Abstract
Human-induced environmental change is occurring at an unprecedented rate and scale. Many freshwater habitats, in particular, have been degraded as a result of increased salinity. Little is known about the effects of anthropogenic salinization on freshwater organisms, especially at sublethal concentrations, where subtle behavioural changes can have potentially drastic fitness consequences. Using a species of Australian frog (Litoria ewingii), we experimentally examined the effects of salinization on tadpole behaviour and their vulnerability to a predatory dragonfly nymph (Hemianax papuensis). We found that tadpoles exposed to an ecologically relevant concentration of salt (15% seawater, SW) were less active than those in our freshwater control (0.4% SW). Tadpoles in elevated salinity also experienced a higher risk of predation, even though the strike rate of the predator did not differ between salt and freshwater treatments. In a separate experiment testing the burst-speed performance of tadpoles, we found that tadpoles in saltwater were slower than those in freshwater. Thus, it would appear that salt compromised the anti-predator response of tadpoles and made them more susceptible to being captured. Our results demonstrate that environmentally relevant concentrations of aquatic contaminants can, even at sublethal levels, severely undermine the fitness of exposed organisms.
Keywords: anthropogenic disturbance, ecotoxicology, predator–prey interaction, tree frog
1. Introduction
Human activities are rapidly accelerating the extent and pace of environmental change. In many parts of the world, freshwater systems are being degraded by an increase in salinity (Hart et al. 2003; Mahajan & Tuteja 2005). Anthropogenic salinization is brought about by the mobilization of salt in the soil profile or groundwater due to activities such as land clearing, the planting of shallow-rooted crops, and/or irrigation practices that raise the water table, causing salt to be transported to the surface (Williams 2001). This salt, in turn, leaches into streams, rivers and wetlands causing naturally ‘fresh’ water to become ‘saline’ (i.e. above 0.5 g NaCl l−1).
Most studies examining the impact of salinity on freshwater organisms have focused on the toxicological effects of salt on development and/or the ‘lethal limit’ of exposed organisms (e.g. Chinathamby et al. 2006). It is now generally accepted that elevated salinity can impact survival by interfering with key physiological and metabolic processes, such as osmoregulation. Less well understood are the behavioural changes that can occur when freshwater animals encounter environmentally relevant concentrations that fall below the lethal limit. The importance of redressing this deficit is underscored by recent studies showing that exposure to various aquatic contaminants, even at sublethal concentrations, can induce fitness-altering changes in organismal behaviour (Fisher et al. 2006; Ward et al. 2008). In this regard, few behaviours have as direct a bearing on individual fitness as those involved in mitigating predation risk (Relyea 2001).
The brown tree frog (Litoria ewingii) is a small anuran amphibian native to southeastern Australia. Within this range, the species is found in a variety of habitats, many of which are currently, or potentially, affected by secondary salinization (NLWRA 2001). Because breeding and tadpole development takes place in shallow water, evidence suggests that heightened salinity may already have excluded the species from certain wetland habitats (Smith et al. 2007). Litoria ewingii tadpoles have also been observed to reduce their activity levels when exposed to salt concentrations comparable to those found in degraded aquatic habitats (Chinathamby et al. 2006). The impact that reduced activity may have on the vulnerability of tadpoles to predation remains unknown. Accordingly, we set out to investigate the behavioural response and vulnerability of L. ewingii tadpoles exposed to a predatory dragonfly nymph under both freshwater and salinized conditions.
2. Material and methods
(a) General methods
Frog eggs were collected from a freshwater pond in Victoria, Australia (salinity level=0.3% seawater (SW), which is equivalent to 0.11 g NaCl l−1). Once hatched, tadpoles were housed in individual tanks (length×width×height=13×13×7 cm) containing 600 ml of freshwater (i.e. at 0.4% SW; equivalent to 0.14 g NaCl l−1). The tadpoles were kept at a constant temperature of 21°C on a 12 L : 12 D cycle, and were fed frozen endive ad libitum. Water quality was maintained by the removal of uneaten food and by carrying out regular partial water changes.
