Abstract
The amniotes generally lay eggs on land and are thereby differentiated from lissamphibians (salamanders, frogs and caecilians) by their developmental pattern. Although a number of 330–300-Myr old fossils are regarded as early tetrapods placed close to amniotes on the basis of anatomical data, we still do not know whether their developmental pattern was more similar to those of lissamphibians or amniotes. Here we report palaeohistological and skeletochronological evidence supporting a salamander-like development in the seymouriamorph Discosauriscus. Its long-bone growth pattern, slow diaphyseal growth rate and delayed sexual maturity (at more than 10 years old) are more comparable with growth features of extant salamanders rather than extant amniotes, even though they are mostly hypothesized to be phylogenetically closer to living amniotes than salamanders.
Keywords: Amniota, salamanders, Discosauriscus, growth evolution, skeletochronology
1. Introduction
The appearance of amniotes is a major evolutionary event in vertebrate history (Ahlberg & Milner 1994) allowing tetrapods to become largely independent of external water for reproduction and development (Sumida & Martin 1997). Knowledge of the ontogeny of early tetrapods has hitherto been based exclusively on the continuous evolution of anatomical features during the ossification, thereby not allowing one to mark precisely the timing of transition from one ontogenetical stage to another (e.g. Steyer 2000; Schoch 2001). Skeletochronology, inferred from hard-tissue analyses, provides detailed information about growth, somatic age and sexual maturity in extant as well as extinct vertebrates (Castanet et al. 1993), especially in dinosaurs (e.g. Padian et al. 2001). However, there are still no such analyses for Palaeozoic vertebrates, even though the bone histology of several Palaeozoic tetrapods is partly known (e.g. de Ricqlès 1981). Here we demonstrate how fossil bone histology can illuminate evolutionary problems, such as those that surround the origin of amniotes.
2. Material and methods
In order to understand the evolution of growth strategies in Palaeozoic tetrapods, this study focus on the seymouriamorph Discosauriscus austriacus (Makowsky 1876), generally more closely related to extant amniotes than salamanders (e.g. Anderson 2007; Ruta & Coates 2007). Long bones of 19 well-preserved specimens (Klembara 1997; Klembara & Bartík 2000), from the Lower Permian (Czech Republic), are studied (c.f. electronic supplementary material). Developmental stages of this growth series have been determined according to anatomical features, and exemplars from all stages were selected (Klembara 1995). Histological and growth patterns in Discosauriscus are compared with that of extant morphotypes of the same size and general developmental stage among amniotes and urodeles. Frogs and caecilians are not considered in this comparison owing to their distinct morphology and locomotion, which could bias the measurements of bone growth.
3. Limb-bone histology of Discosauriscus
This is the first skeletochronological study of a large fossil growth series of such an ancient early tetrapod. The mid-shaft bone tissue organization of Discosauriscus is very similar to that of extant tetrapods of small body size: saurians (e.g. Lacerta viridis; Castanet 1985) and urodeles (e.g. Euproctus asper; Montori 1990; Desmognathus monticola, Castanet et al. 1996). Primary bone tissue is mostly sub-lamellar (even if locally lamellar or parallel fibred; figure 1a), indicating a relatively slow, well-organized bone deposition (de Ricqlès et al. 1991). Numerous radial Sharpey's fibres (S.f.), showing muscle attachments, are revealed under polarized light (PL) in many long-bone diaphyses (figure 1b). At the periphery of the medullary cavity, bone remodelling (erosion/reconstruction process) leaves a distinct endosteal margin visible under PL (figure 1a). This endosteal tissue, made of parallel-fibred bone, shows that remodelling, mainly linked to morphogenesis and mechanical constraints (Francillon-Vieillot et al. 1990), had already occurred in late larval specimens. The periosteal cortex is split into numerous growth layers bordered by lines of arrested growth (LAGs; figure 1). The K-index of the cortical thinness (Currey & Alexander 1985) indicates that bone compacta is thinner in the humeri, femora and radii (0.33<K<0.73) than in the ulnae, fibulae and tibiae (0.24<K<0.55). Bone trabeculae (b.t.) are visible in the marrow cavity of the femora and humeri. A sparse vascularization, composed of primary radial and longitudinal vascular canals in young specimens, intensifies in the femur, humerus and tibia towards a mainly radial arrangement among the largest specimens (figure 1d). The bone cortex (b.c.) in older specimens is relatively compact (up to 93%) and characteristic of mostly terrestrial extant tetrapods (Germain & Laurin 2005).
