Abstract
Vertebrate claws are used in a variety of important behaviours and are typically composed of a keratinous sheath overlying the terminal phalanx of a digit. Keratinous claws, however, are rare in living amphibians; their microstructure and other features indicate that they probably originated independently from those in amniotes. Here we show that certain African frogs have a different type of claw, used in defence, that is unique in design among living vertebrates and lacks a keratinous covering. These frogs have sectorial terminal phalanges on their hind feet that become functional by cutting through the skin. In the resting state, the phalanx is subdermal and attached to a distal bony nodule, a neomorphic skeletal element, via collagen-rich connective tissue. When erected, the claw breaks free from the nodule and pierces the ventral skin. The nodule, suspended by a sheath attached to the terminal phalanx and supported by collagenous connections to the dermis, remains fixed in place. While superficially resembling the shape of claws in other tetrapods, these are the only vertebrate claws known to pierce their way to functionality.
Keywords: Arthroleptidae, functional morphology, limb anatomy, morphological novelty
1. Introduction
Claws and hooves formed by a keratinous sheath covering the terminal phalanges are common among terrestrial vertebrates, but especially amniotes. They are variously employed in locomotion, prey capture, feeding, defence and other behaviours. By contrast, similar structures are rarely present in recent amphibians (Noble 1931). Indeed, the microstructure and growth of the keratinized claws in the pipid frog Xenopus laevis differ from those of amniotes and may provide the evidence of the independent derivation of such claws in the two groups (Maddin et al. 2007). Here we describe a unique form of tetrapod claw. Certain African frogs (Ranoidea: Arthroleptidae) struggle and kick violently when picked up, raking their erectile, bony claws to inflict cuts in their antagonist's skin. Our investigation provides both the first detailed anatomical study and first interpretation of these specialized structures, which have remained enigmatic since their initial discovery more than 100 years ago (Boulenger 1900, 1901, 1918; Mocquard 1902).
2. Material and methods
We examined museum specimens of 63 species in seven arthroleptid frog genera. Morphology of the terminal pedal phalanges was determined from both digital radiographs and skeletal preparations. Skeletal preparations consisted of cleared-and-stained whole mounts in which the ‘soft’ tissues were macerated and cleared; cartilage and bone were then stained using Alcian blue and Alizarin red, respectively (Klymkowsky & Hanken 1992). The presence and distribution of recurved terminal phalanges on the toes as well as the presence of the associated bony nodule were determined for each specimen (see table 1 in the electronic supplementary material).
Images of whole mounts (figure 1a,b) were obtained with a JVC 3-CCD digital camera mounted on a dissecting microscope using AutoMontage Pro v. 5.0 (Synoptics). Images of stained histological sections were obtained using a Hamamatsu digital camera (Model C4742-95) with a Micro-colour filter (Model RGB-MS-C; CRI, Inc.) mounted on a Leica DMRE compound microscope and acquired through Openlab v. 5.0.0 (Improvision). Images were cropped and brightness, coloration and contrast adjusted using Photoshop v. 7.0 (Adobe). In addition to the examination of skeletal preparations, digital radiography was used to determine the skeletal anatomy of a wide range of frogs of the Arthroleptidae. Digital radiographic analysis was conducted using a Thermo Kevex digital X-ray (Model PXS10) in combination with a PaxScan amorphous silicon sensor array (Model 4030R) and ViVa v. 2.0 (Varian Medical Systems, Inc.); specimens were X-rayed using 40 kV.
Figure 1.