Four days after hatching, tadpoles (mean length±s.d.=11.94±1.19 mm) were randomly assigned to one of two treatment groups. The tadpoles allocated to the ‘freshwater’ treatment were kept in 0.4% SW (i.e. a concentration typical of freshwater systems unaffected by increased salinity). The tadpoles in the saline treatment were maintained in 15% SW (equivalent to 5.25 g NaCl l−1). This is an environmentally meaningful concentration (Smith et al. 2007) that falls below the lethal limit for the species (Chinathamby et al. 2006). Water in both groups was prepared using Coralife Scientific Grade Marine Salt (ESU Inc., USA). Salt concentrations were checked with a Hanna H198130 conductivity meter every 4 days and kept within 0.01 g NaCl l−1 of the desired concentration by the addition of either salt or distilled water. To prevent osmotic shock, the tadpoles in the saline treatment were gradually introduced to 15% SW by incrementally increasing the level of salt by 5% SW (i.e. 1.75 g NaCl l−1) per day over 3 days. The tadpoles in both groups were then acclimatized to their respective treatments for a further 16 days before the start of any experimental trials.
(b) Predation experiment
Predation experiments were carried out by exposing tadpoles to dragonfly nymphs (Hemianax papuensis). We chose H. papuensis because its range and habitat preferences overlap with that of L. ewingii (Theischinger & Hawking 2006). Moreover, the nymphs of this species are known to feed on tadpoles (including those of L. ewingii) and, like the nymphs of many other odonates, ambush their prey by adopting a sit-and-wait strategy.
Nymphs (mean length±s.d.=38.83±4.62 mm) were housed in individual tanks (length×width×height=13×13×7 cm) containing a piece of artificial vegetation. All nymphs were initially housed in freshwater. Nymphs randomly assigned to the saline treatment were then acclimatized to 15% SW over 3 days (as described for tadpoles). Thereafter, all nymphs were fed one tadpole to standardize hunger levels and then starved for 4 days before the start of experiments.
We monitored the fate of tadpoles from each of the two treatment groups (fresh versus salt) in both the presence and absence of predatory nymphs. In predation trials, individual tadpoles (n=20 per treatment) were placed into a container housing a single nymph and observed for 30 min. Any tadpole that was eaten during this time was noted, along with the time taken for the tadpole to be captured. A 10 min video recording made at the start of each trial was later used to determine tadpole activity (as a percentage of time spent swimming), nymph strike rate and the amount of time the tadpole spent within 5 cm of the nymph (as a measure of opportunity for prey capture). We also measured the activity levels of tadpoles (n=20 per treatment) in the absence of a predator. As in the predation trials, tanks were videotaped for 10 min and the recordings analysed to determine the time tadpoles spent active.
(c) Burst-speed performance
In this experiment, we measured the burst-speed performance (i.e. ‘flight’ response) of tadpoles when exposed to external stimuli. The tadpoles were placed into individual containers, the bottom of which was marked with 1 cm grid lines. After a 2 min acclimation period, we videotaped the tadpoles as they were poked at the base of the tail with a pipette. The burst speed (cm s−1) from the initial poke to the time the tadpole stopped swimming was calculated from the recordings using the video timer and grid lines on the images as reference.
(d) Statistical analyses
All data were checked for normality and homogeneity of variances. Proportional data were analysed following arcsine square root transformation (Crawley 2002). Time to capture was used in a non-parametric survival analysis to test the vulnerability of tadpoles in the predation experiment. Any tadpoles not consumed were ‘right censored’ for analysis (SYSTAT 2002). All tests were two-tailed, with results presented as mean±s.e.
3. Results
(a) Predation experiment
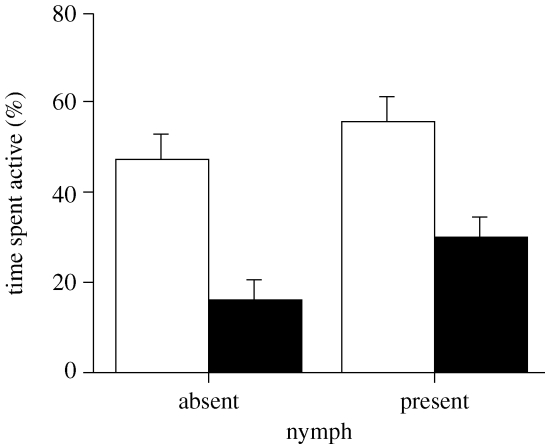
There was no significant interaction between the effects of salinity and predation on tadpole activity levels (factorial two-way ANOVA, F1,72=0.67, p=0.41). The interaction term was therefore removed from the model. Tadpole activity was affected by both salinity and predator presence, with individuals spending more time active in freshwater (factorial two-way ANOVA, F1,73=29.17, p<0.001; figure 1) and in the presence of a nymph (factorial two-way ANOVA, F1,73=5.41, p=0.023; figure 1).
