Abstract
Thick molar enamel is among the few diagnostic characters of hominins which are measurable in fossil specimens. Despite a long history of study and characterization of Paranthropus molars as relatively ‘hyper-thick’, only a few tooth fragments and controlled planes of section (designed to be proxies of whole-crown thickness) have been measured. Here, we measure molar enamel thickness in Australopithecus africanus and Paranthropus robustus using accurate microtomographic methods, recording the whole-crown distribution of enamel. Both taxa have relatively thick enamel, but are thinner than previously characterized based on two-dimensional measurements. Three-dimensional measurements show that P. robustus enamel is not hyper-thick, and A. africanus enamel is relatively thinner than that of recent humans. Interspecific differences in the whole-crown distribution of enamel thickness influence cross-sectional measurements such that enamel thickness is exaggerated in two-dimensional sections of A. africanus and P. robustus molars. As such, two-dimensional enamel thickness measurements in australopiths are not reliable proxies for the three-dimensional data they are meant to represent. The three-dimensional distribution of enamel thickness shows different patterns among species, and is more useful for the interpretation of functional adaptations than single summary measures of enamel thickness.
Keywords: hominin evolution, microtomography, enamel distribution
1. Introduction
Thick molar enamel is among the few diagnostic characters of hominins observable in the fossil record (e.g. Martin 1985; Strait et al. 1997). Relatively thick enamel distinguishes hominins from thin-enamelled African apes, and enamel thickness is discussed in the seminal diagnosis of nearly every newly described hominin taxon (e.g. Leakey et al. 2001; Senut et al. 2001; Brunet et al. 2002). Thick molar enamel is often associated with the mastication of hard or abrasive foodstuffs, although recent evidence indicates that enamel thickness may not relate directly to the primary dietary strategies of hominins (Ungar et al. 2008). Although much is said about thick enamel in hominins, few fossil molars have been measured using standardized techniques. Previous studies employed tooth fragments or cross sections produced manually or via medical CT (e.g. Beynon & Wood 1986; Grine & Martin 1988; Macho & Thackeray 1992; Schwartz et al. 1998). These cross sections were designed to yield proxy measures of the entire tooth crown, since medical imaging techniques were insufficient to record the whole-crown measurements at the time of their development (Martin 1985). These two-dimensional methods prohibit the examination of enamel thickness distribution over the entire molar crown (sensu Kono 2004; Olejniczak et al. 2008a).
Recently, non-destructive three-dimensional micro-CT techniques have been applied to the study of enamel thickness in several taxa, including recent and fossil hominoids (Kono 2004; Tafforeau 2004; Olejniczak et al. 2008a,b), recent humans (Suwa & Kono 2005), Neandertals (Olejniczak et al. 2008c) and fossil Homo sapiens (Smith et al. 2006, 2007). The australopith fossil record, however, has not been systematically studied using modern techniques (e.g. Macchiarelli et al. 2004). Here, we measure molars of two australopith taxa (Australopithecus africanus and Paranthropus robustus), with the aim of documenting fossil hominin enamel thickness using a whole-crown, three-dimensional technique. We also compare two-dimensional sections (analogous to those of previous studies) with three-dimensional data, to assess the impact of methodological differences on the enamel thickness measurements.
2. Material and methods
(a) Study sample
Fully formed and unworn fossil teeth are rare; but three-dimensional measurements require that the entire volume of the enamel cap is present with only minor breakage and attrition. From the Transvaal Museum and University of the Witwatersrand, 18 molars were suitable for measurement (table 1). In some cases, molars from the same individual were included (e.g. STW 421a and STW 421b). Digital reconstructions of broken cervical enamel were made when necessary. Scans were produced with a Sky scan 1172 at 100 kV and 100 mA, with aluminium and copper filters. Voxel dimensions for each specimen were 13.98 μm3. Enamel and dentine were digitally segmented; virtual models depicting the three-dimensional distribution of enamel were created (figure 1). Comparative data for extant primates derive from Olejniczak et al. (2008b); modern human and Neandertal data are from Olejniczak et al. (2008c).
Table 1.