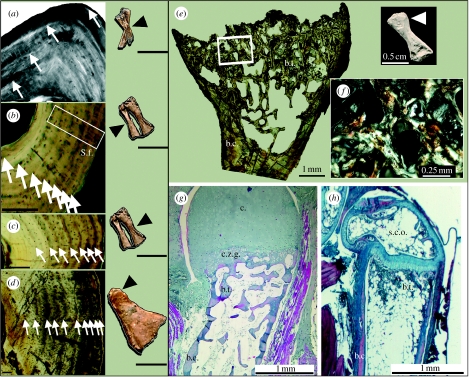
Figure 1.
Diaphyseal and epiphyseal bone histology of D. austriacus. (a) Radius, (under polarized light, PL) of the youngest larval specimen (RCZ6) showing four LAGs underlined by lamellar bone (in white) and spaced out by sub-lamellar bone. (b) and (c) Tibia and fibula, respectively (under natural light, NL), of the late larval specimen KO88 showing eight LAGs in both cortices and radial Sharpey's fibres (S.f.). (d) Tibia (NL) of the oldest subadult SNM Z 15568 showing 10 LAGs and a radial vascularization. (e) Distal femoral end (NL) of the metamorphic specimen (KO224) showing bone trabeculae (b.t., built by endochondral ossification) and the metaphyseal bone cortex (b.c.). (f) Detail of the trabeculae (PL) made of parallel-fibred bone (in white). (g) Comparative stained distal humeral end (NL) of a metamorphosed urodele, Pleurodeles waltl, showing a cartilaginous zone of growth (c.z.g.) and a cartilaginous top (c.). (h) Comparative stained distal humeral end (NL) of a juvenile amniote, Anolis sp., showing a secondary centre of ossification (s.c.o.). Scale bars: (sections in (a–d)), 0. 1 mm; (long bones in (a–d)), 0.5 cm.
The long-bone epiphyseal organization of Discosauriscus resembles that of urodeles (e.g. Pleurodeles waltl; de Ricqlès 1965; figure 1e–g), but the numerous trabeculae made of parallel-fibred bone and the absence of calcified cartilage in juvenile individuals suggest a relatively faster endochondral ossification and epiphyseal growth. As in living salamanders, the epiphyses of Discosauriscus were probably covered by a cartilaginous structure that extended to the metaphysis (figure 1g). Discosauriscus' epiphyses have no secondary centre of ossification, which so far has only been observed in amniotes (figure 1h).
4. Bone growth of the seymouriamorph Discosauriscus and evolutionary implications
Long-bone diaphyses of Discosauriscus show a simple classic LAG pattern as in extant tetrapods (Castanet et al. 1993). LAGs, separating two tissue types of differential bone densities, punctuate quiescent osteogeneses followed by sudden resumptions, expressed in living poikilotherms during annual aestivations or hibernations (Castanet et al. 1993). The LAGs of similar structure in Discosauriscus thus imply a probable annual periodicity. The skeletochronological analysis indicates that Discosauriscus, which lived under a tropical climate (Ziegler 1990), shows a seasonal annual life cycle similar to that of extant tropical Caudata (Castanet et al. 2003). The youngest sampled specimen was at least 4 years old when it died (four LAGs; figure 1a), whereas the oldest one was at least 10 years old (10 LAGs; figure 1d). A decrease in the growth-mark width towards the cortical periphery is obvious in the oldest specimens (figure 1d), suggesting that the diaphyseal growth strongly and definitively slows in thickness from the seventh or eighth year on. In extant tetrapods, such a transition in LAG spacing is linked with the acquisition of sexual maturity (Castanet et al. 2003). By comparison, it can be assumed that sexual maturity was reached late in development and probably not before 9 or 10 years old, allowing extended larval (until 6 years old) and juvenile stages (figure 2a). Palaeohistology therefore elaborates upon anatomical data (Klembara 1997) by showing that specimens up to 10 years old were subadults. Owing to their longevity, at least twice the age of acquisition of sexual maturity in extant urodeles (e.g. E. asper; Castanet et al. 2003), it can be reasonably estimated that the overall longevity of Discosauriscus may also be at least twice the immature period, i.e. at least 20 years. Finally, the life-history traits revealed by palaeohistology support the idea that young individuals lived for a long time until they reached the subadult stage (figure 2a) and then left the lacustrine environment for a more terrestrial life, as already suggested by anatomical data (Klembara et al. 2001). These observations confirm previous assertions concerning changes in environmental habitat co-occurring with metamorphosis (e.g. Schoch 2002). Discosauriscus probably became adult during its terrestrial style of life.