Figure 1.
Activity level of tadpoles in the presence and absence of a dragonfly nymph under freshwater (white bars) and saltwater (black bars) conditions.
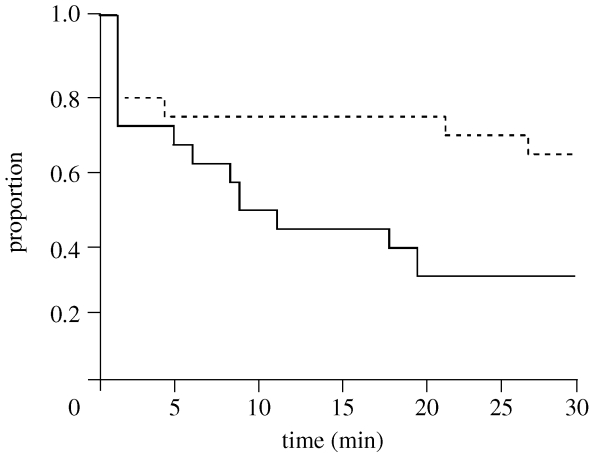
The tadpoles in the saltwater treatment were captured at a higher rate than those in freshwater (survival analysis: z=−2.33, p=0.02; figure 2). This was true even though the percentage of time spent by tadpoles within 5 cm of the nymph did not differ significantly between treatments (freshwater=16.9±3.0%, saltwater=13.5±4.3%; two-sample t-test, t38=0.66, p=0.51). Similarly, there was no significant difference in the number of strikes directed by nymphs towards tadpoles in freshwater (0.53±0.15 strikes) compared with those in saltwater (0.45±0.11 strikes; two-sample t-test, t38=1.62, p=0.12). However, nymphs were significantly less successful at seizing their prey in freshwater, with 19% of the strikes successful in freshwater compared with 52.9% in saltwater (Χ2-test, Χ2=4.80, p=0.03).
Figure 2.
Tadpole survival in the presence of a nymph under both freshwater (stippled line) and saltwater (solid line) conditions.
(b) Burst-speed performance
We found a significant difference in the burst-speed performance of tadpoles responding to external stimuli, with those in the freshwater control moving faster than those in saltwater (burst speed in freshwater=3.04±0.21 cm s−1, saltwater=2.35±0.20 cm s−1; two-sample t-test, t38=2.38, p=0.023).
4. Discussion
We found that L. ewingii tadpoles were less active under saline conditions (15% SW) than in freshwater. This result is concordant with an earlier study in which L. ewingii tadpoles were observed to be ‘sluggish’ under saline conditions (Chinathamby et al. 2006). In anurans, exposure to salt is known to affect both muscles and nerves, leading to physiological adjustments and biochemical changes that are often energetically demanding (Dole et al. 1994). These adjustments come at a cost by taking energy away from activities such as somatic growth, swimming and feeding, and necessitating a reallocation of energy to meet increased osmoregulatory demands (Gomez-Mestre et al. 2004). Here, we show, for the first time, that salt exposure may also have consequences for the anti-predator responses of tadpoles.
A decrease in activity is often used by tadpoles as an anti-predator defence to reduce the risk of detection (e.g. Relyea 2001). In our study, rapid detection by nymphs might explain why tadpoles were more active in the predator's presence. Nevertheless, tadpoles in saltwater were more vulnerable to being captured than those in freshwater. Why? It seems unlikely that this was due to the effects of salt on the nymphs themselves, as nymphs survived equally well in freshwater as they did in saline water. In addition, we found no difference in the strike rate of nymphs between fresh and saltwater treatments. Indeed, nymphs had equal opportunity for attack, with tadpoles in both treatments spending equal amounts of time within 5 cm of the potential predator.
