Abstract
Electrical activity has numerous roles in early neuronal development. Calcium transients generated at low frequencies regulate neural induction and neuronal proliferation, migration and differentiation. Recent work demonstrates that these signals participate in specification of the transmitters expressed in different classes of neurons. Matching of postsynaptic receptor expression with the novel expression of transmitters ensues. These findings have intriguing implications for development, mature function and evolution of the nervous system.
Keywords: calcium spikes, neurotransmitters, neurotransmitter receptors, homeostatic receptor plasticity, neuromuscular junction, spinal cord
1. Introduction
With 100 billion neurons in the human nervous system, each one of which receives 10 000 synapses, matching presynaptic neurotransmitters with the appropriate postsynaptic neurotransmitter receptors is a considerable challenge. The problem is complicated by the existence of roughly 100 neurotransmitters and 1000 neurotransmitter receptors from which to choose. Our recent studies suggest that embryonic electrical activity may have a key role in making the match. Focusing on a classic preparation, the vertebrate neuromuscular junction (NMJ), we find that embryonic striated muscle cells express a number of transmitter receptors in addition to those for acetylcholine that have been recognized for many years. When the presynaptic transmitter is changed by altering electrical activity (Borodinsky et al. 2004), appropriate transmitter receptors are selected from the available population (Borodinsky & Spitzer 2007). Here we discuss the explanatory power and some of the potential implications of these findings.
2. Activity-dependent neurotransmitter specification
Specification of neurotransmitters represents a fundamental developmental process because it allows the establishment of functional connections at synapses. How is this specification determined? A combinatorial code of transcription factor expression, triggered by secreted morphogenetic proteins, has been shown to control the differentiation of different neuronal phenotypes within the developing spinal cord and brain (Pierani et al. 2001; Thaler et al. 2002; Gunhaga et al. 2003; Novitch et al. 2003). Disruption of this code leads to ectopic neurotransmitter expression (Tanabe et al. 1998; Cheng et al. 2003).
On the other hand, electrical activity appears to be a potent regulator of neurotransmitter expression in developing and mature neurons, both in culture and in the intact nervous system. Activity-dependent choices between cholinergic or adrenergic, GABAergic or glutamatergic, and excitatory versus inhibitory phenotypes have been reported in diverse developing nervous system structures such as neonatal rat superior cervical ganglion neurons, rat dentate gyrus granule cells and Xenopus embryonic spinal neurons, respectively (Walicke & Patterson 1981; Gutierrez et al. 2003; Borodinsky et al. 2004).
These choices seem to follow a homeostatic paradigm: when activity is suppressed, more neurons express excitatory neurotransmitters and less express inhibitory neurotransmitters; the opposite outcome results when activity is enhanced (Spitzer et al. 2004). For these changes in neurotransmitter specification to be functional, one hypothesizes that they must be accompanied by appropriate changes in postsynaptic neurotransmitter receptor expression. We have recently tested this hypothesis by studying activity dependence of neurotransmitter receptor expression in developing Xenopus skeletal muscle.
3. Activity-dependent receptor selection
Neurotransmitter receptor expression is modulated by activity during both the development and plasticity of mature synapses. N-methyl-d-aspartic acid (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), kainate, γ-aminobutyric acid A-type (GABAA) and acetylcholine (ACh) receptor expression are regulated by synaptic activity in a variety of nervous system structures such as the hippocampus, visual cortex, superior colliculus, prefrontal cortex and even in skeletal muscles (Broadie & Bate 1993; Bessereau et al. 1994; Catalano et al. 1997; Kidd & Isaac 1999; Shi et al. 2000; Brumwell et al. 2002).