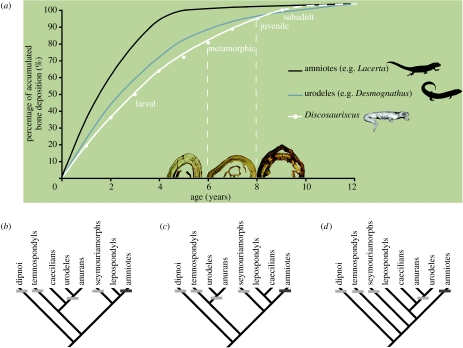
Figure 2.
(a) Comparative diaphyseal femoral growth curves of D. austriacus, an amniote (L. viridis) and a urodele (D. monticola), of nearly the same size, and their implication in elaborating different scenarios of early tetrapod growth strategy according to the different phylogenetic hypotheses: (b) Ruta & Coates (2007), (c) Anderson (2007) and (d) Vallin & Laurin (2004). (a) Desmognathus, whose histological organization is typical (i.e. not derived) of other urodeles, is morphologically similar to Discosauriscus. The reconstruction of the curves is explained in the electronic supplementary material. Femora of three individuals of Discosauriscus illustrate each growth stage: larval (KO96, 5 years old), metamorphic (Z15697, 7 years old) and juvenile (KO153, 9 years old). The tightening of the last peripheral LAGs indicates that long bones of the largest specimens, aged up to 10 years, are no longer intensely growing in thickness, i.e. individuals are not far from sexual maturity. The growth-curve tendency of Discosauriscus is similar to that of Desmognathus and other urodeles: slow bone deposition and late acquisition of sexual maturity. (b–d) Light-grey bars indicate an endochondral ossification with cartilaginous epiphyses (Haines 1942; Sanchez et al. 2007) and dark-grey bars, secondary centres of epiphyseal ossification (Haines 1942). All character optimizations, with Dipnoi as an out-group, show the salamander-like ossification as a primitive trait compared to that of amniotes.
The similarities in bone growth pattern between this seymouriamorph and urodeles are also confirmed by a similar average diaphyseal growth rate (26–82 μm yr−1 on average in juvenile femora of Discosauriscus; 48–97 μm yr−1 in lissamphibians of the same size; de Ricqlès et al. (1991); and 73–120 μm yr−1 in saurians of the same size; Castanet 1985; figure 2a).
In conclusion, this new palaeohistological analysis clearly shows that seymouriamorphs retained a salamander-like developmental pattern, although their limbs were certainly already largely adapted to terrestrial locomotion. Although the phylogeny of early tetrapods is still debated (e.g. Vallin & Laurin 2004; Anderson 2007; Ruta & Coates 2007), this study concludes that the salamander-like ossification (epiphyseal structure, figure 2b–d; diaphyseal deposition) is a shared primitive trait through the evolution of tetrapods until closer to the amniote crown. Next it will be interesting to complete these results by applying skeletochronology on a growth series of lepospondyls (remaining up to now impossible given the scarcity of material) to further test the different phylogenetic hypotheses.
