Abstract
Soil respiration is responsible for recycling considerable quantities of carbon from terrestrial ecosystems to the atmosphere. There is a growing body of evidence that suggests that the richness of plants in a community can have significant impacts on ecosystem functioning, but the specific influences of plant species richness (SR), plant functional-type richness and plant community composition on soil respiration rates are unknown. Here we use 10-year-old model plant communities, comprising mature plants transplanted into natural non-sterile soil, to determine how the diversity and composition of plant communities influence soil respiration rates. Our analysis revealed that soil respiration was driven by plant community composition and that there was no significant effect of biodiversity at the three levels tested (SR, functional group and species per functional group). Above-ground plant biomass and root density were included in the analysis as covariates and found to have no effect on soil respiration. This finding is important, because it suggests that loss of particular species will have the greatest impact on soil respiration, rather than changes in biodiversity per se.
Keywords: grassland, microbial activity, plant community composition
1. Introduction
Annual carbon (C) flux through soil respiration is 10 times greater than fossil fuel combustion and recycles approximately 10% of atmospheric carbon dioxide (CO2). The regulation of soil respiration by plants is particularly important because they are the principal pathway through which C enters soil. It is well known that particular species of plants can modify the size and activity of soil microbial populations, and in so doing have the potential to affect key ecosystem processes such as soil respiration. However, the relative importance of plant diversity and community composition in regulating soil respiration rates is unknown. Understanding these relationships is important because extinction rates are increasing and much conservation legislation aims to restore or maintain plant species diversity. Recent evidence suggests that the diversity of plants in a community can have significant impacts on ecosystem functioning (Spehn et al. 2005), although there remains intense debate about the interpretation of many findings (Thompson et al. 2005). In particular, experiments using unnatural soils, sterilization pre-treatments and immature plants may have little ecological relevance (Read 2002). Furthermore, most of the past studies have focused on productivity, soil nutrient status and leaching as indicators of ecosystem function, yet soil respiration has been studied only rarely, often in short-term studies (e.g. Craine et al. 2001a,b; De Boeck et al. 2007). Soil respiration is important as a measure of ecosystem function, since it integrates key properties including microbial activity and C inputs to soils from litter, roots and root exudates, in addition to being a globally important C input to the atmosphere.
Here we use ecologically relevant experimental plant communities to test how soil respiration is regulated by three levels of plant diversity (species richness, SR; functional group richness, FGR and species per functional group, S/FG) and by plant community composition (i.e. the identity of the plants in the community). Since rates of C transfer to soils should be primarily influenced by the capacity of component species to fix C, we hypothesize that community composition will be the dominant regulating factor for soil C flux, and that the diversity of plants (species or FGR and species per functional type) will be of little importance.
2. Material and methods
(a) Mesocosm communities
In 1995, model communities were constructed within propylene mesocosms (60 cm×60 cm×15 cm deep) filled with homogenized natural, sieved (10 mm) rendzina soil removed from calcareous grassland near Sheffield (UK). The 32 mesocosms were arranged randomly in five rows within a 7 m×15 m open compound at the University of Sheffield botanic gardens. The plant communities (table 1) comprised mature individuals transplanted from calcareous grassland and measurements were not taken until the tenth year of establishment. This design therefore reproduces several essential components of mature grassland communities that have been missing from previous studies using experimental communities, while avoiding the confounding effects in the field of pre-existing soil properties determining local species distributions.
Table 1.
Composition of the experimental communities consisting of either one or three plant functional groups, and one or four species representing each functional type. (Functional group of each species in parentheses: (g) grass; (s) sedge; (f) forb. Each community is replicated four times.)
