Abstract
Maternal dermatophagy, the eating of maternal skin by offspring, is an unusual form of parental investment involving co-evolved specializations of both maternal skin and offspring dentition, which has been recently discovered in an African caecilian amphibian. Here we report the discovery of this form of parental care in a second, distantly related Neotropical species Siphonops annulatus, where it is characterized by the same syndrome of maternal and offspring specializations. The detailed similarities of skin feeding in different caecilian species provide strong evidence of its homology, implying its presence in the last common ancestor of these species. Biogeographic considerations, the separation of Africa and South American land masses and inferred timescales of amphibian diversification all suggest that skin feeding is an ancient form of parental care in caecilians, which has probably persisted in multiple lineages for more than 100 Myr. These inferences support the hypotheses that (i) maternal dermatophagy is widespread in oviparous direct-developing caecilians, and (ii) that viviparous caecilians that feed on the hypertrophied maternal oviduct evolved from skin-feeding ancestors. In addition to skin-feeding, young S. annulatus were observed to congregate around, and imbibe liquid exuded from, the maternal cloacal opening.
Keywords: dermatophagy, skin, teeth, behaviour, Siphonops
1. Introduction
Kupfer et al. (2006) recently described an unusual form of parental investment in the oviparous and direct-developing East African caecilian amphibian Boulengerula taitanus. In this species, hatchlings are altricial and they remain with their mothers in underground nest chambers, feeding periodically upon the skin of their attending parent until they have grown and developed sufficiently to assume an independent existence. During this extended parental care, the mothers' epidermis is hypertrophied and heavily invested with lipids; the offspring possess distinctive teeth, with multiple cusps, which they use to peel the outermost layer (stratum corneum) of the epidermis of their attending parent.
Foetuses of viviparous caecilians also have a specialized dentition (Parker 1956) that they use to feed on the hypertrophied lining of the maternal oviduct (e.g. Wake & Dickie 1998). Convergent evolution of this complex trait seems implausible and yet inferred phylogenetic relationships imply that viviparity has evolved independently in multiple lineages of caecilians (e.g. Wilkinson & Nussbaum 1998). Kupfer et al. (2006) suggested that the specialized dentition of skin-feeding, oviparous caecilians is homologous to that of oviduct-feeding foetuses of viviparous species and that viviparous caecilians evolved from skin-feeding ancestors. This implies that the evolution of viviparity did not involve the de novo acquisition of the specialized foetal dentition, rendering its convergent evolution more plausible. They also suggested that maternal dermatophagy might be fairly widespread in oviparous caecilians, based primarily on preliminary observations of the Neotropical caecilian Siphonops annulatus. Here we report new behavioural and anatomical observations of this species and consider their significance. We also report an additional form of maternal–offspring interaction previously unknown in any amphibian species.
2. Material and methods
Family groups (one mother, from 5 to 16 young) were maintained in artificial nests covered by half coconut shells that could be readily lifted to enable direct observation. During filming for the BBC's Life in Cold Blood, families were checked at intervals of between 15 and 60 min over a 96 hour period. For six families, body lengths and mass (to the nearest 1 mm and 0.01 g, respectively) were recorded every two days for one week subsequent to collection.
Fragments of dorsal skin of females were fixed in 4% paraformaldehyde in PBS 0.1 M, pH 7.2 for 24 hours, dehydrated in ethanol and embedded in glycol methacylate (Leica). Sections of 2 μm were stained with toluidine blue-fuchsin. In order to preserve lipid content, some skin fragments were postfixed with 1% osmium tetroxide prior to dehydration and embedding and stained with Sudan black B. Tooth morphology was examined with a scanning electron microscope (Hitachi 2500). Samples were transferred through an acetone series and critical point dried using carbon dioxide, mounted on aluminium stubs and sputter coated with gold–palladium.
3. Results
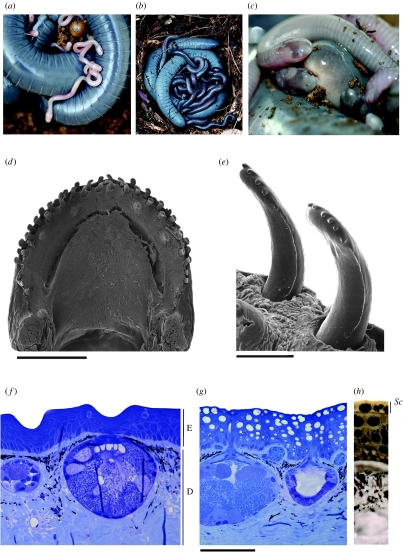
Hatchling S. annulatus are altricial, small (ca 40 mm long) and unpigmented, with heads that are much wider than their bodies (figure 1a). In the wild they grow substantially and develop a more adult pigmentation and body shape while remaining with their mothers (figure 1b). Growth is rapid. For six families that upon capture had between 8 and 16 (mean=13, s.d.=3) young, ranging from 75 to 136 mm (mean=123.2, s.d.=9.08, n=76) total length and 0.9 to 4 g (mean=3.04, s.d.=0.52) mass, litters increased in total length between 0.1 and 0.8% (0.1–0.9 mm) and in body mass between 0.2 and 2.3% (5–47 mg) per day.
