Abstract
The scientific study of animal emotion is an important emerging discipline in subjects ranging from neuroscience to animal welfare research. In the absence of direct measures of conscious emotion, indirect behavioural and physiological measures are used. However, these may have significant limitations (e.g. indicating emotional arousal but not valence (positivity versus negativity)). A new approach, taking its impetus from human studies, proposes that biases in information processing, and underlying mechanisms relating to the evaluation of reward gains and losses, may reliably reflect emotional valence in animals. In general, people are more sensitive to reward losses than gains, but people in a negative affective state (e.g. depression) are particularly sensitive to losses. This may underlie broader findings such as an enhanced attention to, and memory of, negative events in depressed individuals. Here we show that rats in unenriched housing, who typically exhibit indicators of poorer welfare and a more negative affective state than those in enriched housing, display a prolonged response to a decrease in anticipated food reward, indicating enhanced sensitivity to reward loss. Sensitivity to reward reduction may thus be a valuable new indicator of animal emotion and welfare.
Keywords: rat, emotion, successive negative contrast, welfare
1. Introduction
The scientific study of animal emotion has been off-limits for many years, but has now emerged as a respectable discipline in subjects ranging from neuroscience to animal welfare research (Panksepp 1998; Mendl & Paul 2004; Rolls 2005). In the latter area, an understanding of emotional states is critical because it is the presumed existence of such states that underlies the public's concerns about animal welfare. Conscious experience of emotion may not be directly measurable, but physiological and behavioural components of emotional responses provide useful proxy indicators. However, these measures are often species specific, sometimes difficult to interpret due to lack of a predictive framework, and do not always reliably reflect whether an emotion is positive or negative (Paul et al. 2005). A novel approach, based on findings from human studies, focuses on changes in information processing and decision making as potential indicators of emotional (affective) state. For example, in comparison to happier people, individuals in a negative affective state attend more to negative stimuli, show enhanced memory for negative events/reduced memory for positive events and make negative judgements about ambiguous or future events (e.g. Mineka et al. 1998; Paul et al. 2005). Such ‘cognitive biases’ may have adaptive value (Haselton & Nettle 2006), and recent studies have indicated similar links between affect and judgement bias in animals, suggesting that judgement of ambiguity may be a good indicator of emotional valence (Harding et al. 2004; Bateson & Matheson 2007; Burman et al. in press).
Inherent in many attention, memory and judgement processes is the evaluation of stimuli, whether they represent or predict rewards or losses. Sensitivity to rewards and losses also appears to be influenced by emotional state. In general, people are more sensitive to possible reward losses than gains (e.g. Dreher 2007), but people in a negative affective state show enhanced sensitivity to loss or failure as evidenced by behavioural and neurophysiological responses (Beck 1967; Wenzlaff & Grozier 1988; Hajcak et al. 2004; Chiu & Deldin 2007; Tucker & Luu 2007). Increased sensitivity to loss thus appears to be symptomatic of negative affective states in people, and may be a useful new measure of such states in animals.
Here, we employ a successive negative contrast (SNC) technique (Flaherty 1996) to investigate this possibility. The instrumental SNC paradigm simulates reward loss by unexpectedly decreasing the size of the food reward for which an animal has been trained to run down a runway. Individuals respond by running more slowly to the new smaller reward than animals that have been trained to run to this same small reward size from the outset (the SNC effect). There is evidence that initial slowing may reflect searching for the previous reward, while the longer term response reflects sensitivity to loss and may involve disappointment-like and frustration-like emotional states (Flaherty 1996). The prolongation of this latter phase would thus be expected for animals in a negative affective state.
In this study, we used environmental enrichment to manipulate affect in laboratory rats. Many studies have demonstrated beneficial effects of enriched housing compared with barren housing for welfare and hence putative affective state (Young 2003). We housed rats in cages containing enrichment objects previously shown to have positive effects on multiple behavioural and physiological measures of welfare (Burman et al. 2006). Half the rats had these objects removed prior to the study so that they were in unenriched conditions. Removing valued enrichment stimuli is likely to induce negative affect (cf. Rolls 2005), and has been shown to lead to diminished welfare (Latham & Mason 2006; Bateson & Matheson 2007; Burman et al. in press). We predicted that unenriched rats would experience poorer welfare and a more negative affective state relative to enriched rats, and would therefore be more sensitive to reward loss.