| community composition (species with functional group) | functional groups in community | functional group richness (FGR) | no. of species for each functional group (S/FG) | species richness (SR) |
|---|---|---|---|---|
| Festuca ovina (g) | grass | 1 | 1 | 1 |
| Carex flacca (s) | sedge | 1 | 1 | 1 |
| Leontodon hispidus (f) | forb | 1 | 1 | 1 |
| Festuca ovina (g) | grass+sedge+forb | 3 | 1 | 3 |
| Carex flacca (s) | ||||
| Leontodon hispidus (f) | ||||
| Festuca ovina (g) | grass | 1 | 4 | 4 |
| Koeleria macrantha (g) | ||||
| Helictotrichon pratense (g) | ||||
| Briza media (g) | ||||
| Carex flacca (s) | sedge | 1 | 4 | 4 |
| C. panacea (s) | ||||
| C. caryophyllea (s) | ||||
| C. pulicaris (s) | ||||
| L. hispidus (f) | forb | 1 | 4 | 4 |
| Succisa pratensis (f) | ||||
| Campanula rotundifolia (f) | ||||
| Viola riviniana (f) | ||||
| Festuca ovina (g) | grass+sedge+forb | 3 | 4 | 12 |
| Koeleria macrantha (g) | ||||
| Helictotrichon pratense (g) | ||||
| Briza media (g) | ||||
| Carex flacca (s) | ||||
| C. panacea (s) | ||||
| C. caryophyllea (s) | ||||
| C. pulicaris (s) | ||||
| Leontodon hispidus (f) | ||||
| Succisa pratensis (f) | ||||
| Campanula rotundifolia (f) | ||||
| Viola riviniana (f) |
Each community (n=4) consisted of 196 individual plants allocated to random locations on a uniform grid within each mesocosm box (plant spacing approx. 4 cm: each corner of the microcosm was unplanted). A mixed bryophyte assemblage collected from calcareous grassland was added to the soil surface. The composition of the plant communities was established in a two-way factorial design so that the community consisted of either one or three plant functional groups (grass, sedge and forb) or one or four species representing each functional group (table 1). These communities also produced a range of SR of 1, 3, 4 and 12 species (table 1).
(b) Soil respiration and plant biomass
Soil respiration rates were measured in situ using a portable infrared gas analyser (Licor LS6400, Licor Biosciences, Inc.) fitted with a soil respiration chamber (Glen Spectra Ltd). Patches of vegetation, which are 10 cm in diameter, were clipped to soil level immediately prior to each measurement, to provide a plant-free patch for the analysis. The chamber was placed directly onto the soil surface to a depth of 1 cm. Mean flux rates were calculated (within a CO2 concentration range of 345–355 ppm) from duplicate measurements taken in June and August (patches re-clipped). The order of the measurements was randomized and these were taken between 10.00 and 13.00. Annual above-ground biomass production was determined every year on dried samples (48 hours at 65°C) from two harvests; first a clip to 2.5 cm above soil in June (of the whole mesocosm; clippings removed) and then a complete harvest to soil level in October within a 10 cm diameter circular sub-area. A soil core (4 cm diameter×10 cm deep) was removed from an adjacent location, from which the roots were removed by wet sieving, dried (48 hours at 65°C) and their density was calculated.
(c) Statistical analyses
The effects of community composition (differences between communities), FGR and S/FG were determined by type I sum of squares (Scherer-Lorenzen et al. 2003) general linear model (SPSS, Chicago, IL). This procedure allows analysis of the hierarchical design with the model terms fitted sequentially. Community composition was fitted as a random factor nested within the fixed factors of FGR and S/FG. To test for the effects of SR, the model was run with and without SR fitted first as a covariate. Shoot biomass, root density and soil temperature data were added as covariates. Differences between each community were determined from post hoc Tukey's HSD tests.
3. Results
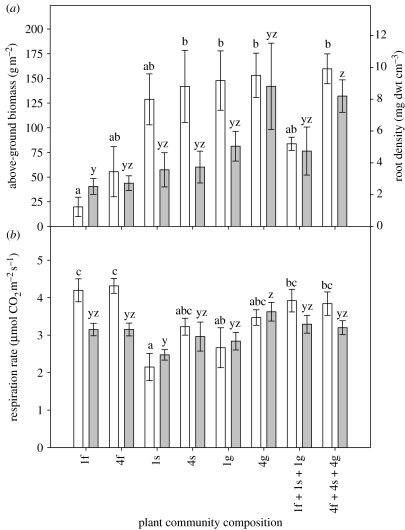
Above-ground plant biomass ranged from 20 to 160 g m−2 across the mesocosm communities and was driven by the composition of the plant communities (p<0.01), that is, there were no significant effects of plant SR, plant functional-type richness or S/FG on plant productivity (figure 1a). Community had a similar effect (p=0.02) on root density, which ranged from 3 to 9 g dwt cm−3 (figure 1a). Soil respiration ranged from 2 to 4.2 μm CO2 m−2 s−1 (figure 1b). The greatest values were obtained in the forb-only communities, and the smallest values obtained from the sedge-only communities. In most communities, we observed little temporal variation between measurements undertaken at different times of the season. Our analysis revealed that soil respiration was affected considerably by plant community composition with major differences occurring between the communities consisting of different plant functional types. This was particularly apparent for the measurements made in June. Here, respiration rates were significantly greater in the one and four forb communities compared with the one sedge and one grass communities (figure 1b). Respiration rates were also significantly smaller in the one sedge community compared with both of the mixed forb/sedge/grass communities. In contrast, there was no significant effect of biodiversity at the three levels tested (SR, functional group and S/FG). Above-ground biomass, root density and soil temperature were included in the analysis as covariates and found to have no significant effect on soil respiration.