Two-dimensional and three-dimensional enamel thickness data for each australopith specimen.
| taxon | tooth | accession | enamel volume (mm3) | dentine volume (mm3) | EDJ surface area (mm2) | three-dimensional average enamel thickness (mm) | three-dimensional relative enamel thickness | mesial section enamel area (mm2) | mesial section dentine area (mm2) | mesial section EDJ length (mm) | mesial section average enamel thickness (mm) | mesial section relative enamel thickness |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. africanus | mandibular LM2 | STW 3 | 540.81 | 711.18 | 363.86 | 1.49 | 16.65 | 43.48 | 44.18 | 20.89 | 2.08 | 31.32 |
| A. africanus | mandibular LM2 | STW 421B | 503.57 | 589.82 | 409.39 | 1.23 | 14.67 | 31.75 | 61.82 | 24.41 | 1.30 | 16.54 |
| A. africanus | mandibular LM3 | STW 412B | 345.47 | 531.32 | 353.52 | 0.98 | 12.07 | 25.57 | 44.36 | 21.43 | 1.19 | 17.91 |
| A. africanus | mandibular LM3 | STW 529 | 554.49 | 561.71 | 347.45 | 1.60 | 19.34 | 42.57 | 51.64 | 22.10 | 1.93 | 26.81 |
| A. africanus | mandibular LM3 | STW 560B | 551.06 | 777.22 | 394.43 | 1.40 | 15.20 | 39.96 | 63.04 | 24.03 | 1.66 | 20.94 |
| A. africanus | mandibular RM1 | STS 9 | 468.27 | 486.31 | 227.88 | 2.05 | 26.13 | 36.01 | 42.59 | 19.87 | 1.81 | 27.76 |
| A. africanus | mandibular RM1 | STW 421A | 483.31 | 668.33 | 368.77 | 1.31 | 14.99 | 30.32 | 60.37 | 24.88 | 1.22 | 15.69 |
| A. africanus | maxillary LM1 | STS 57 | 382.29 | 491.08 | 240.58 | 1.59 | 20.14 | 33.93 | 46.61 | 22.05 | 1.54 | 22.54 |
| A. africanus | maxillary LM3 | STW 179 | 480.39 | 573.15 | 288.14 | 1.67 | 20.07 | 42.23 | 55.78 | 22.08 | 1.91 | 25.60 |
| P. robustus | mandibular RM1 | DNH 60B | 383.29 | 387.34 | 283.16 | 1.35 | 18.57 | 31.10 | 38.50 | 20.09 | 1.55 | 24.95 |
| P. robustus | mandibular RM1 | DNH 67 | 469.41 | 383.89 | 201.39 | 2.33 | 32.07 | 33.21 | 36.49 | 18.77 | 1.77 | 29.29 |
| P. robustus | mandibular RM1 | SK 3974 | 563.39 | 445.73 | 303.17 | 1.86 | 24.33 | 40.77 | 44.26 | 20.12 | 2.03 | 30.47 |
| P. robustus | mandibular RM2 | DNH 60C | 485.52 | 410.35 | 312.07 | 1.56 | 20.94 | 39.93 | 36.30 | 18.57 | 2.15 | 35.68 |
| P. robustus | maxillary LM1 | SK 102 | 591.98 | 498.18 | 248.18 | 2.39 | 30.09 | 49.81 | 56.79 | 23.10 | 2.16 | 28.61 |
| P. robustus | maxillary LM1 | SK 832 | 642.42 | 544.84 | 381.78 | 1.68 | 20.60 | 50.72 | 54.75 | 23.61 | 2.15 | 29.03 |
| P. robustus | maxillary LM3 | SK 41 | 574.60 | 620.92 | 419.66 | 1.37 | 16.05 | 47.18 | 67.07 | 23.40 | 2.02 | 24.62 |
| P. robustus | maxillary RM3 | SK 31 | 624.15 | 783.59 | 320.62 | 1.95 | 21.12 | 48.50 | 75.04 | 24.90 | 1.95 | 22.49 |
| P. robustus | maxillary RM3 | TM 1517C | 682.82 | 493.60 | 336.20 | 2.03 | 25.70 | 61.54 | 69.90 | 24.28 | 2.53 | 30.31 |
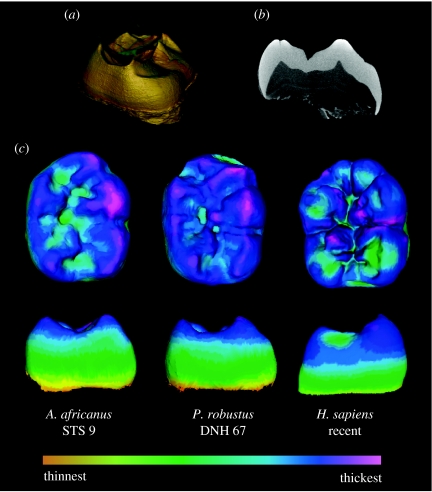
Figure 1.