At the NMJ, pre- and postsynaptic differentiation appear to be mutually inductive events. Agrin from innervating motor neurons is the trigger for localization of receptors and the associated postsynaptic machinery in muscle cells (Sanes & Lichtman 2001). Prior to neuronal contact, ACh receptors are distributed largely uniformly over the entire surface of the muscle cell. After neuromuscular contact, ACh receptors cluster rapidly at these sites in the postsynaptic membrane and the density of extrajunctional ACh receptors decreases gradually (Diamond & Miledi 1962) in a process regulated by muscle cell activity (Lomo & Rosenthal 1972; Schuetze & Role 1987; Hall & Sanes 1993). The role of synaptic transmission in formation of the NMJ was tested in mutant mice lacking choline acetyltransferase activity, the ACh-synthesizing enzyme. The numbers of motor axons, myotubes and Schwann cells were altered. ACh synthesis also seems to have a role in stabilization of nerve–muscle contacts (Misgeld et al. 2002). In zebrafish embryos, migrating motor axons form en passant synaptic contacts with myotomal muscle before establishing terminal synapses with their final muscle targets. In the twister mutant, neuromuscular transmission is prolonged at these synapses, and aberrant motor axon trajectories and muscular degeneration ensue (Lefebvre et al. 2004). In Caenorhabditis elegans, the postsynaptic muscles can induce sprouting and synaptogenesis in innervating motor neurons (Plunkett et al. 1996). These studies define clearly the importance of neural activity and cellular interactions in the assembly and refinement of newly formed synapses, but much less is known about the specification of properties of pre- and postsynaptic cells at earlier stages of development.
The vertebrate NMJ has been extensively characterized as cholinergic (Misgeld et al. 2002). However, several reports have described the expression in skeletal muscle cells of neurotransmitter receptors other than the classical nicotinic receptors. Metabotropic glutamate receptors have been described in the adult frog NMJ (Pinard et al. 2003), while muscarinic receptors have been found in primary cultures of rat myotubes (Reyes & Jaimovich 1996). The role of these novel transmitter receptors is unclear, but the investigators suggested a modulation of synaptic events. More recently, Brunelli et al. (2005) have found AMPA receptors expressed in adult rat skeletal muscle.
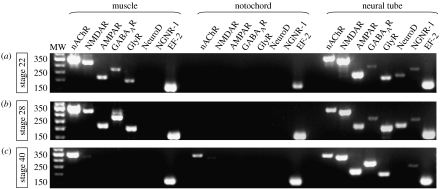
We have recently investigated neurotransmitter receptor expression in vertebrate skeletal muscle. Surprisingly, transcripts of several different neurotransmitter receptors in addition to nicotinic cholinergic receptors are present in developing Xenopus skeletal muscle at an early stage of development, at the onset of muscle innervation (figure 1). Moreover, immunolabelling of glutamate and glycine receptor subunits and labelling with tagged muscimol, a GABAA receptor-specific agonist, reveals that the receptors are present at the protein level in immature skeletal muscle. As maturation and innervation progress, the nicotinic phenotype prevails, while glutamate, GABA and glycine receptor expression is downregulated (figure 2a; Borodinsky & Spitzer 2007).
Figure 1.
Expression of nAChR, NMDAR, AMPAR, GABAAR and GlyR transcripts in skeletal muscle during normal development. RT-PCR was used for detection of subunit transcripts of five neurotransmitter receptors in muscle, notochord and neural tube at three stages of development. Tissue-specific RNA was analysed from embryos (a) at 1 day (stage 22), (b) at 1.3 days (stage 28) and from larvae (c) at 3 days (stage 40). Primers were designed from predicted Xenopus sequences for nAChRα1, NR1, GluR1, GABAARβ2 and GlyRα1 subunits and for neuronal markers NeuroD and Neurogenin-related protein-1 (NGNR-1). EF-2, elongation factor 2. Reproduced with permission from Borodinsky & Spitzer (2007).
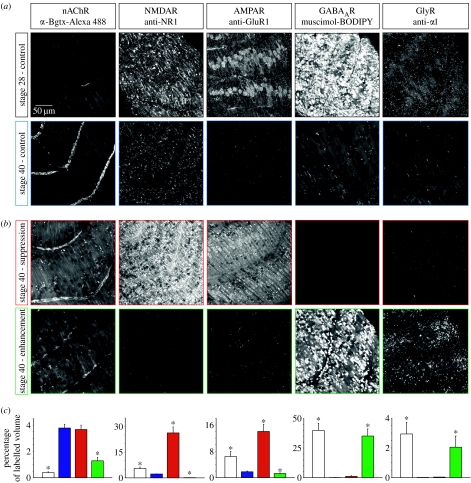
Figure 2.
