Abstract
Unrelated same-sex individuals pairing together and cooperating to raise offspring over many years is a rare occurrence in the animal kingdom. Cooperative breeding, in which animals help raise offspring that are not their own, is often attributed to kin selection when individuals are related, or altruism when individuals are unrelated. Here we document long-term pairing of unrelated female Laysan albatross (Phoebastria immutabilis) and show how cooperation may have arisen as a result of a skewed sex ratio in this species. Thirty-one per cent of Laysan albatross pairs on Oahu were female–female, and the overall sex ratio was 59% females as a result of female-biased immigration. Female–female pairs fledged fewer offspring than male–female pairs, but this was a better alternative than not breeding. In most female–female pairs that raised a chick in more than 1 year, at least one offspring was genetically related to each female, indicating that both females had opportunities to reproduce. These results demonstrate how changes in the sex ratio of a population can shift the social structure and cause cooperative behaviour to arise in a monogamous species, and they also underscore the importance of genetically sexing monomorphic species.
Keywords: cooperation, same-sex pairing, female–female pairing, Laysan albatross, sex ratio, seabirds
1. Introduction
Unrelated same-sex individuals pairing together and cooperating to raise offspring over many years is a rare occurrence in the animal kingdom (Bagemihl 1999). In birds, female–female pairs have occurred in socially monogamous species with bi-parental care when there was a female-biased sex ratio (Hunt & Hunt 1977; Conover & Hunt 1984; Nisbet & Hatch 1999). Female–female pairing has not been documented previously in albatross, which is often regarded as an icon of monogamy. Laysan albatross (Phoebastria immutabilis) are indeed characterized by their social monogamy, longevity and obligate bi-parental care (Whittow 1993). However, unlike other bird species in which female–female pairs have been documented, Laysan albatross can only lay and incubate one egg per year, and will not re-lay if the egg is lost (Fisher 1971; Ryan et al. 2007).
Here we document the first record of cooperative breeding in Laysan albatross, in which unrelated females paired with one another for multiple years and cooperated in chick rearing. For unrelated female–female pairing to arise in a population, a surplus of females is often a prerequisite. For this phenomenon to persist in a population, two criteria must be met. First, females in same-sex pairs must have higher fitness than females that do not attempt to nest. Second, both females in a pair should have opportunities to reproduce during the period for which the pair persists, i.e. females must reciprocate (Ligon & Ligon 1978; Nowak et al. 2004). Examination of the circumstances in which female–female pairing occurred in Laysan albatross can provide insights into the mechanisms through which cooperative breeding may arise in other systems.
2. Material and methods
(a) Study locations and sampling
Laysan albatross at Kaena Point Natural Area Reserve on Oahu, Hawaii, were monitored from 2004 to 2007 for the duration of the breeding season (Young & VanderWerf 2008). Each bird was ringed and a 400 μl blood sample was collected from the tarsal vein. At Kilauea Point National Wildlife Refuge on Kauai, feather samples were collected from a portion of breeding pairs that had a history of two-egg clutches to confirm whether they were female–female pairs. In 2007, all birds on both Oahu and Kauai were observed daily during the entire egg-laying period to determine the order of egg laying and the fate of all eggs laid. Individuals that shared incubation of an egg and feeding of a chick were considered to be a pair. Hatching (percentage of eggs incubated and hatched), fledging (percentage of chicks hatched and fledged) and reproductive success (percentage of eggs that resulted in chicks fledged) were compared using Χ2 tests.
(b) DNA extraction, amplification, sexing and genotyping
DNA was extracted using ID Labs DNA isolation kit eluted in 50 μl of H2O. All individuals were independently sexed at least twice using protocols described by Fridolfsson & Ellegren (1999) and all putative female–female pairs were sexed four times. Thirty-six microsatellite loci were screened (Burg 1999; Abbott & Double 2003; Dubois et al. 2005) and five loci were selected, which were polymorphic from 4 to 11 alleles. Reaction conditions and microsatellite characteristics can be found in the electronic supplementary material. All loci were scored independently at least twice with GeneMapper (Applied Biosystems) and tested for departures from the Hardy–Weinberg equilibrium, linkage disequilibrium and null alleles in GenePop (Raymond & Rousset 1997) and Cervus v. 2.0 (Marshall et al. 1998); no locus showed significant deviations from the above assumptions.