Figure 1.
Parental care in S. annulatus. (a) mother and partially hatched clutch showing both eggs and unpigmented altricial hatchlings. (b) Late clutch photographed in the field with well-developed young (the pale colour of the mother most likely indicates she was still skin feeding). (c) Young aggregating around the exposed vent on the elevated body terminus of their mother. (d) View of the lower jaw of a nestling (120 mm total length) showing alternating dental tooth rows. Scale bar, 1.20 mm. (e) Detail of two dentary teeth. Scale bar, 120 μm. Histological comparison of the skin of (f) non-brooding and (g,h) brooding females showing differences in structure and histochemistry. In the latter the epidermis is full of inclusions stained in black after osmium tetroxide and Sudan black B treatment (g,h). E=epidermis, D=dermis, Sc=stratum corneum. Scale bar, 120 μm.
Adult S. annulatus have a single row of gently recurved monocuspid teeth in the lower jaw (e.g. Jared et al. 1999). In contrast, a 120 mm (TL) dermatophagic nestling has 44 spoon-shaped teeth on the lower jaw, 22 on each side, arranged alternately in three rows and each bearing multiple small claw-like cusps distally (figure 1c,d). The lower jaw of a 40 mm (TL) hatchling has only six spoon-shaped teeth with a similar crown form observed in the older young.
The skin of attending mothers is typically much paler than that of other adults (figure 1b). Histological examination indicates that during parental care the epidermal cells are much enlarged (figure 1g,h) and show many inclusions in the cytoplasm with a great affinity for osmium tetroxide and Sudan black B, which indicate the presence of lipids (figure 1h). The number of cell layers, however, remains the same. No other significant changes were observed in the skin.
We initially observed a partial bout of skin feeding in which the young were moving rapidly over and around the mother, biting her skin and peeling the outer layer of it as they moved. Subsequently, we observed and filmed one complete and seven partial bouts of feeding in seven different clutches, once at night and the others during daylight hours. Feeding bouts involve the simultaneous activity of all members of a clutch. Feeding behaviour is quite frenetic with the young frequently tearing pieces of skin by spinning along their long axes and sometimes struggling over the same piece of skin. The mother remains calm during this activity. When the mother has been peeled, the young continue to search for and eat fragments of skin on the substrate. Feeding bouts are short: just seven minutes for the only complete bout observed and interspersed with long periods of quiescence. Skin feeding was seen twice in one family group separated by approximately 64 hours.
We also observed several bouts of a previously unreported form of maternal–offspring interaction (figure 1c), in which a coiled female raised its body terminus vertically, exposing its vent (the opening of the cloaca) laterally, and its offspring aggregated around the vent area, pressing against its surface and sometimes opening and closing their mouths. In several of the observed bouts of this behaviour, we noted that the heads of the young were moist without ascertaining the source of the moisture. In two bouts we observed clear fluid exuded from the maternal cloaca and imbibed by young animals, one watery and another more viscous offering. Edited highlights of this behaviour and of one of these bouts of skin feeding can be found online (http://news.bbc.co.uk/2/hi/science/nature/7235205.stm).
4. Discussion
Following its initial discovery in B. taitanus (Kupfer et al. 2006), our observations clearly demonstrate the presence of maternal dermatophagy in a second species of caecilian. The detailed similarities of the skin-feeding behaviour and of associated anatomical features of mothers and young provide strong evidence of the homology of skin feeding in the East African B. taitanus and the Neotropical S. annulatus, and suggest its presence in their last common ancestor. The fossil record of caecilians is too poor to be relevant to the understanding of this ancestor, but both species have been included in a broad, multi-gene, molecular study of the timescale of amphibian diversification (Roelants et al. 2007). Confidence intervals on divergence date estimates varied slightly with the particular relaxed clock method used and when using an alternative tree (Frost et al. 2006); but all methods placed the timing of the divergence of S. annulatus and B. taitanus at between 156 and 162 Myr ago with 95% confidence intervals spanning 134–186 Myr ago. The timing of the breakup of South America and Africa is controversial (Upchurch 2008), but in most palaeogeographic reconstructions the South Atlantic forms between 100 and 140 Myr ago (Smith et al. 1994; Hay et al. 1999) and would probably have been an effective barrier to the dispersal of most amphibians owing to their salt intolerance. The independent estimates of the minimum age of the divergence of Siphonops and Boulengerula are consistent with the hypothesis that skin feeding is an ancient form of parental care in caecilians that have survived in at least two lineages for more than 100 Myr.
Despite both being caeciliids, S. annulatus and B. taitanus are not particularly closely related. According to molecular phylogenies (Frost et al. 2006; Roelants et al. 2007), they diverged with the deepest/earliest split in Caeciliidae such that all caeciliids are inferred to be descendants of a common skin-feeding ancestor. This is consistent with the hypotheses that caecilian viviparity evolved (repeatedly) from skin feeding and that skin feeding may be quite widespread among oviparous, direct-developing caecilians. Thus we predict that variants of skin feeding will be discovered in many caeciliid species for which there are currently no or only incomplete life-history data, providing a test of our hypotheses and the alternative that maternal dermatophagy is a spectacular convergence between Boulengerula and Siphonops.