Figure 1.
(a) Above-ground plant biomass (open bars) and root density (shaded bars) and (b) soil respiration rates in June (open bars) and August (shaded bars) in mesocosm plant communities (±s.e.m.). f, forb; s, sedge and g, grass and the numbers refer to the number of species represented. Bars sharing a letter are not significantly different (p>0.05). Each data series is interpreted independently.
4. Discussion
Previous short-term (approx. 2 years) experiments investigating how soil respiration responds to plant SR have found either positive (Craine et al. 2001b) or no effects (Gastine et al. 2003; De Boeck et al. 2007). In one of the few long-term (7 years) studies, rates of soil respiration increased alongside plant SR, although the measurements were made in vitro (Zak et al. 2003). Our experiment was designed to determine how plant community composition and biodiversity, measured at three levels (SR, FGR and S/FG), affect in vivo soil respiration rates in established species-rich grassland mesocosms. Our analysis demonstrated that soil respiration was regulated by plant community composition, with large differences between communities consisting of different plant functional types, and that there was no significant effect of biodiversity at any of the three levels tested. Although we only measured soil respiration twice, this effect was consistent between sampling periods. The forb communities in particular (Leontodon hispidus, Succisa pratensis, Campanula rotundifolia and Viola riviniana) were associated with the greatest soil respiration rates, regardless of their SR. The contrast between communities of different functional types indicates an important role played by functional traits of the component plants that may influence C transfer below ground. Potential maximum relative growth rates of individual species have been shown to scale-up to predict ecosystem productivity (Vile et al. 2006) and there is no reason to suspect that species traits that influence soil respiration should not similarly scale-up to predict community-level soil respiration. These findings suggest that loss of a particular species or functional group may have the greatest impact on ecosystem functioning, rather than changes in biodiversity per se. This is important because species loss is likely to occur in a non-random predictable way (Grime 2002), and so identifying the plant communities that have the greatest influence on soil respiration may further our understanding of the functional consequences of species changes.
However, it is important to consider the experimental mesocosms in context with the natural environment. Although we attempted to increase the ecological relevance of the study by using mature plants established for 10 years on natural soil, the impact of loss of particular plant species in their natural, heterogeneous environment may differ from our observations. In addition, our study used a maximum of 12 species, whereas the calcareous grassland typically contains approximately 20 higher plant species m−2, and so there may be other species-specific interactions to consider; these possibilities clearly require further study.
We would expect the large variation in above- and below-ground plant biomass observed between mesocosm communities to have a significant impact on processes that are dependent on inputs of C. In our mesocosms, in which environmental variables were constant between experimental units, we would predict that the plant communities with the greatest biomass would result in greater soil respiration. Some studies have found productivity to be correlated with soil respiration (e.g. Craine et al. 2001a), and root biomass can contribute up to 51% of soil respiration (Wang et al. 2005). Our analysis revealed that the effects of plant community on soil respiration were over and above those of shoot biomass and root density. This observation suggests that C inputs from litter and roots are not the main factors in regulating soil respiration in these experimental mesocosm systems.
This raises the question as to the source of CO2 flux from our mesocosms. It is recognized that the clipping treatment may lead to a small increase in CO2 flux, but this is unlikely to be a major contributor to respiration rates, particularly in the August measurement. An important abiotic driver that we did not consider in our analysis is variation in soil water content. Leachate volume from the mesocosms was quantified for the duration of the experiment and this was found to range from 280 to 340 l m−2 yr−1 (Phoenix et al. in press), and together with the contrasting plant cover, imply that the dynamics of soil water differs between mesocosms. Many of the species used in this experiment also have different phenology and this may also explain some of the variation in soil respiration. Other important biological drivers of soil respiration are arbuscular mycorrhizal fungi, which form mutualistic relationships with all plant species used in the mesocosms except the sedges (Johnson et al. 2004). Although rarely considered in models of C fluxes, there is evidence that mycorrhizal fungi can contribute significantly to soil respiration rates by rapidly using recent plant assimilate (Johnson et al. 2002). The fact that the sedges are non-mycorrhizal may also partly explain why soil respiration rates from these mesocosms were smallest.
This study has highlighted that plant community composition is an important driver of soil respiration, and that biodiversity at all levels tested has little effect. There is now clearly a need to identify and quantify pathways of below-ground C flux in the contrasting plant assemblages to gain a mechanistic understanding of these findings.
Acknowledgments
D.J. is supported by a NERC Advanced Fellowship and G.K.P. by an RCUK Academic Fellowship.