(c) Relatedness and parentage analysis
Relatedness values were calculated with Kinship v. 1.2 (Queller & Goodnight 1989) for all female–female pairs using a log-likelihood ratio which tests the null hypothesis that the individuals are unrelated. Parentage assignments were done in Cervus v. 2.0 (Marshall et al. 1998) using 2 candidate mothers and 70 candidate fathers (all males in the colony), and confirmed whether parents shared an allele at each locus with the chick.
3. Results
Genetic sexing revealed that 31% (N=39/125) of Laysan albatross nests on Oahu from 2004 to 2007 were attended by female–female pairs (figure 1). The proportion of female–female pairs was similar among years. The presence of two eggs in 44% of female–female nests indicated that in some instances both pair members laid an egg, but only one egg was incubated in each case. Eggs that were not incubated either became buried within the nest cup or accidentally rolled out of the nest when the pairs switched incubation duties. All females in these pairs were unrelated (p>0.05 for all pairwise comparisons). Female–female pairs were also present in Laysan albatross on Kauai (N=18).
Figure 1.
A female–female Laysan albatross pair at Kaena Point.
A shortage of males on Oahu was confirmed by genetic sexing of all adult birds in the colony, which revealed a female-biased sex ratio (59%, N=102/172). Paternity analysis showed that 10 of the 16 chicks produced by female–female pairs were fathered by paired males, probably due to a scarcity of unpaired males at the colony during the copulatory period. This observation also provides confirmation of extra-pair copulations in Laysan albatross. Fathers of chicks in female–female pairs were located at varying distances from the nest and were not simply the nearest neighbours. No male fathered more than three of the chicks during the course of the study, indicating that females did not prefer any one male.
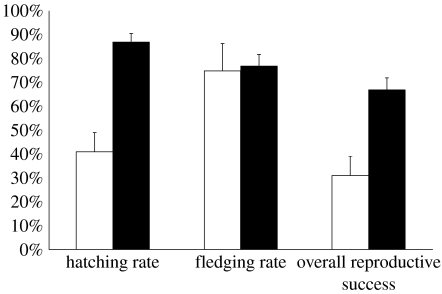
Females in same-sex pairs had higher reproductive success than those that did not attempt to nest and thus did not reproduce, but they had lower reproductive success than male–female pairs (figure 2). Fledging rates were similar in female–female and male–female pairs, indicating comparable ability to care for chicks, but hatching success was lower in female–female pairs, leading to lower overall reproduction (figure 2).
Figure 2.
Reproductive success of female–female and male–female Laysan albatross pairs on Oahu. Female–female pairs (open bars, N=39) had a lower hatching rate (41% versus 87%, Χ12=28.90, p<0.001), a similar fledging rate (75% versus 77%, Χ12=0.04, p=0.84), but lower overall reproductive success (31% versus 67%, Χ12=14.65, p<0.001) than male–female pairs (filled bars, N=86).
Maternity analyses revealed that three out of five female–female pairs that fledged chicks in more than 1 year raised at least one chick from each female, indicating that both females had opportunities to reproduce during the study and thus cooperated. Almost half of the female–female pairs on Oahu were together for the duration of this study (4 years). Several pairs on Kauai have been together for at least 8 years, and one pair on Kauai has been together for 19 years (B. Zaun 2007, unpublished data), indicating that female–female pairing is a long-term strategy.
4. Discussion
Here we have reported the first record, to our knowledge, of female–female pairing and cooperative breeding in albatross and in the order Procellariiformes. This is the first study of female–female pairing in birds to combine multiple years of reproductive outcomes with parentage of the offspring produced and relatedness of the pair members. The proportion of pairs exhibiting this behaviour in Laysan albatross (31%) was more than double the highest proportion of female–female pairing previously known in any animal (14% in western gulls, Larus occidentalis; Hunt & Hunt 1977). As in the previously reported cases (Hunt & Hunt 1977; Conover & Hunt 1984; Nisbet & Hatch 1999), a female-biased sex ratio appeared to be the driving force behind this phenomenon. We therefore suggest that a skewed sex ratio may be causing this behaviour, and that the natural tendency towards social monogamy in this species is what causes it to persist.