The repeated observation of young gathering around the exposed vent of the mother when the body terminus is elevated, and the finding that they sometimes imbibe liquid released from the cloaca are noteworthy. Such behaviour is unknown in other amphibians. We do not know whether the imbibed fluid was urine or something else, and we might postulate many possible functions (e.g. in nutrition, microbial transfer, water balance and/or communication), but the phenomenon requires further study if it is to be understood. That skin feeding may be combined with feeding upon some internally derived fluid in S. annulatus is fascinating and might provide insight into the transition from extracorporeal skin feeding to intracorporeal oviduct feeding in the evolution of caecilian viviparity. If, as we argue, oviduct feeding evolved from skin feeding, we might expect the tissue-specific changes in the gene expression associated with hypertrophy and lipid production (Wake & Dickie 1998) to be the same in both skin and oviduct.
Siphonops annulatus has the broadest distribution of any terrestrial caecilian species, is comparatively well represented in scientific collections and is one of only a handful of caecilians for which there is sufficient information to enable a conservation assessment (as of least concern, Lavilla et al. 2004). Since Göldi (1899) it has been known that it is oviparous, but its parental care has never been previously observed. Although the subterranean lifestyles of caecilians can make observation of their behaviours difficult, recent discoveries suggest that their reproductive diversity merits much further attention.
Acknowledgments
We thank Marli Encarnação and the Comissão Executiva do Plano da Lavoura Cacaueira (CEPLAC, Ilhéus, Brazil) for facilitating the collection of animals (under IBAMA permits 0110/2004—CGFAU/LIC, and 107/05—IBAMA/RAN), and gratefully acknowledge the assistance of José Abade, Luiz F. Santos, Simone Jared, Simon Loader and Hana Suzuki. We thank Ron Nussbaum for helpful discussions and sharing observations that helped direct attention to Siphonops, and we dedicate this work to the memory of Claudio Zamprogno, who was instrumental in the discovery of altriciality in Siphonops.
References
- Frost D.R, et al. The amphibian tree of life. Bull. Am. Mus. Nat. Hist. 2006;297:1–370. doi:10.1206/0003-0090(2006)297[0001:TATOL]2.0.CO;2 [Google Scholar]
- Göldi E.A. Über die Entwicklung von Siphonops annulatus. Zool. Jahrb. (Abt. Syst.) 1899;12:170–173. [Google Scholar]
- Hay W.W, et al. Alternative global Cretaceous paleogeography. Geological Society America Special Paper. 1999;332:1–47. [Google Scholar]
- Jared C, Navas C.A, Toledo R.C. An appreciation of the physiology and morphology of the caecilians (Amphibia: Gymnophiona) Comp. Biochem. Physiol. 1999;123:313–328. doi:10.1016/S1095-6433(99)00076-8 [Google Scholar]
- Kupfer A, Müller H, Jared C, Antoniazzi M, Nussbaum R.A, Greven H, Wilkinson M. Parental investment by skin feeding in a caecilian amphibian. Nature. 2006;440:926–929. doi: 10.1038/nature04403. doi:10.1038/nature04403 [DOI] [PubMed] [Google Scholar]
- Lavilla, E., Hoogmoed, M., Reichle, S., Baldo, D., Wilkinson, M. & Measey, G. J. 2004 Siphonops annulatus In: IUCN 2007. 2007 IUCN Red List Of Threatened Species. See http://www.iucnredlist.org
- Parker H.W. Viviparous caecilians and amphibian phylogeny. Nature. 1956;178:250–252. doi:10.1038/178250a0 [Google Scholar]
- Roelants K, Gower D.J, Wilkinson M, Loader S.P, Biju S.D, Guillaume K, Moriau L, Bossuyt F. Global patterns of diversification in the history of modern amphibians. Proc. Natl Acad. Sci. USA. 2007;104:887–892. doi: 10.1073/pnas.0608378104. doi:10.1073/pnas.0608378104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.G, Smith D.G, Funnell B.M. Cambridge University Press; Cambridge, UK: 1994. Atlas of Mesozoic and Cenozoic Coastlines. [Google Scholar]
- Upchurch P. Godwanan break-up: legacies of a lost world? Trends Evol. Ecol. 2008;23:229–236. doi: 10.1016/j.tree.2007.11.006. doi:10.1016/j.tree.2007.11.006 [DOI] [PubMed] [Google Scholar]
- Wake M.H, Dickie R. Oviduct structure and function and reproducive modes in amphibians. J. Exp. Zool. 1998;282:477–506. doi:10.1002/(SICI)1097-010X(199811/12)282:4/5<477::AID-JEZ6>3.3.CO;2-R [PubMed] [Google Scholar]
- Wilkinson M, Nussbaum R.A. Caecilian viviparity and amniote origins. J. Nat. Hist. 1998;32:1403–1409. doi:10.1080/00222939800770701 [Google Scholar]