2. Material and methods
We used 24 three-month-old male Lister hooded rats (Harlan, UK) housed in groups of three in standard cages (33×50× 21 cm3) on a 12 hour reversed light cycle (lights off 08.00–20.00) with food (Harlan Teklad Laboratory Diet) and water available ad libitum. The rats were housed for 12 weeks with enrichment items (Burman et al. 2006). Prior to the start of the experiment, 12 of the animals experienced removal of these objects (unenriched: U), while the other 12 continued to live in the enriched (E) environment.
Following habituation to the test apparatus in a separate room, the rats were then individually trained to run from a start box down a 2 m long black Perspex runway to obtain 45 mg food pellets from a metal bowl at the far end. Half of the E rats were trained to run for 12 food pellets on each trial and half for one pellet, and likewise for the U rats. Each rat received six trials per day. On each trial, the time taken for the subject to move from the start box to the food bowl and start feeding was recorded. All the subjects had their food removed 1 hour prior to training to ensure that all food pellets were eaten. To ensure that all rats received the same number of pellets each day, supplementary feeding was provided to those subjects receiving the one-pellet reward on all the training days. This occurred at least 30 min after training.
When rats receiving 12 pellets were running significantly faster than those receiving one pellet, the instrumental SNC technique was applied and all rats received one pellet only in all the subsequent trials. The time taken to reach the food bowl and feed was measured to compare the responses of E(12-1) and U(12-1) rats to this unexpected decrease in reward size from 12 pellets to one pellet, with those of the E(1-1) and U(1-1) rats that ran for one pellet throughout.
During training, prior to implementation of the shift in reward size, mean latency to feed was analysed each day using an ANOVA with treatment (E/U) and original reward size (12 pellets/1 pellet) as between-subject factors. On the day of the reward size shift (day 13), latency to feed was analysed using a repeated measures general linear model (GLM) with treatment (E/U) and original reward size (12/1) as between-subject factors and trial (1–6) as a within-subject factor. Mean daily latencies to feed from the day of the reward size shift to the day on which no treatment effects on latency were detected (day 17) were analysed using a repeated measures GLM with treatment (E/U) and original reward size (12/1) as between-subject factors, and day (13–17) as a within-subjects factor. Adjusted F-statistics (Greenhouse Geisser) were used when data were non-spherical, and t-tests were used for post hoc tests. All data (transformed if necessary) satisfied the requirements for parametric tests. The statistics package used was SPSS v. 12.
3. Results
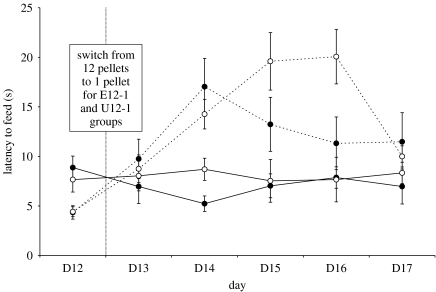
On day 12 of training, rats receiving 12 pellets ran significantly faster than those receiving one pellet (F1,19=15.6, p=0.001; figure 1). There was no difference between E and U rats, and no housing treatment×reward size interaction. From day 13 onwards, all E and U rats that had been trained to run for 12 pellets were unexpectedly given only one pellet. On day 13 itself, these rats ran significantly slower on the third trial after the drop in food reward than the E and U rats that had been trained to run for one pellet from the outset (reward size×trial: F2.25,42.7=3.5, p=0.035; post hoc: t21=−2.62, p=0.016). This SNC effect was then observed for mean daily latencies on all days until day 17, by which time no differences in running speed were evident (F1,21=3.2, n.s.) and testing was stopped. However, and critically, a repeated measures GLM analysis of mean daily latencies to reach the food bowl revealed a highly significant interaction between treatment (E/U), original reward size (12/1) and test day (13–17; F4,76=4.96, p=0.001), indicating that U rats showed a significantly prolonged response to reward loss than E rats (figure 1). Post hoc analysis showed that while E12-1 rats were significantly slower to feed than both E1-1 (t9=4.36, p=0.002) and U1-1 (t9=2.93, p=0.017) rats for day 14 only, U12-1 rats were significantly slower than E1-1 and U1-1 rats for day 14 (E1-1: t10=5.35, p<0.001; U1-1: t10=2.98, p=0.014), day 15 (E1-1: t10=3.99, p=0.003; U1-1: t10=3.34, p=0.008) and day 16 (E1-1: t10=4.14, p=0.002; U1-1: t10=3.49, p=0.006). U12-1 rats were also significantly slower than E12-1 rats on day 16 (t9=2.26, p=0.05). U1-1 rats differed significantly from E1-1 rats on only one occasion (day 14: t10=2.54, p=0.029).
Figure 1.