Expression of nAChR, NMDAR, AMPAR, GABAAR and GlyR protein in skeletal muscle during normal development and after perturbations of Ca spike activity. Whole mounts from 1.3 day (stage 28) embryos and 3 day (stage 40) control (a) and activity-manipulated (b) larvae were labelled for nAChR, NMDAR, AMPAR, GABAAR and GlyR with probes noted above each column. Manipulation of activity was achieved by implanting beads impregnated with 30 μM TTX (tetrodotoxin), 200 nM calcicludine, 10 μM GVIA ω-conotoxin and 10 μM flunarizine ((i–v), Ca2+ spike activity suppression) or with 1 mM veratridine ((vi–x), Ca2+ spike activity enhancement). Images of chevrons of mononucleate muscle cells are representative Z-series projections obtained from confocal stacks of 20 optical sections of 62 500 μm2 area. (c) Bar graphs indicate the percentage of labelled volume. Colours link to stages in (a) and (b). Values are mean±s.e.m., n≥5 embryos for each probe. *p<0.001 when compared with stage-40 control for each probe. Reproduced with permission from Borodinsky & Spitzer (2007).
We then investigated whether this developmental selection of neurotransmitter receptor expression in muscle is activity dependent. Indeed, early neuronal activity regulates neurotransmitter receptor selection in the skeletal muscle. When activity is suppressed, leading to increases in the number of cholinergic and glutamatergic neurons, glutamate receptors remain. In contrast, when activity is enhanced, leading to increases in the number of GABAergic and glycinergic neurons, GABAA and glycine receptors remain, in addition to nicotinic cholinergic receptors (figure 2b).
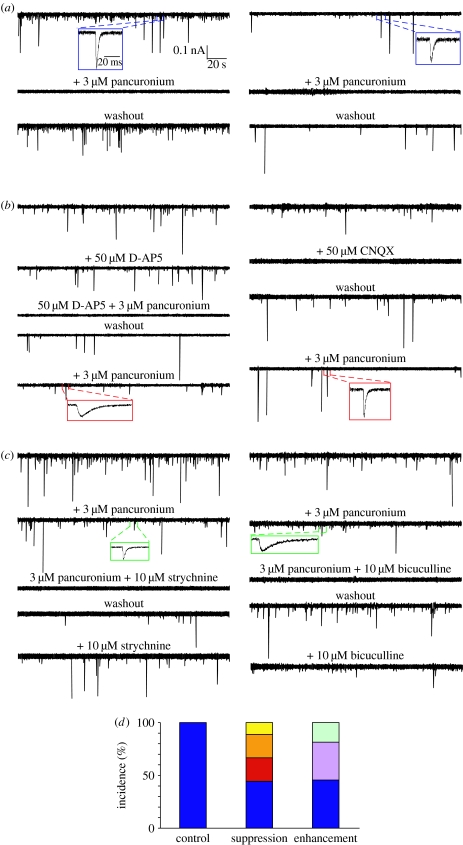
Since synaptic boutons immunopositive for glutamate, GABA or glycine were detected in embryos in which activity has been perturbed, we next determined whether non-cholinergic NMJs were formed upon alterations of neuronal activity. Remarkably, glutamatergic synaptic currents were recorded following suppression of activity, and GABAergic and glycinergic synaptic currents were evident when activity was enhanced (figure 3; Borodinsky & Spitzer 2007). The findings are consistent with observations of the release of glutamate from neonatal mammalian motor neurons (Mentis et al. 2005; Nishimaru et al. 2005). Glutamatergic innervation of adult rat skeletal muscle via peripheral nerve grafts (Brunelli et al. 2005) may result from these activity-dependent processes.
Figure 3.