Micro-CT reconstructions are as follows: (a) segmentation of enamel and dentine; (b) section through the mesial cusps of an Australopithecus molar; enamel over cusp tips in this section is thicker than the lateral enamel; (c) enamel thickness distribution in mandibular molars; the scale of enamel thickness is relative to each tooth. Paranthropus and Australopithecus molars have thicker enamel at the cusp tips than at the lateral aspect of the cusps.
(b) Three-dimensional measurements
Enamel thickness data were recorded for each molar as follows: enamel volume (mm3), coronal dentine volume (mm3) and enamel–dentine junction (EDJ) surface area (mm2). Two indices were calculated as follows: average enamel thickness (three-dimensional AET; the volume of the enamel cap divided by the surface area of the EDJ, yielding the average straight-line distance between the EDJ and the outer enamel surface); and relative enamel thickness (three-dimensional RET; three-dimensional AET divided by the cube root of coronal dentine volume, then multiplied by 100, yielding a scale-free enamel thickness index suitable for intertaxon comparisons).
(c) Two-dimensional measurements
Virtual mesial-cusp planes of section mimicking the physically produced sections of earlier studies (figure 1) were created using VoxBlast software (Vaytek, Inc.); the accuracy of virtual sections and the compatibility of virtual and manually produced sections have been established elsewhere (Olejniczak & Grine 2006). Following Martin (1985), two-dimensional measurements include: enamel cap area (mm2), coronal dentine area (mm2) and EDJ length (mm). Average enamel thickness (two-dimensional AET) is the area of the enamel cap divided by the length of the EDJ, yielding the average straight-line distance from the EDJ to the outer enamel surface. Relative enamel thickness (two-dimensional RET) is two-dimensional AET divided by the square root of coronal dentine area, then multiplied by 100, yielding a scale-free index suitable for interspecies comparisons.
(d) Statistical analysis
Species differences in three-dimensional AET and three-dimensional RET were tested using the rank-based Kruskal–Wallis test with post hoc comparisons. Bootstrap resampling was performed on species mean values of three-dimensional AET and three-dimensional RET to determine confidence intervals (electronic supplementary material (esm)).
3. Results and discussion
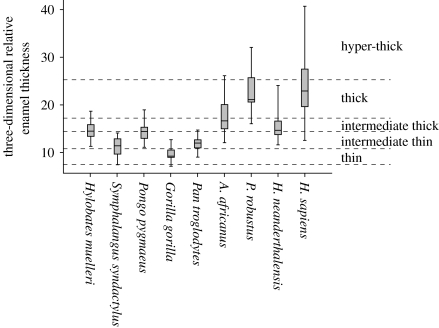
Among hominins, P. robustus has the thickest enamel on average (two-dimensional AET and three-dimensional AET). Interspecies overlap in three-dimensional RET is abundant (figure 2), but statistically significant differences are apparent. Paranthropus robustus has significantly greater three-dimensional RET than all taxa except H. sapiens; A. africanus has significantly thinner three-dimensional RET than other hominins except Homo neanderthalensis (esm). The P. robustus molars derive from three localities and (potentially) three different geological ages, but relative enamel thickness does not appear to differ substantially among localities (table 1). The australopiths evince no clear differences in three-dimensional AET and three-dimensional RET among tooth positions, although small sample sizes prevent rigorous intrataxon analyses.
Figure 2.
Plot depicting three-dimensional RET ranges. Line inside the box represents the mean, ends of boxes are 25th and 75th percentiles and whiskers represent the range. Martin's (1985) thickness categories (and Grine & Martin's 1988 hyper-thick category) are shown to the right, and as dashed lines.
Measurements in two and three dimensions yield different results (table 2). Three-dimensional RET shows that H. sapiens and P. robustus have similarly thick enamel, while A. africanus and H. neanderthalensis have thinner enamel. However, two-dimensional RET is greatest in P. robustus, followed by A. africanus, H. sapiens and H. neanderthalensis, in rank order. Enamel distribution maps show that australopith molar enamel is thickest over cusp tips, while recent humans have thicker enamel surrounding cusp bases (figure 1). Previous studies employed cross sections deliberately made through cusp tips; hence the thickness of molar enamel is exaggerated in those sections. Our two-dimensional results are similar to those of Grine & Martin (1988), but such cross-sectional proxy measures do not reliably predict three-dimensional enamel thickness in these australopiths. The ‘hyper-thick’ category devised to accommodate P. robustus (Grine & Martin 1988) is not warranted when three-dimensional data are considered (figure 2).