Mean (±s.e.m.) daily latency (s) for rats in different treatment groups (see text) to feed from the bowl at the end of the runway. Solid line with filled circle, E1-1; dashed line with filled circle, E12-1; solid line with open circle, U1-1; dashed line with open circle, U12-1.
4. Discussion
In §1, we argued that the unenriched rats had poorer welfare and were in a negative affective state relative to the enriched rats, and predicted that they would therefore be more sensitive to reward loss. Our finding that unenriched rats showed a more prolonged SNC response to reward loss than enriched rats supports this prediction. A prominent theory of response to reward loss in the SNC paradigm suggests that the decrease in reward size is aversive (see also Rolls 2005), leading to disappointment-like or frustration-like affective responses (reversible with anxiolytic drugs). The animal associates this aversiveness with the goal box and apparatus and consequently slows its running speed. With time, the rewarding properties of the new smaller reward override the effects of aversion (Flaherty 1996; Tucker & Luu 2007). The degree of aversion experienced is thus likely to affect the size and duration of the response to reward loss (Flaherty 1996). Recent data from human studies have indicated that people in negative affective states show stronger behavioural and neurophysiological responses to loss, error and failure (Hajcak et al. 2004; Chiu & Deldin 2007; Tucker & Luu 2007). A similar relationship in animals would thus result in a stronger aversive response to loss in subjects in a negative affective state, as observed here. Findings that rats from high-anxiety lines, or experiencing the negative effects of drug withdrawal, show enhanced and prolonged SNC responses (Barr & Phillips 2002; Rosas et al. 2007) may also be explained in this way. Animals in a negative state may also have a lower expectation that the reward will return to its original size (Paul et al. 2005), hence lengthening the response to reward loss.
Although unenriched rats showed a prolonged SNC response, we did not observe a difference in peak response as might be expected. However, it has been proposed that the initial response to SNC reward reduction involves searching for the lost reward, and is a ‘non-emotional’ process (insensitive to anxiolytics) that is thus likely to be less responsive to differences in background affect (see Flaherty 1996). Correspondingly, humans in negative affective states also show delayed recovery from negative events (e.g. longer lasting event-related negative cognitions and affect; Gunthert et al. 2007), despite no differences in immediate response, or even a blunted initial response (Peeters et al. 2003).
Differences in general activity and a general decrease in reward valuation are unlikely explanations for our findings because enriched and unenriched rats did not differ in their running speeds during initial training, and eventually showed similar running speeds to the new small reward. Explanations in terms of associative generalization decrements, the idea that the new small reward disrupts associative links between the context (apparatus), reward and response and hence interferes with the response subsequently shown, also appear unlikely since differences in such effects are predicted to occur immediately (Flaherty 1996).
In summary, our results indicate that sensitivity to reward reduction may be a valuable new indicator of animal emotion and welfare. It remains to be seen whether other reward evaluation processes involving contrasts between expected and actual rewards (e.g. sensitivity to reward gain; removal of aversive stimuli) also reflect background affective state. Parallel studies using this approach in humans and animals may also reveal cross-species commonalities in the influence of affect on reward evaluation.
Acknowledgments
This work followed the ASAB guidelines for the use of animals in research and was performed under a UK Home Office licence.
We thank the BBSRC for supporting this work and funding O.H.P.B., R.P. and E.S.P.
References
- Barr A.M, Phillips A.G. Increased successive negative contrast in rats withdrawn from an escalating-dose schedule of d-amphetamine. Pharmacol. Biochem. Behav. 2002;71:301–307. doi: 10.1016/s0091-3057(01)00664-5. doi:10.1016/S0091-3057(01)00664-5 [DOI] [PubMed] [Google Scholar]