Matching of neurotransmitters and receptors at NMJs in vivo. Whole-cell recordings from muscle cells of the axial musculature of 3 day (stage 40) (a) control, (b) Ca2+ spike-suppressed and (c) Ca2+ spike-enhanced larvae were performed in the presence of 2 mM Ca2+, Mg2+-free saline and 3 μM TTX; Vh=−80 mV. Examples illustrate the pharmacological blockade of the miniature postsynaptic currents (mpscs) from two muscle cells in each group of embryos. Single mpscs are shown on an expanded time base to illustrate their kinetics. (d) Incidence of cholinergic, glutamatergic, GABAergic and glycinergic NMJs. Bars represent the percentage of each class of NMJ in each group based on blockade by different receptor antagonists (blue, nAChR; red, AMPAR; light orange, NMDAR; yellow, NMDAR+nAChR; lavender, GABAAR+nAChR; light turquoise, GlyR+nAChR). Values are from 6 control, 12 spike-suppressed and 13 spike-enhanced NMJs. CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione. Reproduced with permission from Borodinsky & Spitzer (2007).
These results demonstrate that (i) multiple classes of receptors are expressed embryonically, (ii) these classes are pruned following innervation, and (iii) there is a mechanism for clustering the appropriate receptors under presynaptic terminals. The matching of transmitters with their cognate receptors by a process of selection from a pool of receptor classes may seem inefficient. However, it could facilitate rapid establishment of connections and has a parallel with other biological processes—most notably antibody selection in the immune system.
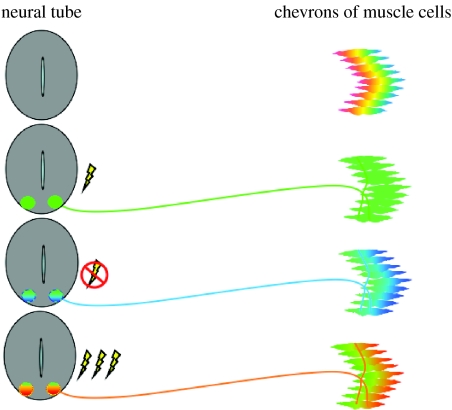
We emphasize that there are limits to what activity can achieve. It does not act on a tabula rasa but is constrained by genetically determined neuronal identity. We model this process as the sequential channelling of cell fates to progressively more restricted phenotypes by differential gene expression, and envisage that different populations of neurons finally have the capability to express one or more of a series of neurotransmitters. Similarly, we propose that populations of neurons, muscles and glands express a substantial number of different transmitter receptors. Patterns of electrical activity in presynaptic neurons then determine both the final outcome of neurotransmitter expression and the match between presynaptic neurotransmitter and postsynaptic receptors (figure 4).
Figure 4.
Model illustrating the process of transmitter–receptor matching at the NMJ. Prior to innervation, muscle cells express multiple classes of receptors. During normal development, motor neurons express ACh and AChR that persist on the muscle fibres. When spontaneous embryonic calcium spike activity is blocked, motor neurons express glutamate in addition to ACh, and both AChR and GluR are found on muscle fibres. When calcium spike activity is increased, motor neurons express GABA and glycine and the cognate receptors are found on muscle fibres. Colour coding of transmitter receptors indicates their presence but not their distribution on the muscle surface (red: GABAR, GABA; yellow: glyR, gly; green: AChR, ACh; blue: gluR, glu.).
4. Implications for development in the peripheral nervous system
Invertebrate NMJs with excitatory glutamatergic and inhibitory GABAergic input or with excitatory cholinergic and inhibitory GABAergic input integrate them in the periphery (Otsuka et al. 1967; Laurienti & Blankenship 1999; Devlin 2001). Inhibitory and excitatory GABAergic innervation of different body muscles coordinate the wave of body muscle contractions involved in locomotion in C. elegans (McIntire et al. 1993). Vertebrate NMJs classically integrate inputs at the level of motor neurons. How does this integration develop? Does integration in vertebrates progress from periphery to motor neurons? The presence of different neurotransmitter receptors in developing skeletal muscle may provide a clue to the answer to this question. The responsiveness of muscle cells to different neurotransmitters could serve as either an early regulatory component or a back-up component in the peripheral nervous system. Conditions leading to the expression of inhibitory transmitters in vertebrate motor neurons would move integration to the periphery as in invertebrates, potentially leading to more finely graded generation of tension over the length of the muscle fibres.