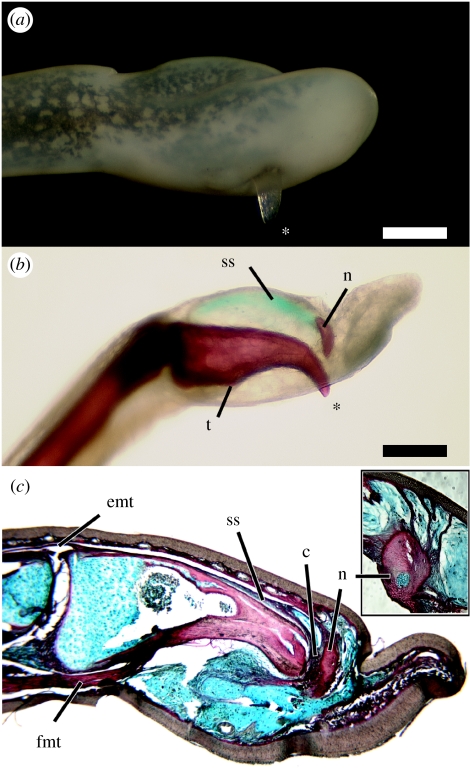
Anatomy of the claws of Astylosternus and Trichobatrachus. (a) Right fourth toe of Astylosternus rheophilus (MCZ A-136934), in lateral view. The exposed bony tip of the terminal phalanx (asterisk) protrudes through the skin on the ventral side. (b) Cleared and stained left fourth toe of Astylosternus laurenti (CAS 158971) in medial view. Bone is red; the suspensory sheath is stained blue. The tendon of the deep digital flexor muscle inserts on a tubercle (t) of the ventral surface of the phalanx. The bony nodule (n) is connected to the proximodorsal surface of the terminal phalanx by a suspensory sheath (ss). (c) Longitudinal section through the left fourth toe of Trichobatrachus robustus (MCZ A-137591) showing the claw in resting state. The terminal phalanx is linked to the bony nodule (n) via collagen-rich tissue (c, red) and is underlain by dense, collagen-poor connective tissue (blue). Activation of the deep digital flexor tendon (fmt) breaks the phalanx free from the bony nodule and exposes the bony tip. The suspensory sheath (ss) connecting the bony nodule to the proximodorsal phalanx shares a common insertion with the tendon of the digital extensor muscle (emt). Inset: longitudinal section through right third toe of T. robustus (MCZ A-137951) with an exposed claw, stained as in c; close-up of the bony nodule. The nodule remains anchored to the dermis via collagenous strands (red). Scale bar, 0.5 mm.
Distal parts of pedal digits of Astylosternus, Trichobatrachus and Leptopelis were amputated from formalin-fixed museum specimens, decalcified overnight in Poly-NoCal (Polysciences, Inc.), and embedded in Paraplast using a Tissue-Tek VIP (Miles Scientific). Ten-micron-thick serial sections were prepared from embedded samples and stained using the following protocol: Clearite (2×, 1 min); 100% EtOH (2×, 2 min); 95% EtOH (1×, 2 min); 70% EtOH (1×, 2 min); 50% EtOH (1×, 2 min); 15% EtOH (1×, 2 min); Mayer haemotoxylin (1×, 5 min); deionized (DI) H20 (2×, 1 min); Alcian blue (1×, 5 min); DI H20 (2×, 1 min); phosphomolybdic acid (1×, 5 min); DI H20 (2×, 1 min); direct red (1×, 5 min); DI H20 (2×, 1 min); 15% EtOH (1×, 2 min); 50% EtOH (1×, 2 min); 70% EtOH (1×, 2 min); 95% EtOH (1×, 2 min); 100% EtOH (2×, 2 min); and Clearite (2×, 1 min). Slides were mounted using mounting medium (Richard-Allan Scientific).
3. Results and discussion
In two genera, Astylosternus and Trichobatrachus, the terminal phalanges of toes II–V are distinctly claw shaped and bear markedly downturned, pointed tips (see table 1 in the electronic supplementary material). In the closely related genus Scotobleps, this feature is variably developed on toes II and III, but other terminal phalanges are conventionally configured as slightly tapering, blunt-ended cylinders. Claw-like phalanges are not present in other closely related genera, including Arthroleptis, Cardioglossa, Nyctibates and Leptodactylodon. We find no evidence of sexual dimorphism in the presence, number or morphology of the terminal phalanges. In addition, these structures are employed similarly by juveniles and adult male and female Astylosternus (D. C. Blackburn 2006, personal observation). Previous authors were aware that the terminal phalanges of Astylosternus and Trichobatrachus sometimes perforated the skin of preserved specimens (figure 1a), though they questioned whether this was pathological or an artefact of preservation (Boulenger 1900; Noble 1931). Noble (1931) was the first to attribute a functional significance to these phalanges in speculating that they may provide a ‘surer grip before leaping’ (p. 517). Durrell (1954) later provided the first report of handling live Trichobatrachus and raised the more likely possibility that these claws are for defence as they can inflict ‘deep bleeding wounds [to] the person holding it’. This claim is verified by Cameroonians who hunt Trichobatrachus for food using long heavy spears (see figure 1 in the electronic supplementary material) or machetes such that they can kill the frogs without handling them and being harmed. Previous authors have made comparisons to the retractile claws of cats (Sanderson 1936; Durrell 1954), but we show that the bony claws of these frogs are different both anatomically and functionally from those in all other vertebrates (Gonyea & Ashworth 1975).