References
- Craine J.M, Wedin D.A, Reich P.B. Grassland species effects on soil CO2 flux track the effects of elevated CO2 and nitrogen. New Phytol. 2001a;150:425–434. doi:10.1046/j.1469-8137.2001.00103.x [Google Scholar]
- Craine J.M, Wedin D.A, Reich P.B. The response of soil CO2 flux to changes in atmospheric CO2, nitrogen supply and plant diversity. Glob. Change Biol. 2001b;7:947–953. doi:10.1046/j.1354-1013.2001.00455.x [Google Scholar]
- De Boeck H.J, Lemmens C.M.H.M, Vicca S, Van den Berge J, Van Dongen S, Janssens I.A, Ceulmans R, Nijs I. How do climate and species richness affect CO2 fluxes in experimental grasslands? New Phytol. 2007;175:512–522. doi: 10.1111/j.1469-8137.2007.02122.x. doi:10.1111/j.1469-8137.2007.02122.x [DOI] [PubMed] [Google Scholar]
- Gastine A, Scherer-Lorenzen M, Leadley P.W. No consistent effects of plant diversity on root biomass, soil biota and soil abiotic conditions in temperate grassland communities. Appl. Soil Ecol. 2003;24:101–111. doi:10.1016/S0929-1393(02)00137-3 [Google Scholar]
- Grime J.P. Declining plant diversity: empty niches or functional shifts? J. Veg. Sci. 2002;13:457–460. doi:10.1658/1100-9233(2002)013[0457:DPDENO]2.0.CO;2 [Google Scholar]
- Johnson D, Leake J.R, Ostle N, Ineson P, Read D.J. In situ 13CO2 pulse-labelling of upland grassland demonstrates a rapid pathway of carbon flux from arbuscular mycorrhizal mycelium to the soil. New Phytol. 2002;153:327–334. doi:10.1046/j.0028-646X.2001.00316.x [Google Scholar]
- Johnson D, Vandenkoornhuyse P.J, Leake J.R, Gilbert L.A, Booth R.E, Grime J.P, Young J.P.W, Read D.J. Plant communities affect arbuscular mycorrhizal fungal diversity and community composition in grassland microcosms. New Phytol. 2004;161:503–516. doi: 10.1046/j.1469-8137.2003.00938.x. doi:10.1046/j.1469-8137.2003.00938.x [DOI] [PubMed] [Google Scholar]
- Phoenix, G. K., Johnson, D., Grime, J. P. & Booth, R. E. In press. Sustaining ecosystem services in ancient limestone grassland: importance of major component plants and community composition. J. Ecol (doi:10.1111/j.1365-2745.2008.01403.x)
- Read, D. J. 2002 Towards ecological relevance—progress and pitfalls in the path towards an understanding of mycorrhizal functions in nature. In Mycorrhizal ecology, (eds M. G. A. van der Heijden & I. Sanders). Ecological studies, vol. 157, pp. 3–29. Berlin, Germany: Springer.
- Scherer-Lorenzen M, Palmborg C, Prinz A, Schulze E.D. The role of plant diversity and composition for nitrate leaching in grasslands. Ecology. 2003;84:1539–1552. doi:10.1890/0012-9658(2003)084[1539:TROPDA]2.0.CO;2 [Google Scholar]
- Spehn E.M, Hector A, Joshi J. Ecosystem effects of biodiversity manipulations in European grasslands. Ecol. Monogr. 2005;75:37–63. doi:10.1890/03-4101 [Google Scholar]
- Thompson K, Askew A.P, Grime J.P, Dunnett N.P, Willis A.J. Biodiversity, ecosystem function and plant traits in mature and immature plant communities. Funct. Ecol. 2005;19:355–358. doi:10.1111/j.0269-8463.2005.00936.x [Google Scholar]
- Vile D, Shipley B, Garnier E. Ecosystem productivity can be predicted from potential relative growth rates and species abundance. Ecol. Lett. 2006;9:1061–1067. doi: 10.1111/j.1461-0248.2006.00958.x. doi:10.1111/j.1461-0248.2006.00958.x [DOI] [PubMed] [Google Scholar]
- Wang W, Ohse K, Liu J.J, Mo W.H, Oikawa T. Contribution of root respiration to soil respiration in a C3/C4 mixed grassland. J. Biosci. 2005;30:507–514. doi: 10.1007/BF02703725. doi:10.1007/BF02703725 [DOI] [PubMed] [Google Scholar]
- Zak D.R, Holmes W.E, White D.C, Peacock A.D, Tilman D. Plant diversity, soil microbial communities, and ecosystem function: are there any links? Ecology. 2003;84:2042–2050. doi:10.1890/02-0433 [Google Scholar]