Processes of regeneration often recapitulate aspects of development. This leads to the prediction that denervation of adult skeletal muscle will lead to its sensitivity to transmitters other than acetylcholine, reflecting a return to the embryonic state in which multiple receptors are expressed. Evidence for upregulation of the expression of ionotropic glutamate receptors has already been reported by Brunelli et al. (2005).
5. Implications for development in the central nervous system
Activity-dependent transmitter–receptor matching may mitigate the requirement for receptor targeting in the formation of central nervous system (CNS) synapses. Without activity-dependent matching, axons would not only have to pathfind to the correct location on postsynaptic neurons, but appropriate receptors would then have to be expressed at the correct postsynaptic locations as well. The matching mechanism may eliminate the latter requirement via the expression of a wide range of receptors that can cluster under the appropriate nerve terminals, achieving more mechanistically parsimonious connections.
The selection of receptors from a pool could represent an effective way of implementing activity-dependent plastic phenomena. For instance, thalamic afferents regulate the expression of GABAA receptors in developing rat neocortex, leading to area-specific expression of receptor subtypes in the primary visual and somatosensory areas. Following unilateral lesions of thalamic nuclei innervating these cortical areas, the boundaries of specific subunit expression are diffuse, and a nearly uniform expression pattern of GABAA receptor subunits becomes apparent (Paysan et al. 1997). Innervation seems ultimately to determine the specific pattern of neurotransmitter receptor expression in developing neurons (Jin et al. 2002; Takayama & Inoue 2004).
6. Implications for function and repair in the mature nervous system
Transmitter–receptor matching may have intriguing clinical implications. If activity changes the identity of the transmitters made by neurons, this new work suggests that the neurons may be able to make functional connections by selecting the appropriate class of receptors. It has been a puzzle to understand how neurons generated in the adult nervous system could integrate into existing functioning networks or how transplantation of neurons could lead to appropriate connectivity. However, if available receptor populations can be reclustered under the newly innervating nerve terminals in these contexts, the formation of functional synapses and networks seems a less daunting problem.
7. Implications for evolution of the nervous system
Did activity first play roles in development or first play roles in mature nervous system function? It seems likely that evolution of mechanisms for generating activity was accompanied by its simultaneous inclusion in both the development of the nervous system and its function in the adult. In this way, diverse forms of development and complex and computationally powerful nervous systems could evolve hand in hand.
Matching may have played a role in facilitating evolution of the nervous system. The iterated microcircuit structure of the CNS (Markram et al. 2004; Pouille & Scanziani 2004; Foldy et al. 2005; Silberberg et al. 2005) is likely to have arisen by duplication. Following duplication, subsequent changes in neurotransmitter expression would be disruptive without matching changes in receptors. The ability to select from a large repertoire of available receptors may have allowed continuity of function and adjustment of the new circuitry, generating behaviours on which natural selection could act.
8. Conclusions
Matching of presynaptic neurotransmitters with postsynaptic transmitter receptors was thought to be achieved entirely by genetic programmes. Identification of a role for electrical activity in this process makes us aware of a new level of plasticity in the developing nervous system. Although the recent findings come from the studies of a model system—the amphibian spinal cord and NMJ—the underlying cell biology is likely to play out similarly in mammalian systems. Many questions remain. What are the mechanisms by which activity reprogrammes the expression of neurotransmitters? How many different classes of receptors are expressed on target cells prior to innervation? What is the machinery for pruning back these classes of receptors? What are the mechanisms by which newly expressed transmitters lead to clustering of the cognate receptors? The answers to these and other questions are likely to reveal further layers of flexibility in the developing nervous system.
Acknowledgments
Support by the NIH (N.C.S.) and the NSF (L.N.B.) is gratefully acknowledged.
Footnotes
One contribution of 11 to a Discussion Meeting Issue ‘Calcium signals and developmental patterning’.