In all cases in which the dermis and epidermis of the toe are preserved intact, claw-like terminal phalanges are associated with a distal bony nodule embedded within the distal toe (figure 1b). The association of the bony nodule with the terminal pedal phalanges is similar to character state 6 of character 119 of Scott (2005; p. 558), which she interprets as the ‘tip [of the phalanx] separated from the body’ of the terminal phalanx. Boulenger (1901) interpreted the bony nodules as ‘hypertrophied intercalary elements’, and thus homologous with the extra digital bones of some tree frogs (Manzano et al. 2007). However, as depicted by Boulenger (1901) and as found in this study, the bony nodule is located distal to the terminal phalanx rather than between the terminal and penultimate phalanges. Because the pedal phalangeal formula is invariant among the genera examined (2-2-3-4-3), we reject the hypothesis that the bony nodule is homologous to the terminal phalanx of ‘non-clawed’ frogs.
We examined in detail the claws of Astylosternus and Trichobatrachus. In the resting (‘retracted’) state, the terminal phalanx lies entirely within the dermal and connective tissue layers of the toe (figure 1c). Between the dermis and the ventral aspect of the phalanx is a mass of loose, predominantly collagen-poor connective tissue. The collagen-rich dermis is thin whereas the epidermis is well developed and thicker on the ventral aspect of the digit tip. This condition is similar to that of the arboreal genus Leptopelis, which is part of the same larger African ranoid frog radiation to which Astylosternus and Trichobatrachus belong (Roelants et al. 2007).
Cortical bone of the proximal half of the terminal phalanx is relatively thin (approx. 0.02 mm), but the cortex begins to thicken in the middle of the phalanx and is thickest near the claw's attenuated tip (approx. 0.13 mm; figure 1c). In the resting state, a nodule distal to the phalanx abuts the claw tip. Both the histology and examination of dried skeletal material (e.g. Trichobatrachus, MCZ A-3368) indicate that this nodule is bony. The phalanx and nodule, which has a cartilaginous core (figure 1c, inset), are linked by collagen-rich tissue (figure 1c). The nodule is embedded in a collagenous suspensory sheath that extends proximally along the upper surface of the phalanx to its proximodorsal edge, where the sheath joins a long digital extensor muscle in a common tendinous insertion on the phalanx (figure 1c). The nodule is anchored further by collagenous strands that connect it to the adjacent dermis (figure 1c, inset). The resting claw is thus supported by an attachment to the nodule, which in turn is anchored by a suspensory sheath and collagenous strands to the dermis. These connections may inhibit the erection of the claw during normal non-defensive behaviours.
A digital flexor muscle inserts via a robust tendon on a tubercle on the ventral surface of the phalanx (figure 1c). We propose that the activation of this muscle flexes the claw that breaks away from the nodule and pierces the ventral skin (figure 1a,b), thus exposing the barb-like tip that is reinforced by cortical thickening. The emergence of the claw causes a traumatic wound in which the skin is torn; there is no consistent pattern in the rupture of the skin across specimens. Because the bony nodule is firmly anchored via collagenous tissue, it remains embedded within the fleshy digit tip when the claw is exposed. Among tetrapods, this defensive mechanism can be compared only to the bony ribs in the salamanders Echinotriton and Pleurodeles, which pierce the skin during defensive display (Leydig 1879; Brodie et al. 1984). Males of many distantly related frog species have bony prepollical or even metacarpal spines that can project through the skin and are probably used in male–male combat (e.g. Wells 2007). However, these secondary sexual characters are not analogous to the erectile claws discussed here as the spines of at least some of these taxa appear to grow through the skin rather than traumatically pierce it (e.g. Petropedetes, Plectrohyla; D. C. Blackburn, 2006, personal observation).
The claws of living specimens of these frogs appear to move in and out of the skin of the digit tip (Durrell 1954; D. C. Blackburn, 2006, personal observation), although it is unclear whether retraction is active, passive or a combination of both. Based on the dissection, the pedal digital extensor musculature of Astylosternus and Trichobatrachus is not more extensively developed than that of other anurans. After becoming erect, the claw may passively return to its resting position. As remarkable regenerative capacity is documented in many amphibians (Gardiner et al. 2002; Brockes & Kumar 2005), the subsequent healing of the epidermis, dermis and connective tissue surrounding the terminal phalanges would be unsurprising, but this has yet to be documented. Similarly, the possible regeneration of the connection between the terminal phalanx and the bony nodule, and thus the return to the resting state of this functional relationship, remains to be evaluated.
These unique claws are comparable with other sectorial claws in shape and in the flexing movement that brings the claw into a functional position. No other vertebrate claw is known, however, that lacks a keratinous sheath, is composed solely of naked bone, and must break free from another skeletal structure to pierce its way to functionality.
