Abstract
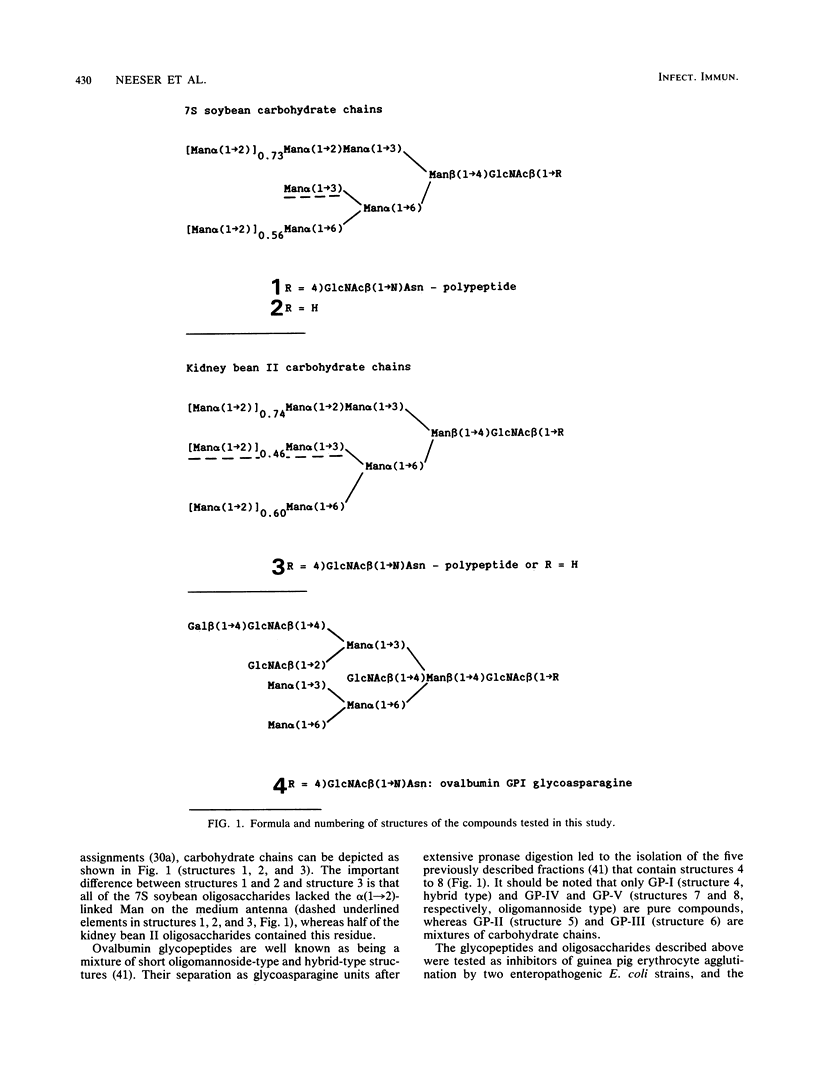