References
- Bessereau J.L, Stratford-Perricaudet L.D, Piette J, Le Poupon C, Changeux J.P. In vivo and in vitro analysis of electrical activity-dependent expression of muscle acetylcholine receptor genes using adenovirus. Proc. Natl Acad. Sci. USA. 1994;91:1304–1308. doi: 10.1073/pnas.91.4.1304. doi:10.1073/pnas.91.4.1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodinsky L.N, Spitzer N.C. Activity-dependent neurotransmitter–receptor matching at the neuromuscular junction. Proc. Natl Acad. Sci. USA. 2007;104:335–340. doi: 10.1073/pnas.0607450104. doi:10.1073/pnas.0607450104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodinsky L.N, Root C.M, Cronin J.A, Sann S.B, Gu X, Spitzer N.C. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;429:523–530. doi: 10.1038/nature02518. doi:10.1038/nature02518 [DOI] [PubMed] [Google Scholar]
- Broadie K, Bate M. Innervation directs receptor synthesis and localization in Drosophila embryo synaptogenesis. Nature. 1993;361:350–353. doi: 10.1038/361350a0. doi:10.1038/361350a0 [DOI] [PubMed] [Google Scholar]
- Brumwell C.L, Johnson J.L, Jacob M.H. Extrasynaptic alpha 7-nicotinic acetylcholine receptor expression in developing neurons is regulated by inputs, targets, and activity. J. Neurosci. 2002;22:8101–8109. doi: 10.1523/JNEUROSCI.22-18-08101.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelli G, Spano P, Barlati S, Guarneri B, Barbon A, Bresciani R, Pizzi M. Glutamatergic reinnervation through peripheral nerve graft dictates assembly of glutamatergic synapses at rat skeletal muscle. Proc. Natl Acad. Sci. USA. 2005;102:8752–8757. doi: 10.1073/pnas.0500530102. doi:10.1073/pnas.0500530102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano S.M, Chang C.K, Shatz C.J. Activity-dependent regulation of NMDAR1 immunoreactivity in the developing visual cortex. J. Neurosci. 1997;17:8376–8390. doi: 10.1523/JNEUROSCI.17-21-08376.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Chen C.L, Luo P, Tan M, Qiu M, Johnson R, Ma Q. Lmx1b, Pet-1, and Nkx2.2 coordinately specify serotonergic neurotransmitter phenotype. J. Neurosci. 2003;23:9961–9967. doi: 10.1523/JNEUROSCI.23-31-09961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin C.L. The pharmacology of gamma-aminobutyric acid and acetylcholine receptors at the echinoderm neuromuscular junction. J. Exp. Biol. 2001;204:887–986. doi: 10.1242/jeb.204.5.887. [DOI] [PubMed] [Google Scholar]
- Diamond J, Miledi R. A study of foetal and new-born rat muscle fibres. J. Physiol. 1962;162:393–408. doi: 10.1113/jphysiol.1962.sp006941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldy C, Dyhrfjeld-Johnsen J, Soltesz I. Structure of cortical microcircuit theory. J. Physiol. 2005;562:47–54. doi: 10.1113/jphysiol.2004.076448. doi:10.1113/jphysiol.2004.076448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunhaga L, Marklund M, Sjodal M, Hsieh J.C, Jessell T.M, Edlund T. Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. Nat. Neurosci. 2003;6:701–707. doi: 10.1038/nn1068. doi:10.1038/nn1068 [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Romo-Parra H, Maqueda J, Vivar C, Ramirez M, Morales M.A, Lamas M. Plasticity of the GABAergic phenotype of the ‘glutamatergic’ granule cells of the rat dentate gyrus. J. Neurosci. 2003;23:5594–5598. doi: 10.1523/JNEUROSCI.23-13-05594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall Z.W, Sanes J.R. Synaptic structure and development: the neuromuscular junction. Cell. 1993;72(Suppl.):99–121. doi: 10.1016/s0092-8674(05)80031-5. doi:10.1016/S0092-8674(05)80031-5 [DOI] [PubMed] [Google Scholar]
