Abstract
The functions of sleep remain an unresolved question in biology. One approach to revealing sleep's purpose is to identify traits that explain why some species sleep more than others. Recent comparative studies of sleep have identified relationships between various physiological, neuroanatomical and ecological traits, and the time mammals spend in rapid eye movement (REM) and non-REM sleep. However, owing to technological constraints, these studies were based exclusively on animals in captivity. Consequently, it is unclear to what extent the unnatural laboratory environment affected time spent sleeping, and thereby the identification and interpretation of informative clues to the functions of sleep. We performed the first electroencephalogram (EEG) recordings of sleep on unrestricted animals in the wild using a recently developed miniaturized EEG recorder, and found that brown-throated three-toed sloths (Bradypus variegatus) inhabiting the canopy of a tropical rainforest only sleep 9.63 h d−1, over 6 h less than previously reported in captivity. Although the influence of factors such as the age of the animals studied cannot be ruled out, our results suggest that sleep in the wild may be markedly different from that in captivity. Additional studies of various species are thus needed to determine whether the relationships between sleep duration and various traits identified in captivity are fundamentally different in the wild. Our initial study of sloths demonstrates the feasibility of this endeavour, and thereby opens the door to comparative studies of sleep occurring within the ecological context within which it evolved.
Keywords: non-rapid eye movement sleep, rapid eye movement sleep, sloth, electroencephalogram, captivity
1. Introduction
The functions of sleep remain a topic of active debate in neurobiology (Siegel 2005; Stickgold 2005; Rattenborg et al. 2007). Recent comparative studies aimed at revealing clues to the functions of sleep through identifying traits that explain why some animals sleep more than others have revealed relationships between the time captive mammals spend in rapid eye movement (REM) and non-REM sleep and various physiological, neuroanatomical and ecological traits (Siegel 2005; Lesku et al. 2006, 2008; Savage & West 2007). However, because all previous electroencephalogram (EEG) recordings of sleep were performed on captive animals, it is unclear to what extent the unnatural laboratory environment affected time spent sleeping (Bert et al. 1975; Campbell & Tobler 1984), and thereby the identification and interpretation of informative clues to the functions of sleep (Horne 1988).
The need for electrophysiological studies of animals sleeping in the wild has been emphasized from the onset of comparative sleep research (Allison 1972; Bert et al. 1975). Unfortunately, several obstacles have prevented researchers from measuring non-REM and REM sleep in unrestricted animals in the wild. Notably, these sleep states can only be distinguished reliably from one another and wakefulness by measuring changes in the EEG and electromyogram (EMG) activity. However, traditional invasive procedures used to obtain stable long-term EEG recordings from the surface of the brain require general anaesthesia and prolonged post-operative recovery, and are therefore unsuitable for field studies wherein the aim is to measure sleep under the most natural conditions possible. Moreover, until recently, lightweight neurophysiological recording systems that animals could carry easily in the field were not readily available.
We resolved these problems by using a minimally invasive EEG and EMG recording technique developed for use in humans (see methods in the electronic supplementary material) in conjunction with a recently developed miniature neurophysiological data recorder (Vyssotski et al. 2006). We deployed this technology on wild brown-throated three-toed sloths (Bradypus variegatus) inhabiting the rainforest at the Smithsonian Tropical Research Institute field station on Barro Colorado Island (BCI), Panama. Sloths are particularly interesting because it is commonly believed that they spend an inordinate amount of time sleeping. Indeed, in the only electrophysiological study of their sleep, sloths (B. variegatus) slept 15.85 h d−1 in captivity (Galvão de Moura Filho et al. 1983).
2. Material and methods
Three adult female three-toed sloths were caught in the forest canopy in the daytime using a snare pole and brought to the forest floor where they were immediately fitted with the EEG/EMG data recorder (figure 1a), a radio-telemetry collar and an accelerometer (see methods in the electronic supplementary material). After completing the 1 h procedure, the sloths were released at the base of the tree where they were captured. The Smithsonian Tropical Research Institute's animal care and use committee approved this research.
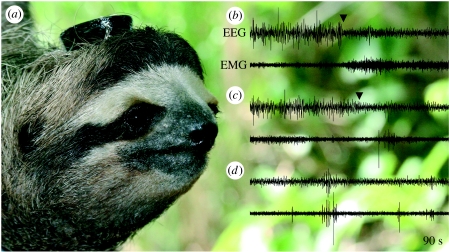
Figure 1.