Table 2.
Two-dimensional and three-dimensional enamel thickness measurements (mean) in fossil hominins and recent hominoids (ordered on three-dimensional relative enamel thickness).
| H. sapiensa | P. robustus | A. africanus | H. neanderthalenisb | Hylobates muelleric,d | Pongo pygmaeusc,e | Pan troglodytesc,e | Symphalangus syndactylusc,d | Gorilla gorillac,e | |
|---|---|---|---|---|---|---|---|---|---|
| three-dimensional sample size | 39 | 9 | 9 | 29 | 11 | 12 | 26 | 17 | 9 |
| enamel volume (mm3) | 218.91 | 557.51 | 478.85 | 225.47 | 26.37 | 197.91 | 137.00 | 62.29 | 372.01 |
| dentine volume (mm3) | 226.83 | 507.60 | 598.90 | 345.65 | 36.88 | 336.05 | 166.05 | 119.60 | 1023.18 |
| EDJ surface area (mm3) | 162.56 | 311.80 | 332.67 | 211.60 | 53.80 | 199.75 | 182.67 | 116.21 | 375.89 |
| three-dimensional AET (mm) | 1.43 | 1.83 | 1.48 | 1.08 | 0.49 | 1.01 | 0.75 | 0.55 | 0.98 |
| three-dimensional RET | 23.97 | 23.27 | 17.70 | 15.55 | 14.72 | 14.49 | 11.80 | 11.15 | 9.77 |
| Two-dimensional sample size | 257 | 9 | 9 | 42 | 11 | 41 | 40 | 17 | 15 |
| Enamel area (mm2) | 24.19 | 44.75 | 36.20 | 21.97 | 4.44 | 23.42 | 14.63 | 7.30 | 29.37 |
| dentine area (mm2) | 38.73 | 53.23 | 52.27 | 41.65 | 8.47 | 50.93 | 36.95 | 18.50 | 79.29 |
| EDJ length (mm) | 19.60 | 21.87 | 22.42 | 20.75 | 10.13 | 21.34 | 19.47 | 13.53 | 28.25 |
| two-dimensional AET (mm) | 1.22 | 2.03 | 1.63 | 1.06 | 0.44 | 1.10 | 0.75 | 0.54 | 1.04 |
| two-dimensional RET | 20.06 | 28.38 | 22.79 | 16.44 | 15.27 | 15.49 | 13.23 | 12.58 | 11.68 |
Two-dimensional data from Smith et al. (2006).
Data from Olejniczak et al. (2008c).
Three-dimensional data from Olejniczak et al. (2008b).
Two-dimensional data from A. J. Olejniczak (2006, unpublished Ph.D. dissertation).
Two-dimensional data from Smith et al. (2005).
Categories erected by Martin (1985) classify hominin molar enamel as relatively thick compared with that of African apes; this dichotomy is widely employed in studies of late Miocene and early Pliocene taxa (e.g. White et al. 1994; Suwa et al. 2007). Our data partially support a ‘thin’ versus ‘thick’ dichotomy, although overlap between species limits the diagnostic use of three-dimensional RET (figure 2; esm). A range of intermediate thickness encompasses the mean values of H. neanderthalensis and P. troglodytes, and parts of the H. sapiens and A. africanus ranges. This intermediate range also overlaps with the values for Pongo, Hylobates and Symphalangus. Species overlap and ambiguous character polarity (see Kono 2004) render summary enamel thickness indices such as three-dimensional RET ineffective in distinguishing species precisely.
While enamel thickness is not necessarily useful for australopith taxonomy, the pattern of molar enamel distribution may yield valuable data for the interpretation of molar adaptation to diet. Among taxa studied in three dimensions, thick enamel over cusp tips is unique to these australopiths and Gigantopithecus (Olejniczak et al. 2008a); these taxa also exhibit relatively short dentine horns (ESM). Thick cuspal enamel coupled with short dentine horns results in a tabular occlusal surface; this molar configuration probably increases molar longevity in response to abrasive diets in small-object feeders, and prevents the formation of cracks at the enamel–dentine junction in large-object feeders (Lucas et al. 2008). Moreover, our observation of thicker enamel at cusp bases in H. sapiens may relate to the distribution of masticatory forces (e.g. Macho & Spears 1999). Although the australopiths studied here share a similar pattern of enamel thickness distribution, differences in their overall enamel thickness are consistent with previous studies reporting the dietary differences between these species (e.g. Scott et al. 2005).