- Jin C.Y, Kalimo H, Panula P. The histaminergic system in human thalamus: correlation of innervation to receptor expression. Eur. J. Neurosci. 2002;15:1125–1138. doi: 10.1046/j.1460-9568.2002.01951.x. doi:10.1046/j.1460-9568.2002.01951.x [DOI] [PubMed] [Google Scholar]
- Kidd F.L, Isaac J.T. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature. 1999;400:569–573. doi: 10.1038/23040. doi:10.1038/23040 [DOI] [PubMed] [Google Scholar]
- Laurienti P.J, Blankenship J.E. Properties of cholinergic responses in isolated parapodial muscle fibers of Aplysia. J. Neurophysiol. 1999;82:778–786. doi: 10.1152/jn.1999.82.2.778. [DOI] [PubMed] [Google Scholar]
- Lefebvre J.L, Ono F, Puglielli C, Seidner G, Franzini-Armstrong C, Brehm P, Granato M. Increased neuromuscular activity causes axonal defects and muscular degeneration. Development. 2004;131:2605–2618. doi: 10.1242/dev.01123. doi:10.1242/dev.01123 [DOI] [PubMed] [Google Scholar]
- Lomo T, Rosenthal J. Control of ACh sensitivity by muscle activity in the rat. J. Physiol. 1972;221:493–513. doi: 10.1113/jphysiol.1972.sp009764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. doi:10.1038/nrn1519 [DOI] [PubMed] [Google Scholar]
- McIntire S.L, Jorgensen E, Kaplan J, Horvitz H.R. The GABAergic nervous system of Caenorhabditis elegans. Nature. 1993;364:337–341. doi: 10.1038/364337a0. doi:10.1038/364337a0 [DOI] [PubMed] [Google Scholar]
- Mentis G.Z, Alvarez F.J, Bonnot A, Richards D.S, Gonzalez-Forero D, Zerda R, O'Donovan M.J. Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc. Natl Acad. Sci. USA. 2005;102:7344–7349. doi: 10.1073/pnas.0502788102. doi:10.1073/pnas.0502788102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld T, Burgess R.W, Lewis R.M, Cunningham J.M, Lichtman J.W, Sanes J.R. Roles of neurotransmitter in synapse formation: development of neuromuscular junctions lacking choline acetyltransferase. Neuron. 2002;36:635–648. doi: 10.1016/s0896-6273(02)01020-6. doi:10.1016/S0896-6273(02)01020-6 [DOI] [PubMed] [Google Scholar]
- Nishimaru H, Restrepo C.E, Ryge J, Yanagawa Y, Kiehn O. Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc. Natl Acad. Sci. USA. 2005;102:5245–5249. doi: 10.1073/pnas.0501331102. doi:10.1073/pnas.0501331102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novitch B.G, Wichterle H, Jessell T.M, Sockanathan S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron. 2003;40:81–95. doi: 10.1016/j.neuron.2003.08.006. doi:10.1016/j.neuron.2003.08.006 [DOI] [PubMed] [Google Scholar]
- Otsuka M, Kravitz E.A, Potter D.D. Physiological and chemical architecture of a lobster ganglion with particular reference to gamma-aminobutyrate and glutamate. J. Neurophysiol. 1967;30:725–752. doi: 10.1152/jn.1967.30.4.725. [DOI] [PubMed] [Google Scholar]
- Paysan J, Kossel A, Bolz J, Fritschy J.M. Area-specific regulation of gamma-aminobutyric acid type A receptor subtypes by thalamic afferents in developing rat neocortex. Proc. Natl Acad. Sci. USA. 1997;94:6995–7000. doi: 10.1073/pnas.94.13.6995. doi:10.1073/pnas.94.13.6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierani A, Moran-Rivard L, Sunshine M.J, Littman D.R, Goulding M, Jessell T.M. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron. 2001;29:367–384. doi: 10.1016/s0896-6273(01)00212-4. doi:10.1016/S0896-6273(01)00212-4 [DOI] [PubMed] [Google Scholar]