Acknowledgments
The authors would like to thank both R. Kerney and A. Herrel for providing helpful comments and R. Kerney for assisting in histology and imaging. The Cameroonian Ministry of Forestry and Wildlife provided permits necessary for portions of this research. Financial support was provided by AmphibiaTree (NSF) and a Putnam Expeditionary Grant (Museum of Comparative Zoology, Harvard University).
Supplementary Material
Supplementary material
Hunting spear and roasted frog
References
- Boulenger G.A. A list of the batrachians and reptiles of Gaboon (French Congo), with descriptions of new genera and species. Proc. Zool. Soc. Lond. 1900;1900:433–456. [Google Scholar]
- Boulenger G.A. Further notes on the African batrachians, Trichobatrachus and Gampsosteonyx. Proc. Zool. Soc. Lond. 1901;1901:709–710. [Google Scholar]
- Boulenger G.A. Aperçu des principes qui doivent régir la classification naturelle des espèces du genre Rana. Bull. Soc. zool. Fr. 1918;43:111–121. [Google Scholar]
- Brockes J.P, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. doi:10.1126/science.1115200 [DOI] [PubMed] [Google Scholar]
- Brodie E.D, Nussbaum R.A, Digiovanni M. Antipredator adaptations of Asian salamanders (Salamandridae) Herpetologica. 1984;40:56–68. [Google Scholar]
- Durrell G. Rupert Hart-Davis Ltd; London, UK: 1954. The Bafut beagles. [Google Scholar]
- Gardiner D.M, Endo T, Bryant S.V. The molecular basis of amphibian limb regeneration: integrating the old with the new. Semin. Cell Dev. Biol. 2002;13:345–352. doi: 10.1016/s1084952102000903. doi:10.1016/S1084952102000903 [DOI] [PubMed] [Google Scholar]
- Gonyea W.J, Ashworth R. The form and function of retractile claws in the Felidae and other representative carnivorans. J. Morphol. 1975;145:229–238. doi: 10.1002/jmor.1051450208. doi:10.1002/jmor.1051450208 [DOI] [PubMed] [Google Scholar]
- Klymkowsky M.W, Hanken J. Whole-mount staining of Xenopus and other vertebrates. Methods Cell Biol. 1992;36:419–441. doi: 10.1016/s0091-679x(08)60290-3. [DOI] [PubMed] [Google Scholar]
- Leydig F. Die Rippenstacheln des Pleurodeles Waltlii. Arch. f. Naturgesch. 1879;45:211–234. [Google Scholar]
- Maddin H.C, Musat-Marcu S, Reisz R.R. Histological microstructure of the claws of the African Clawed Frog, Xenopus laevis (Anura: Pipidae): implications for the evolution of claws in tetrapods. J. Exp. Zool. (Mol. Dev. Evol.) 2007;308B:259–268. doi: 10.1002/jez.b.21145. doi:10.1002/jez.b.21145 [DOI] [PubMed] [Google Scholar]
- Manzano A.S, Fabrezi M, Vences M. Intercalary elements, treefrogs, and the early differentiation of a complex system in the Neobatrachia. Anat. Rec. 2007;290:1551–1567. doi: 10.1002/ar.20608. doi:10.1002/ar.20608 [DOI] [PubMed] [Google Scholar]
- Mocquard M.F. Sur des reptiles et batraciens de l'Afrique orientale anglaise du Gabon et de la Guinée française (region de Kouroussa) Bull. Mus. Hist. Nat. (Paris) 1902;8:404–417. [Google Scholar]
- Noble G.K. McGraw-Hill Book Co; New York, NY: 1931. The biology of the Amphibia. [Google Scholar]
- Roelants K, Gower D.J, Wilkinson M, Loader S.P, Biju S.D, Guillaume K, Moriau L, Bossuyt F. Global patterns of diversification in the history of modern amphibians. Proc. Natl Acad. Sci. USA. 2007;104:887–892. doi: 10.1073/pnas.0608378104. doi:10.1073/pnas.0608378104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson I.T. The amphibians of the Mamfe division, Cameroons—II. Ecology of the frogs. Proc. Zool. Soc. Lond. 1936;1936:165–208. [Google Scholar]
- Scott E. A phylogeny of ranid frogs (Anura: Ranoidea: Ranidae), based on a simultaneous analysis of morphological and molecular data. Cladistics. 2005;21:507–574. doi: 10.1111/j.1096-0031.2005.00079.x. doi:10.1111/j.1096-0031.2005.00079.x [DOI] [PubMed] [Google Scholar]
- Wells K.D. University of Chicago Press; Chicago, IL: 2007. The ecology and behavior of amphibians. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Hunting spear and roasted frog