(a) Photograph of the first brown-throated three-toed sloth (B. variegatus) with an EEG/EMG recorder attached to its head. Three representative 90 s recordings of the EEG and EMG activity from a sloth showing (b) a transition (black arrowhead) from non-REM sleep to wakefulness, (c) a transition (black arrowhead) from non-REM to REM sleep and (d) a period of stable REM sleep. In contrast to wakefulness and REM sleep, the EEG during non-REM sleep exhibits high-amplitude, low-frequency activity. REM sleep is distinguished from wakefulness by the low-amplitude EMG activity, intermittently interrupted by brief high-amplitude twitches. The respective amplitude scales for the EEG and EMG recordings are the same in (b)–(d).
The EEG/EMG was recorded continuously from each sloth for 3.1–5.1 d, for a total of 12.8 d of recording. The state of each sloth was visually scored in 10 s epochs across all 12.8 d of recording, and categorized as wakefulness, non-REM or REM sleep. For figure 2a, time spent in each state was first averaged across the corresponding hours of successive days for each sloth. The hourly means for each sloth were then averaged across all three sloths. Because non-REM and REM sleep were reduced following release for 12 and 24 h, respectively (see results in the electronic supplementary material), data for the first 24 h were excluded. Time spent feeding based on the occurrence of mastication artefacts in the EEG/EMG recording was analysed in a similar manner. A REM sleep episode was defined as a period of REM sleep separated from prior and subsequent REM sleep by more than 5 min of any other state. A sleep cycle was calculated as the cumulative amount of sleep time from the start of one REM sleep episode to the start of the next.
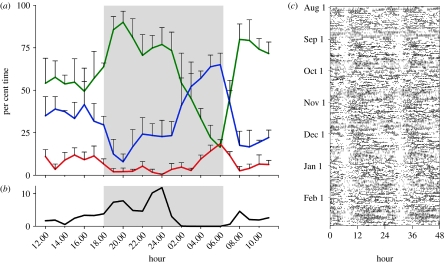
Figure 2.
(a) The per cent time (mean+s.e.m.) spent in wakefulness (green), non-REM sleep (blue) and REM sleep (red) for each hour of the day. (b) The time spent feeding (black) based on the occurrence of mastication artefacts in the EEG/EMG recordings. The values are plotted at the beginning of each hour. Data for the first 24 h following release were omitted. The area shaded in grey shows the time from sunset (18.00) to sunrise (06.20). (c) Actogram showing seven months of activity recorded from a sloth with an automated radio-telemetry system. Each horizontal line is data from one 24 hour period plotted twice. The black lines indicate periods when activity was detected. Note that the two white vertical bands at 6 and 30 h correspond to the clear peak in sleep (a) and absence of feeding (b) occurring at this time. See methods in the electronic supplementary material.
Finally, the activity patterns of two additional adult female three-toed sloths were recorded continuously for approximately seven months (August 2006–February 2007) via radio collars and the automated radio-telemetry system (ARTS) in place on BCI (see methods in the electronic supplementary material).
3. Results
The quality of the EEG/EMG recordings remained high throughout the experiment, and each brain state was readily identifiable (figure 1b–d) and remarkably similar to that described in captive sloths of the same species (Galvão de Moura Filho et al. 1983). (Bradypus tridactylus reported in Galvão de Moura Filho et al. (1983) were captured in the State of Pernambuco, Brazil, where they are now considered to be B. variegatus (see Gilmore et al. 2000; Gardner 2005).) Wakefulness was characterized by low-amplitude, high-frequency EEG activity occurring in conjunction with high levels of EMG activity superimposed on the posterior EEG recordings (figure 1b). Sustained periods of wakefulness often included periods of stereotypical rhythmic (1.54±0.03 Hz) high-amplitude bursts of EMG activity that obscured all recordings. In the previous study of captive sloths (Galvão de Moura Filho et al. 1983), this unique activity occurred in conjunction with rapid mastication during feeding. Consequently, this pattern was scored as wakefulness and used to estimate time spent feeding. During non-REM sleep (figure 1b,c), EMG activity decreased and the EEG showed a progressive increase in amplitude and decrease in frequency. Sleep spindles (8–12 Hz) were also evident. Non-REM sleep always developed simultaneously in the left and right hemispheres. REM sleep was characterized by low-amplitude, mixed-frequency EEG activity (figure 1b,c). As in the previous study of sloths, a hippocampal theta rhythm was not observed during REM sleep or wakefulness. EMG activity during REM sleep either declined further from the preceding non-REM sleep level or remained unchanged if the preceding non-REM sleep level was already low. In addition, intermittent brief bursts of EMG activity occurred during REM sleep. This phasic activity often exhibited a distinct pattern similar to that characteristic of mastication, but occurring for much shorter durations and with markedly lower amplitude (figure 1d). Non-REM sleep preceded episodes of REM sleep.
