in Xenopus. Curr. Biol. 2001a;11:652–661. doi: 10.1016/s0960-9822(01)00201-9. doi:10.1016/S0960-9822(01)00201-9 [DOI] [PubMed] [Google Scholar]
- Wallingford J.B, Vogeli K.M, Harland R.M. Regulation of convergent extension in Xenopus by Wnt5a and Frizzled-8 is independent of the canonical Wnt pathway. Int. J. Dev. Biol. 2001b;45:225–227. [PubMed] [Google Scholar]
- Westfall T.A, Brimeyer R, Twedt J, Gladon J, Olberding A, Furutani-Seiki M, Slusarski D.C. Wnt-5/pipetail functions in vertebrate axis formation as a negative regulator of Wnt/β-catenin activity. J. Cell Biol. 2003a;162:889–898. doi: 10.1083/jcb.200303107. doi:10.1083/jcb.200303107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall T.A, Hjertos B, Slusarski D.C. Requirement for intracellular calcium modulation in zebrafish dorsal–ventral patterning. Dev. Biol. 2003b;259:380–391. doi: 10.1016/s0012-1606(03)00209-4. doi:10.1016/S0012-1606(03)00209-4 [DOI] [PubMed] [Google Scholar]
- Wright C.V, Halpern M.E. Specification of left–right asymmetry. Results Probl. Cell Differ. 2002;40:96–116. doi: 10.1007/978-3-540-46041-1_6. [DOI] [PubMed] [Google Scholar]