Acknowledgments
All animals were anaesthetized and killed by deep freezing before dissection and did not belong to the CITES list of protected animals. All procedures were carried out under the ethics guidelines of France.
We thank the Comenius University and Slovak National Museum (Bratislava) for allowing us to section material from their collections, A. Abourachid, G. Clément, H. Francillon-Vieillot, D. Germain, P. Janvier, M. Laurin, M. Lemoine, M.-M. Loth, P. Loubry, A. de Ricqlès, R. Vacant (MNHN, CNRS, Paris), K. Padian (Berkeley), R. Debruyne (Hamilton) and J. S. Anderson (Calgary). S.S. thanks S. Štamberg (Hradec Králové) and J. Schneider (Freiberg). S.S. was funded by a fellowship of the French Ministry of Education and Research, and UMR 5143 & 7179 (CNRS); J.K., by a grant of the Ministry of Education and the Slovak Academy of Sciences (1/3285/06); and J.S.S., by a European Marie-Curie fellowship (HPMF-CT-2002-01797).
Supplementary Material
Material; preparation; assessment of individual age; assessment of sexual maturity; reconstruction of the growth curves
References
- Ahlberg P.E, Milner A.R. The origin and early diversification of tetrapods. Nature. 1994;368:507–514. doi:10.1038/368507a0 [Google Scholar]
- Anderson J.S. Incorporating ontogeny into the matrix: a phylogenetic evaluation of developmental evidence for the origin of modern amphibians. In: Anderson J.S, Sues H.-D, editors. Major transitions in vertebrate evolution. Indiana University Press; Bloomington, IN: 2007. pp. 182–227. [Google Scholar]
- Castanet J. La squelettochronologie chez les reptiles I. Résultats expérimentaux sur la signification des marques de croissance squelettiques chez les lézards et les tortues. Ann. Sci. Nat. Zool. 1985;13:23–40. [Google Scholar]
- Castanet J, Francillon-Vieillot H, Meunier F.-J, de Ricqlès A. Bone and individual aging. In: Hall B.K, editor. Bone. CRC Press; Boca Raton, FL: 1993. pp. 245–283. [Google Scholar]
- Castanet J, Francillon-Vieillot H, Bruce R.C. Age estimation in Desmognathine salamanders assessed by skeletochronology. Herpetologica. 1996;52:160–171. [Google Scholar]
- Castanet J, Francillon-Vieillot H, de Ricqlès A. The skeletal histology of the Amphibia. In: Heatwole H, Davies M, editors. Amphibian biology. Surrey Beatty & Sons; Chipping Norton, UK: 2003. pp. 1598–1683. [Google Scholar]
- Currey J.D, Alexander R.M. The thickness of the walls of tubular bones. J. Zool. 1985;206:453–468. [Google Scholar]
- de Ricqlès A. La formation des os longs des membres de Pleurodeles waltlii (Michahelles)—Deuxième partie. Bull. Soc. Zool. Fr. 1965;90:267–286. [Google Scholar]
- de Ricqlès A. Recherches paléohistologiques sur les os longs des tétrapodes—VI.- Les Stégocéphales. Ann. Paléont. 1981;67:141–157. [Google Scholar]
- de Ricqlès A, Meunier F.-J, Castanet J, Francillon-Vieillot H. Comparative microstructures of bone. In: Hall B.K, editor. Bone matrix and bone specific products. CRC Press; London, UK: 1991. pp. 1–78. [Google Scholar]
- Francillon-Vieillot H, de Buffrenil V, Castanet J, Géraudie J, Meunier F.-J, Sire J.-Y, Zylberberg L, de Ricqlès A. Microstructure and mineralization of vetebrate skeletal tissues. In: Carter J.G, editor. Skeletal biomineralization: patterns, processes and evolutionary trends. Van Nostrand Reinhold; New York, NY: 1990. pp. 471–530. [Google Scholar]