The risk of tadpoles being eaten was probably due, instead, to reduced ability to successfully escape the predator's attack under saline conditions. Burst speed is an important anti-predator response in tadpoles (e.g. Van Buskirk 2002) and, in our burst-speed experiment, we found that tadpoles in saltwater were slower than those in the freshwater control. This suggests that salt reduced the burst-speed performance of tadpoles and, as a result, compromised the ability of individuals to successfully evade a predator's attack. More broadly, our study highlights the importance of considering the impact of sublethal concentrations of aquatic contaminants, which may affect individual fitness through subtle, but important, changes in organismal behaviour.
Acknowledgments
This study conforms to all the relevant State and Federal legislation of Australia and was conducted under Monash University animal ethics approval (project no. BSCI2004/10).
We thank Ian Stewart, Andreas Svensson, Graeme Farrington, Sheila Hamilton-Brown, Murray Logan and Oz Water Gardens. Funding was provided by grants from Monash University (to R.D.R.) and the Australian Research Council (to B.B.M.W.).
References
- Chinathamby K, Reina R.D, Bailey P.C.E, Lees B.K. Effects of salinity on the survival, growth and development of tadpoles of the brown tree frog, Litoria ewingii. Aust. J. Zool. 2006;54:97–105. doi:10.1071/ZO06006 [Google Scholar]
- Crawley M.J. Wiley; Chichester, UK: 2002. Statistical computing: an introduction to data analysis using S-plus. [Google Scholar]
- Dole J.W, Palmer B, Rose B. The effect of hyperosmotic stress on tongue extension in the western toad, Bufo boreas. J. Herpetol. 1994;28:261–264. doi:10.2307/1564634 [Google Scholar]
- Fisher H.S, Wong B.B.M, Rosenthal G.G. Alteration of the chemical environment disrupts communication in a freshwater fish. Proc. R. Soc. B. 2006;273:1187–1193. doi: 10.1098/rspb.2005.3406. doi:10.1098/rspb.2005.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Mestre I, Tejedo M, Ramayo E, Estepa J. Developmental alterations and osmoregulatory physiology of a larval anuran under osmotic stress. Physiol. Biochem. Zool. 2004;77:267–274. doi: 10.1086/378143. doi:10.1086/378143 [DOI] [PubMed] [Google Scholar]
- Hart B.T, Lake P.S, Webb J.A, Grace M.R. Ecological risk to aquatic systems from salinity increases. Aust. J. Bot. 2003;51:689–702. doi:10.1071/BT02111 [Google Scholar]
- Mahajan S, Tuteja N. Cold, salinity and drought stresses: an overview. Arch. Biochem. Biophys. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. doi:10.1016/j.abb.2005.10.018 [DOI] [PubMed] [Google Scholar]
- NLWRA. Land and Water Resources Australia; Canberra, Australia: 2001. National land and water resources audit. [Google Scholar]
- Relyea R.A. Morphological and behavioral plasticity of larval anurans in response to different predator environment. Ecology. 2001;82:523–540. doi:10.2307/2679877 [Google Scholar]
- Smith M.J, et al. Associations between anuran tadpoles and salinity in a landscape mosaic of wetlands impacted by secondary salinisation. Freshw. Biol. 2007;52:75–84. doi:10.1111/j.1365-2427.2006.01672.x [Google Scholar]
- SYSTAT. SYSTAT Software, Inc; Richmond, CA: 2002. SYSTAT 10.2. Statistics I. [Google Scholar]
- Theischinger T, Hawking J. CSIRO; Collingwood, Australia: 2006. The complete field guide to dragonflies of Australia. [Google Scholar]
- Van Buskirk J. Phenotypic lability and the evolution of predator-induced plasticity in tadpoles. Evolution. 2002;56:361–370. doi: 10.1111/j.0014-3820.2002.tb01346.x. doi:10.1111/j.0014-3820.2002.tb01346.x [DOI] [PubMed] [Google Scholar]
- Ward A.J.W, Duff A.J, Horsfall J.S, Currie S. Scents and scents-ability: pollution disrupts chemical social recognition and shoaling in fish. Proc. R. Soc. B. 2008;275:101–105. doi: 10.1098/rspb.2007.1283. doi:10.1098/rspb.2007.1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams W.D. Anthropogenic salinisation of inland waters. Hydrobiologia. 2001;446:329–337. doi:10.1023/A:1014598509028 [Google Scholar]