The Laysan albatross colony on Oahu is only 17 years old, and while recruitment of chicks hatched at the colony is beginning to occur, most of the increase in colony size was due to immigration (L. Young 2007, unpublished data). The majority of immigrants thus far have been females, and the female–female pairs are almost certainly a by-product of a female-biased sex ratio. This hypothesis is supported by the presence of female–female pairs on Kauai where the colony is of similar age and has a similar immigration rate (B. Zaun 2007, unpublished data), and by the lack of female–female pairs on Guadalupe Island (B. Henry 2008, personal communication) and in larger colonies (Whittow 1993), which have even sex ratios and low immigration rates.
Owing to the stable long-term pair bond exhibited by albatross, female–female pairs may persist in this population past the point when the sex ratio evens out. This work demonstrates that not only is cooperation a long-term strategy in this population of Laysan albatross, but also that a lengthy time period is actually required for cooperation to be adaptive in this species by allowing each female the opportunity to reproduce. Although female–female pairing results in lower reproductive success than male–female pairing, in situations where males are in short supply, female–female pairing in the interim appears to make the best of a bad job.
Many questions remain surrounding the mechanism behind female–female pairing; additional data on chick parentage and long-term fitness are needed to better understand the nature and adaptive value of this strategy. Despite these uncertainties, these results still provide insight into how changes in the sex ratio of a population can shift the social structure and cause cooperative behaviour to arise. In other species of albatross, skewed sex ratios have been achieved through both natural and anthropogenically influenced differences in mortality (Mills & Ryan 2005; Awkerman et al. 2007). With sea-level rise threatening, more than 65% of seabird breeding sites in the Hawaiian archipelago (Baker et al. 2006) and other low lying tropical atolls, colonization events will become increasingly common as displaced birds search for safe locations to breed. Whether a skew in sex ratio is due to differential mortality or dispersal, there exists an opportunity to study the appearance of cooperation and changes in mating systems.
Acknowledgments
All research was conducted under necessary state, federal and institutional protected wildlife permits.
We thank B. Bowen., S. Conant, K. Hayes, D. Kapan, S. Karl, J. Reece, A. Taylor and two anonymous reviews for their helpful comments on the manuscript, D. Carlon for laboratory space, C. Lippe and N. Yeung for their help with laboratory work and analyses, B. Henry for unpublished data from Guadalupe Island and the Hawaii Natural Area Reserves Commission for permission to conduct research at Kaena Point. L.C.Y is funded by the Kilauea Point Natural History Association, and the University of Hawaii Ecology, Evolution and Conservation Biology program research award through NSF grant DGE02-32016 to K. Y. Kaneshiro.