- Germain D, Laurin M. Microanatomy of the radius and lifestyle in amniotes (Vertebrata, Tetrapoda) Zool. Scripta. 2005;34:335–350. doi:10.1111/j.1463-6409.2005.00198.x [Google Scholar]
- Haines R.W. The evolution of epiphyses and of endochondral bone. Biol. Rev. 1942;174:267–292. doi:10.1111/j.1469-185X.1942.tb00440.x [Google Scholar]
- Klembara J. The external gills and ornementation of skull roof bones of the Lower Permian tetrapod Discosauriscus (Kuhn 1933) with remarks to its ontogeny. Paläontol. Z. 1995;69:265–281. [Google Scholar]
- Klembara J. The cranial anatomy of Discosauriscus Kuhn, a seymouriamorph tetrapod from the Lower Permian of the Boskovice Furrow (Czech Republic) Phil. Trans. R. Soc. B. 1997;352:257–302. doi:10.1098/rstb.1997.0021 [Google Scholar]
- Klembara J, Bartík I. The postcranial skeleton of Discosauriscus Kuhn, a seymouriamorph tetrapod from the Lower Permian of the Boskovice Furrow (Czech Republic) Trans. R. Soc. Edinb. Earth Sci. 2000;90:287–316. [Google Scholar]
- Klembara J, Martens T, Bartík I. The postcranial remains of a juvenile seymouriamorph tetrapod from the Lower Permian Rotliegend of the Tambach Formation of Central Germany. J. Vert. Paleontol. 2001;21:521–527. doi:10.1671/0272-4634(2001)021[0521:TPROAJ]2.0.CO;2 [Google Scholar]
- Makowsky A. Über einen neuen Labyrinthodonten “Archegosaurus austriacus nov. spec.”. Sitzungsber. Akad. Wiss. 1876;73:155–166. [Google Scholar]
- Montori A. Skeletochronological results in the pyrenean newt Euproctus asper (Dugès, 1852) from one prepyrenean population. Ann. Sci. Nat. Zool. 1990;11:209–211. [Google Scholar]
- Padian K, de Ricqlès A, Horner J.R. Dinosaurian growth rates and bird origins. Nature. 2001;412:405–408. doi: 10.1038/35086500. doi:10.1038/35086500 [DOI] [PubMed] [Google Scholar]
- Ruta M, Coates M.I. Dates, nodes and character conflict: adressing the Lissamphibian origin problem. J. Syst. Palaeontol. 2007;5:69–122. doi:10.1017/S1477201906002008 [Google Scholar]
- Sanchez S, Steyer J.-S, de Ricqlès A, Schoch R.R. Life history-traits of Apateon (Lower Permian of Europe), a key-genus among dissorophoids, revealed by bone histology. J. Vert. Paleontol. 2007;27(Suppl. 3):139A. [Google Scholar]
- Schoch R.R. Can metamorphosis be recognised in Palaeozoic amphibians? Neues Jb. Geol. Paläontol. Abh. 2001;220:335–367. [Google Scholar]
- Schoch R.R. The evolution of metamorphosis in temnospondyls. Lethaia. 2002;35:309–327. doi:10.1080/002411602320790634 [Google Scholar]
- Steyer J.-S. Ontogeny and phylogeny in temnospondyls: a new method of analysis. Zool. J. Linn. Soc. 2000;130:449–467. doi:10.1006/zjls.1999.0220 [Google Scholar]
- Sumida S.S, Martin K.L.M. Academic Press; San Diego, CA: 1997. Amniote origins. [Google Scholar]
- Vallin G, Laurin M. Cranial morphology and affinities of Microbrachis, and a reappraisal of the phylogeny and lifestyle of the first amphibians. J. Vert. Paleontol. 2004;24:56–72. doi:10.1671/5.1 [Google Scholar]
- Ziegler A.M. Phytogeographic patterns and continental configurations during the Permian Period. In: McKerrow W.S, Scotese C.R, editors. Palaeozoic palaeogeography and biogeography. The Geological Society; London, UK: 1990. pp. 363–379. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Material; preparation; assessment of individual age; assessment of sexual maturity; reconstruction of the growth curves