- Bateson M, Matheson S.M. Performance of a categorisation task suggests that removal of environmental enrichment induces ‘pessimism’ in captive European starlings (Sturnus vulgaris) Anim. Welf. 2007;16(Suppl.):33–36. [Google Scholar]
- Beck A. Harper Row; New York, NY: 1967. Depression: clinical, experimental and theoretical aspects. [Google Scholar]
- Burman O.H.P, AbouIsmail U, Nicol C, Day M, Owen D, Bailey M, Mendl M. A multidisciplinary study of the long-term effects of environmental enrichment on laboratory rat welfare. In: Mendl M, et al., editors. Proc. 40th Int. Congress of the ISAE. ISAE; Bristol, UK: 2006. p. 72. [Google Scholar]
- Burman, O. H. P., Parker, R., Paul, E. S. & Mendl, M. In press. A spatial judgement task to determine background emotional state in laboratory rats, Rattus norvegicus Anim. Behav (doi:10.1016/j.anbehav.2008.02.014)
- Chiu P.H, Deldin P.J. Neural evidence for enhanced error detection in major depressive disorder. Am. J. Pyschiatry. 2007;164:608–616. doi: 10.1176/ajp.2007.164.4.608. doi:10.1176/appi.ajp.164.4.608 [DOI] [PubMed] [Google Scholar]
- Dreher J.-C. Sensitivity of the brain to loss aversion during risky gambles. Trends Cogn. Sci. 2007;11:270–272. doi: 10.1016/j.tics.2007.05.006. doi:10.1016/j.tics.2007.05.006 [DOI] [PubMed] [Google Scholar]
- Flaherty C.F. Cambridge University Press; Cambridge, UK: 1996. Incentive relativity. [Google Scholar]
- Gunthert K.C, Cohen L.H, Butler A.C, Beck J.S. Depression and next-day spillover of negative mood and depressive cognitions following interpersonal stress. Cognit. Ther. Res. 2007;31:521–532. doi:10.1007/s10608-006-9074-1 [Google Scholar]
- Hajcak G, McDonald N, Simons R.F. Error-related psychophysiology and negative affect. Brain Cogn. 2004;56:189–197. doi: 10.1016/j.bandc.2003.11.001. doi:10.1016/j.bandc.2003.11.001 [DOI] [PubMed] [Google Scholar]
- Harding E.J, Paul E.S, Mendl M. Cognitive bias and affective state. Nature. 2004;427:312. doi: 10.1038/427312a. doi:10.1038/427312a [DOI] [PubMed] [Google Scholar]
- Haselton M.G, Nettle D. The paranoid optimist: an integrative evolutionary model of cognitive biases. Pers. Soc. Psychol. Rev. 2006;10:47–66. doi: 10.1207/s15327957pspr1001_3. doi:10.1207/s15327957pspr1001_3 [DOI] [PubMed] [Google Scholar]
- Latham N, Mason G. We've got to get out of this place: frustration, enrichment and the development of stereotypies in laboratory mice (Mus musculus) In: Mendl M, et al., editors. Proc. 40th Int. Congress of the ISAE. ISAE; Bristol, UK: 2006. p. 125. [Google Scholar]
- Mendl M, Paul E.S. Consciousness, emotion and animal welfare: insights from cognitive science. Anim. Welf. 2004;13:S17–S25. [Google Scholar]
- Mineka S, Watson D, Clark L.A. Comorbidity of anxiety and unipolar mood disorders. Annu. Rev. Psychol. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. doi:10.1146/annurev.psych.49.1.377 [DOI] [PubMed] [Google Scholar]
- Panksepp J. Oxford University Press; New York, NY: 1998. Affective neuroscience. [Google Scholar]
- Paul E.S, Harding E.J, Mendl M. Measuring emotional processes in animals: the utility of a cognitive approach. Neurosci. Biobehav. Rev. 2005;29:469–491. doi: 10.1016/j.neubiorev.2005.01.002. doi:10.1016/j.neubiorev.2005.01.002 [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicolson N.A, Berkhof J, Delespaul P, deVries M. Effects of daily events on mood states in major depressive disorder. J. Abnorm. Psychol. 2003;112:203–211. doi: 10.1037/0021-843x.112.2.203. doi:10.1037/0021-843X.112.2.203 [DOI] [PubMed] [Google Scholar]
- Rolls E.T. Oxford University Press; Oxford, UK: 2005. Emotion explained. [Google Scholar]
- Rosas J.M, Callejas-Aguilera J.E, Escarabajal M.D, Gomez M.J, de la Torre L, Aguero A, Tobeira A, Fernandez-Teruel A, Torres C. Successive negative contrast effect in instrumental runway behaviour: a study with Roman high-(RHA) and Roman low-(RLA) avoidance rats. Behav. Brain Res. 2007;185:1–8. doi: 10.1016/j.bbr.2007.07.027. doi:10.1016/j.bbr.2007.07.027 [DOI] [PubMed] [Google Scholar]
- Tucker D.M, Luu P. Neurophysiology of motivated learning: adaptive mechanisms underlying cognitive bias in depression. Cognit. Ther. Res. 2007;31:189–209. doi:10.1007/s10608-006-9115-9 [Google Scholar]
- Wenzlaff R.M, Grozier S.A. Depression and the magnification of failure. J. Abnorm. Psychol. 1988;97:90–93. doi: 10.1037//0021-843x.97.1.90. doi:10.1037/0021-843X.97.1.90 [DOI] [PubMed] [Google Scholar]
- Young R.J. Blackwell Publishing; Oxford, UK: 2003. Environmental enrichment for captive animals. [Google Scholar]