Supplementary Material
Characteristics of dinucleotide microsatellite loci
References
- Abbott C.L, Double M.C. Genetic structure, conservation genetics, and evidence of speciation by range expansion in shy and white-capped albatrosses. Mol. Ecol. 2003;12:2953–2962. doi: 10.1046/j.1365-294x.2003.01980.x. doi:10.1046/j.1365-294X.2003.01980.x [DOI] [PubMed] [Google Scholar]
- Awkerman J.A, Hobson K.A, Anderson D.J. Isotopic (δ15N and δ13C) evidence for intersexual foraging differences and temporal variation in habitat use in waved albatrosses. Can. J. Zool. 2007;85:273–279. doi:10.1139/Z06-202 [Google Scholar]
- Bagemihl B. St. Martins' Press; New York, NY: 1999. Biological exuberance. Animal homosexuality and natural diversity. [Google Scholar]
- Baker J.D, Littman C.L, Johnston D.W. Potential effects of sea level rise on the terrestrial habitats of endangered and endemic megafauna in the Northwestern Hawaiian Islands. Endanger. Species Res. 2006;4:1–10. doi:10.3354/esr002021 [Google Scholar]
- Burg T.M. Isolation and characterization of microsatellites in albatrosses. Mol. Ecol. 1999;8:338–341. doi:10.1046/j.1365-294X.1999.00534.x [PubMed] [Google Scholar]
- Conover M.R, Hunt G.L., Jr Experimental evidence that female–female pairs in gulls result from a shortage of breeding males. Condor. 1984;86:472–476. doi:10.2307/1366828 [Google Scholar]
- Dubois M.-P, Jarne P, Jouventin P. Ten polymorphic microsatellite markers in the wandering albatross Diomedea exulans. Mol. Ecol. Notes. 2005;5:905–907. doi:10.1111/j.1471-8286.2005.01108.x [Google Scholar]
- Fisher H.I. The Laysan Albatross: its incubation, hatching, and associated behaviors. Living Bird. 1971;10:19–78. [Google Scholar]
- Fridolfsson A.K, Ellegren H. A simple and universal method for molecular sexing of non-ratite birds. J. Avian Biol. 1999;30:116–121. doi:10.2307/3677252 [Google Scholar]
- Hunt G.L, Jr, Hunt M.W. Female–female pairing in Western Gulls (Larus occidentalis) in Southern California. Science. 1977;196:1466–1467. doi: 10.1126/science.196.4297.1466. doi:10.1126/science.196.4297.1466 [DOI] [PubMed] [Google Scholar]
- Ligon J.D, Ligon S.H. Communal breeding in the green woodhoopoes as a case of reciprocity. Nature. 1978;276:496–498. doi:10.1038/276496a0 [Google Scholar]
- Marshall T.C, Slate J, Kruuk L.E.B, Pemberton J.M. Statistical confidence for likelihood-based paternity inference in natural populations. Mol. Ecol. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. doi:10.1046/j.1365-294x.1998.00374.x [DOI] [PubMed] [Google Scholar]
- Mills M.S.L, Ryan P.G. Modelling impacts of long-line fishing: what are the effects of pair-bond disruption and sex-biased mortality on albatross fecundity? Anim. Conserv. 2005;8:359–367. doi:10.1046/j.0021-8901.2001.00661.x [Google Scholar]
- Nisbet I.C.T, Hatch J.J. Consequences of a female-biased sex-ratio in a socially monogamous bird: female–female pairs in the Roseate Tern Sterna dougallii. Ibis. 1999;141:307–320. doi:10.1111/j.1474-919X.1999.tb07553.x [Google Scholar]
- Nowak M.A, Sasaki A, Taylor C, Fudenber D. Emergence of cooperation and evolutionary stability in finite populations. Nature. 2004;428:646–650. doi: 10.1038/nature02414. doi:10.1038/nature02414 [DOI] [PubMed] [Google Scholar]
- Queller D.C, Goodnight K.F. Estimating relatedness using genetic markers. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. doi:10.2307/2409206 [DOI] [PubMed] [Google Scholar]
- Raymond M, Rousset F. GenePop (version 3.1b). An updated version of GenePop version 1.2: population genetics software for exact tests and ecumenicism. J. Hered. 1997;86:248–249. http://jhered.oxfordjournals.org/cgi/reprint/86/3/248 [Google Scholar]
- Ryan P.G, Cuthbert R, Cooper J. Two-egg clutches among albatrosses. Emu. 2007;107:210–213. doi:10.1071/MU07018 [Google Scholar]
- Whittow G.C. Laysan albatross (Phoebastria immutabilis) In: Poole A, Gill F, editors. The birds of North America, no. 66. The Academy of Natural Sciences and The American Ornithologists' Union; Washington, DC: 1993. pp. 1–20. [Google Scholar]
- Young L.C, VanderWerf E.A. Prevalence of avian pox virus and effect on fledging success in Laysan albatross. J. Field Ornithol. 2008;79:93–98. doi:10.1111/j.1557-9263.2008.00149.x [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of dinucleotide microsatellite loci