Acknowledgments
This research is supported by the Max Planck Society, the EVAN Marie Curie Research Training Network MRTN-CT-019564, the Transvaal Museum (S. Potze) and the University of the Witwatersrand (M. Raath). H. Temming provided scanning support.
Supplementary Material
Additional tables, statistical analyses, and figure
References
- Beynon A.D, Wood B.A. Variations in enamel thickness and structure in East African hominids. Am. J. Phys. Anthropol. 1986;70:177–193. doi: 10.1002/ajpa.1330700205. doi:10.1002/ajpa.1330700205 [DOI] [PubMed] [Google Scholar]
- Brunet M, et al. A new hominid from the Upper Miocene of Chad, central Africa. Nature. 2002;418:145–151. doi: 10.1038/nature00879. doi:10.1038/nature00879 [DOI] [PubMed] [Google Scholar]
- Grine F.E, Martin L.B. Enamel thickness and development in Australopithecus and Paranthropus. In: Grine F, editor. Evolutionary history of the “robust” australopithecines. Aldine de Gruyter; New York, NY: 1988. pp. 3–42. [Google Scholar]
- Kono R. Molar enamel thickness and distribution patterns in extant great apes and humans: new insights based on a 3-dimensional whole crown perspective. Anthropol. Sci. 2004;112:121–146. doi:10.1537/ase.03106 [Google Scholar]
- Leakey M.G, Spoor F, Brown F.H, Gathogo P.N, Kiarie C, Leakey L.N, McDougall I. New hominin genus from eastern Africa shows diverse middle Pliocene lineages. Nature. 2001;410:433–440. doi: 10.1038/35068500. doi:10.1038/35068500 [DOI] [PubMed] [Google Scholar]
- Lucas P, Constantino P, Wood B, Lawn B. Dental enamel as a dietary indicator in mammals. BioEssays. 2008;30:374–385. doi: 10.1002/bies.20729. doi:10.1002/bies.20729 [DOI] [PubMed] [Google Scholar]
- Macchiarelli R, et al. Early pliocene hominid tooth from Galili, Somali region, Ethiopia. Coll. Antropol. 2004;28:65–76. [PubMed] [Google Scholar]
- Macho G.A, Spears I.R. Effects of loading on the biochemical behavior of molars of Homo, Pan, and Pongo. Am. J. Phys. Anthropol. 1999;109:211–227. doi: 10.1002/(SICI)1096-8644(199909)110:1<117::AID-AJPA13>3.0.CO;2-R. doi:10.1002/(SICI)1096-8644(199906)109:2<211::AID-AJPA6>3.0.CO;2-B [DOI] [PubMed] [Google Scholar]
- Macho G.A, Thackeray J.F. Computed tomography and enamel thickness of maxillary molars of Plio- Pleistocene hominids from Sterkfontein, Swartkrans, and Kromdraai (South Africa): an exploratory study. Am. J. Phys. Anthropol. 1992;89:133–143. doi: 10.1002/ajpa.1330890202. doi:10.1002/ajpa.1330890202 [DOI] [PubMed] [Google Scholar]
- Martin L.B. Significance of enamel thickness in hominoid evolution. Nature. 1985;314:260–263. doi: 10.1038/314260a0. doi:10.1038/314260a0 [DOI] [PubMed] [Google Scholar]
- Olejniczak A.J, Grine F.E. Assessment of the accuracy of dental enamel thickness measurements using micro-focal X-Ray computed tomography. Anat. Rec. A. 2006;288A:263–275. doi: 10.1002/ar.a.20307. doi:10.1002/ar.a.20307 [DOI] [PubMed] [Google Scholar]
- Olejniczak A.J, Smith T.M, Wang W, Potts R, Ciochon R, Kullmer O, Schrenk F, Hublin J.-J. Molar enamel thickness and dentine horn height in Gigantopithecus blacki. Am. J. Phys. Anthropol. 2008a;135:85–91. doi: 10.1002/ajpa.20711. doi:10.1002/ajpa.20711 [DOI] [PubMed] [Google Scholar]
- Olejniczak A.J, Tafforeau P, Feeney R.N.M, Martin L.B. Three-dimensional primate molar enamel thickness. J. Hum. Evol. 2008b;54:187–195. doi: 10.1016/j.jhevol.2007.09.014. doi:10.1016/j.jhevol.2007.09.014 [DOI] [PubMed] [Google Scholar]