The 24 h pattern of sleep and wakefulness is shown in figure 2a. The sloths' sleep patterns parallel the activity patterns recorded for a period of seven months with ARTS (figure 2c; figure 4 of the electronic supplementary material) and in an earlier study of sloths (B. variegatus) on BCI (Sunquist & Montgomery 1973). Notably, although sleep could occur at any time of the day or night, the sloths were more likely to be awake and feeding (figure 2a,b) during the first two-thirds of the night, and sleeping during the last, a pattern also reflected in the activity plots by the relative absence of activity towards the end of the night. The sloths spent 14.37±0.51 h awake, 7.78±0.68 h in non-REM sleep and 1.85±0.27 h in REM sleep, for a total sleep time of 9.63±0.51 h. REM sleep encompassed 19.45±3.53% of the total sleep time. Episodes of REM sleep lasted 7.82±0.88 min and the sleep cycle was 46.24±10.26 min.
4. Discussion
Our results from wild sloths inhabiting the canopy of a tropical rainforest demonstrate for the first time that electrophysiologically defined wakefulness, non-REM and REM sleep can be effectively measured using minimally invasive techniques in unrestricted animals in the wild. Interestingly, in contrast to the 15.85 h of sleep shown by captive sloths (Galvão de Moura Filho et al. 1983), sloths in the wild only slept 9.63 h d−1. This discrepancy of over 6 h does not appear to be a response to our recording procedure, because the time spent in non-REM and REM sleep was stable starting 24 h after release (see results in the electronic supplementary material). Differences in the age of the animals in the respective studies may have contributed to the difference in sleep duration. Based on size, the 10 sloths (five males and five females) studied in captivity included an unspecified mix of adults and juveniles (Galvão de Moura Filho et al. 1983). Because juvenile mammals typically sleep longer than adults (Zepelin et al. 2005), the inclusion of juveniles may have increased the mean sleep duration in the study of captive sloths. Although the influence of age and other factors cannot be ruled out, sloths in the wild may sleep less than sloths in captivity due to increased ecological demands such as the need to forage and monitor the environment for predators.
A fundamental assumption in comparative studies of sleep is that the time spent sleeping in captivity reflects a largely inflexible species-specific need for sleep. However, in a critical assessment of comparative sleep studies, Horne (1988) noted that captive animals may sleep longer than required to fulfil their essential need for sleep because they do not have to forage or remain vigilant for predators. Although additional studies are needed to determine whether mammals in general sleep less in the wild, this potential capacity to extend sleep in captivity is a likely source of noise in comparative studies aimed at identifying traits that predict sleep requirements, especially if traits theorized to be linked to sleep function predict whether some species engage in proportionately more excess sleep than others (Allison 1972; Horne 1988). In contrast to animals in captivity, animals in the wild are faced with ecological pressures, particularly predation risk (Lima et al. 2005), that may force them to engage in the minimum amount of sleep required to complete the functions performed by sleep. Given that such niche-adapted sleep may be more directly related to the need for sleep than sleep in captivity, comparative studies of sleep duration in the wild may be more likely to reveal informative clues to the functions of sleep. Consequently, we contend that the comparative approach to understanding the functions of sleep would benefit greatly from additional electrophysiological studies of animals sleeping in the wild. Such studies would at least alleviate persistent concerns over the functional implications of correlations derived from mammals sleeping in captivity if mammals in the wild spend the same amount of time sleeping. Alternatively, if mammals in the wild sleep less, and presumably closer to their essential need for sleep, such studies may provide novel insight into the functions of sleep. Our initial study of sloths sleeping in the canopy of a rainforest demonstrates the feasibility of this endeavour, and thereby heralds a new age of comparative sleep research.
Acknowledgments
The Smithsonian Tropical Research Institute's animal care and use committee approved this research.
We are indebted to Dolores Martinez-Gonzalez and John A. Lesku for their valuable comments on the manuscript. J. A. L. also created the figures and Erich Koch provided excellent technical assistance. We also thank the staff of the Smithsonian Tropical Research Institute field station on BCI, Panama. This work was funded in part by the Max Planck Society and the Frank Levinson Family Foundation.