- Pinard A, Levesque S, Vallee J, Robitaille R. Glutamatergic modulation of synaptic plasticity at a PNS vertebrate cholinergic synapse. Eur. J. Neurosci. 2003;18:3241–3250. doi: 10.1111/j.1460-9568.2003.03028.x. doi:10.1111/j.1460-9568.2003.03028.x [DOI] [PubMed] [Google Scholar]
- Plunkett J.A, Simmons R.B, Walthall W.W. Dynamic interactions between nerve and muscle in Caenorhabditis elegans. Dev. Biol. 1996;175:154–165. doi: 10.1006/dbio.1996.0103. doi:10.1006/dbio.1996.0103 [DOI] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–723. doi: 10.1038/nature02615. doi:10.1038/nature02615 [DOI] [PubMed] [Google Scholar]
- Reyes R, Jaimovich E. Functional muscarinic receptors in cultured skeletal muscle. Arch. Biochem. Biophys. 1996;331:41–47. doi: 10.1006/abbi.1996.0280. doi:10.1006/abbi.1996.0280 [DOI] [PubMed] [Google Scholar]
- Sanes J.R, Lichtman J.W. Induction, assembly, maturation and maintenance of a postsynaptic apparatus. Nat. Rev. Neurosci. 2001;2:791–805. doi: 10.1038/35097557. doi:10.1038/35097557 [DOI] [PubMed] [Google Scholar]
- Schuetze S.M, Role L.W. Developmental regulation of nicotinic acetylcholine receptors. Annu. Rev. Neurosci. 1987;10:403–457. doi: 10.1146/annurev.ne.10.030187.002155. doi:10.1146/annurev.ne.10.030187.002155 [DOI] [PubMed] [Google Scholar]
- Shi J, Townsend M, Constantine-Paton M. Activity-dependent induction of tonic calcineurin activity mediates a rapid developmental downregulation of NMDA receptor currents. Neuron. 2000;28:103–114. doi: 10.1016/s0896-6273(00)00089-1. doi:10.1016/S0896-6273(00)00089-1 [DOI] [PubMed] [Google Scholar]
- Silberberg G, Grillner S, LeBeau F.E, Maex R, Markram H. Synaptic pathways in neural microcircuits. Trends Neurosci. 2005;28:541–551. doi: 10.1016/j.tins.2005.08.004. doi:10.1016/j.tins.2005.08.004 [DOI] [PubMed] [Google Scholar]
- Spitzer N.C, Root C.M, Borodinsky L.N. Orchestrating neuronal differentiation: patterns of Ca2+ spikes specify transmitter choice. Trends Neurosci. 2004;27:415–421. doi: 10.1016/j.tins.2004.05.003. doi:10.1016/j.tins.2004.05.003 [DOI] [PubMed] [Google Scholar]
- Takayama C, Inoue Y. Morphological development and maturation of the GABAergic synapses in the mouse cerebellar granular layer. Brain Res. Dev. Brain Res. 2004;150:177–190. doi: 10.1016/j.devbrainres.2004.03.011. doi:10.1016/j.devbrainres.2004.03.011 [DOI] [PubMed] [Google Scholar]
- Tanabe Y, William C, Jessell T.M. Specification of motor neuron identity by the MNR2 homeodomain protein. Cell. 1998;95:67–80. doi: 10.1016/s0092-8674(00)81783-3. doi:10.1016/S0092-8674(00)81783-3 [DOI] [PubMed] [Google Scholar]
- Thaler J.P, Lee S.K, Jurata L.W, Gill G.N, Pfaff S.L. LIM factor Lhx3 contributes to the specification of motor neuron and interneuron identity through cell-type-specific protein–protein interactions. Cell. 2002;110:237–249. doi: 10.1016/s0092-8674(02)00823-1. doi:10.1016/S0092-8674(02)00823-1 [DOI] [PubMed] [Google Scholar]
- Walicke P.A, Patterson P.H. On the role of Ca2+ in the transmitter choice made by cultured sympathetic neurons. J. Neurosci. 1981;1:343–350. doi: 10.1523/JNEUROSCI.01-04-00343.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]