- Olejniczak A.J, et al. Dental tissue proportions and enamel–thickness in Neandertal and modern human molars. J. Hum. Evol. 2008c;55:12–23. doi: 10.1016/j.jhevol.2007.11.004. doi:10.1016/j.jhevol.2007.11.004 [DOI] [PubMed] [Google Scholar]
- Schwartz G.T, Thackeray J.F, Reid C, Van Reenan J.F. Enamel thickness and the topography of the enamel–dentine junction in South African Plio-Pleistocene hominids with special reference to the Carabelli trait. J. Hum. Evol. 1998;35:523–542. doi: 10.1006/jhev.1998.0239. doi:10.1006/jhev.1998.0239 [DOI] [PubMed] [Google Scholar]
- Scott R.S, Ungar P.S, Bergstrom T.S, Brown C.A, Grine F.E, Teaford M.F, Walker A. Dental microwear texture analysis shows within-species diet variability in fossil hominins. Nature. 2005;436:693–695. doi: 10.1038/nature03822. doi:10.1038/nature03822 [DOI] [PubMed] [Google Scholar]
- Senut B, Pickford M, Gommery D, Mein P, Cheboi K, Coppens Y. First hominid from the Miocene (Lukeino Formation, Kenya) Comptes Rendus Acad. Sci. Ser. II-A. 2001;332:137–144. [Google Scholar]
- Smith T.M, Olejniczak A.J, Martin L.B, Reid D.J. Variation in hominoid molar enamel thickness. J. Hum. Evol. 2005;48:575–592. doi: 10.1016/j.jhevol.2005.02.004. doi:10.1016/j.jhevol.2005.02.004 [DOI] [PubMed] [Google Scholar]
- Smith T.M, Olejniczak A.J, Tafforeau P, Reid D.J, Grine F.E, Hublin J.-J. Molar crown thickness, volume, and development in South African Middle Stone Age humans. S. Afr. J. Sci. 2006;102:513–518. [Google Scholar]
- Smith T.M, Tafforeau P, Reid D.J, Grün R, Eggins S, Boutakiout M, Hublin J.-J. Earliest evidence of modern human life history in North African early Homo sapiens. Proc. Natl Acad. Sci. USA. 2007;104:6128–6133. doi: 10.1073/pnas.0700747104. doi:10.1073/pnas.0700747104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strait D.S, Grine F.E, Moniz M.A. A reappraisal of early hominid phylogeny. J. Hum. Evol. 1997;32:17–82. doi: 10.1006/jhev.1996.0097. doi:10.1006/jhev.1996.0097 [DOI] [PubMed] [Google Scholar]
- Suwa G, Kono R.T. A micro-CT based study of linear enamel thickness in the mesial cusp section of human molars: reevaluation of methodology and assessment of within-tooth, serial, and individual variation. Anthropol. Sci. 2005;113:273–289. doi:10.1537/ase.050118 [Google Scholar]
- Suwa G, Kono R.T, Katoh S, Asfaw B, Beyene Y. A new species of great ape from the late Miocene epoch in Ethiopia. Nature. 2007;448:921–924. doi: 10.1038/nature06113. doi:10.1038/nature06113 [DOI] [PubMed] [Google Scholar]
- Tafforeau, P. 2004 Phylogenetic and functional aspects of tooth enamel microstructure and three-dimensional structure of modern and fossil primate molars. Ph.D. dissertation, Université de Montpellier II.
- Ungar P.S, Grine F.E, Teaford M.F. Dental microwear and diet of the Plio-Pleistocene hominin Paranthropus boisei. PLoS ONE. 2008;3:e2044. doi: 10.1371/journal.pone.0002044. doi:10.1371/journal.pone.0002044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.D, Suwa G, Asfaw B. Australopithecus ramidus, a new species of early hominid from Aramis, Ethiopia. Nature. 1994;371:306–312. doi: 10.1038/371306a0. doi:10.1038/371306a0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional tables, statistical analyses, and figure