Supplementary Material
Supplementary methods, results, references and figure captions
Computed tomographic scanner image of a sloth skull showing the electrode placement
Stabilization of sleep patterns following release
Concurrent EEG and activity recordings in a sloth
Long-term activity recorded with ARTS
References
- Allison T. Comparative and evolutionary aspects of sleep. In: Chase M.H, editor. Perspectives in the brain sciences: the sleeping brain. Brain Information Service; Los Angeles, CA: 1972. pp. 1–57. [Google Scholar]
- Bert J, Balzamo E, Chase M, Pegram V. Sleep of baboon, Papio papio, under natural conditions and in laboratory. Electroencephalogr. Clin. Neurophysiol. 1975;39:657–662. doi: 10.1016/0013-4694(75)90079-6. doi:10.1016/0013-4694(75)90079-6 [DOI] [PubMed] [Google Scholar]
- Campbell S.S, Tobler I. Animal sleep—a review of sleep duration across phylogeny. Neurosci. Biobehav. Rev. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. doi:10.1016/0149-7634(84)90054-X [DOI] [PubMed] [Google Scholar]
- Galvão de Moura Filho A.G, Huggins S.E, Lines S.G. Sleep and waking in the three-toed sloth, Bradypus tridactylus. Comp. Biochem. Physiol. A. 1983;76:345–355. doi: 10.1016/0300-9629(83)90336-5. doi:10.1016/0300-9629(83)90336-5 [DOI] [PubMed] [Google Scholar]
- Gardner A. Order Pilosa. In: Wilson D.E, Reeder D.M, editors. Mammal species of the world: a taxonomic and geographic reference. 3rd edn. The Johns Hopkins University Press; Baltimore, MD: 2005. pp. 100–101. [Google Scholar]
- Gilmore D.P, Da-Costa C.P, Duarte D.P. An update on the physiology of two- and three-toed sloths. Braz. J. Med. Biol. Res. 2000;33:129–146. doi: 10.1590/s0100-879x2000000200001. doi:10.1590/S0100-879X2000000200001 [DOI] [PubMed] [Google Scholar]
- Horne J. Oxford University Press; Oxford, UK: 1988. Why we sleep: the function of sleep in humans and other mammals. [Google Scholar]
- Lesku J.A, Roth T.C, II, Amlaner C.J, Lima S.L. A phylogenetic analysis of sleep architecture in mammals: the integration of anatomy, physiology, and ecology. Am. Nat. 2006;168:441–453. doi: 10.1086/506973. doi:10.1086/506973 [DOI] [PubMed] [Google Scholar]
- Lesku J.A, Roth T.C, II, Rattenborg N.C, Amlaner C.J, Lima S.L. Phylogenetics and the correlates of mammalian sleep: a reappraisal. Sleep Med. Rev. 2008 doi: 10.1016/j.smrv.2007.10.003. doi:10.1016/j.smrv.2007.10.003 [DOI] [PubMed] [Google Scholar]
- Lima S.L, Rattenborg N.C, Lesku J.A, Amlaner C.J. Sleeping under the risk of predation. Anim. Behav. 2005;70:723–736. doi:10.1016/j.anbehav.2005.01.008 [Google Scholar]
- Rattenborg N.C, Lesku J.A, Martinez-Gonzalez D, Lima S.L. The non-trivial functions of sleep. Sleep Med. Rev. 2007;11:405–409. doi: 10.1016/j.smrv.2007.04.003. doi:10.1016/j.smrv.2007.04.003 [DOI] [PubMed] [Google Scholar]
- Savage V.M, West G.B. A quantitative, theoretical framework for understanding mammalian sleep. Proc. Natl Acad. Sci. USA. 2007;104:1051–1056. doi: 10.1073/pnas.0610080104. doi:10.1073/pnas.0610080104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J.M. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. doi:10.1038/nature04285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437:1272–1278. doi: 10.1038/nature04286. doi:10.1038/nature04286 [DOI] [PubMed] [Google Scholar]
- Sunquist M.E, Montgomery G.G. Activity patterns and rates of movement of 2-toed and 3-toed sloths (Choloepus hoffmanni and Bradypus infuscatus) J. Mam. 1973;54:946–954. doi:10.2307/1379088 [PubMed] [Google Scholar]
- Vyssotski A.L, Serkov A.N, Itskov P.M, Dell'Omo G, Latanov A.V, Wolfer D.P, Lipp H.P. Miniature neurologgers for flying pigeons: multichannel EEG and action and field potentials in combination with GPS recording. J. Neurophysiol. 2006;95:1263–1273. doi: 10.1152/jn.00879.2005. doi:10.1152/jn.00879.2005 [DOI] [PubMed] [Google Scholar]
- Zepelin H, Siegel J.M, Tobler I. Mammalian sleep. In: Kryger M.H, Roth T, Dement W.C, editors. Principles and practice of sleep medicine. 4th edn. Elsevier Saunders; Philadelphia, PA: 2005. pp. 91–100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods, results, references and figure captions
Computed tomographic scanner image of a sloth skull showing the electrode placement
Stabilization of sleep patterns following release
Concurrent EEG and activity recordings in a sloth
Long-term activity recorded with ARTS