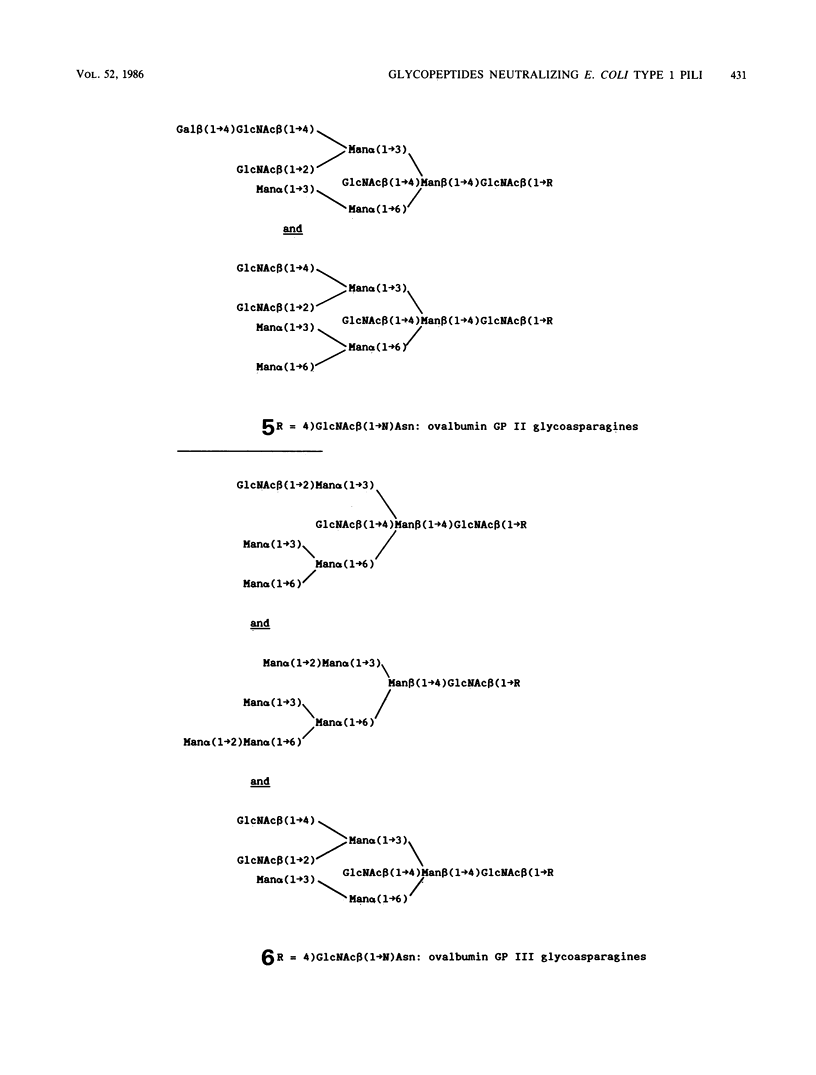
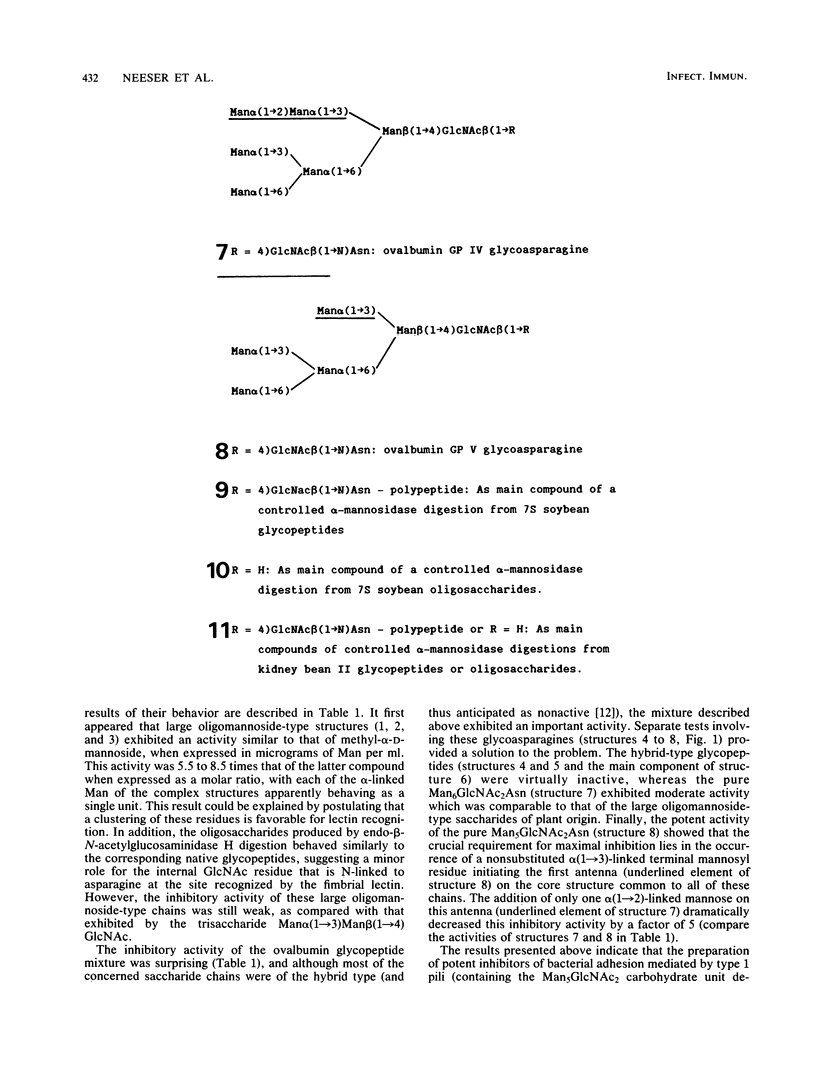
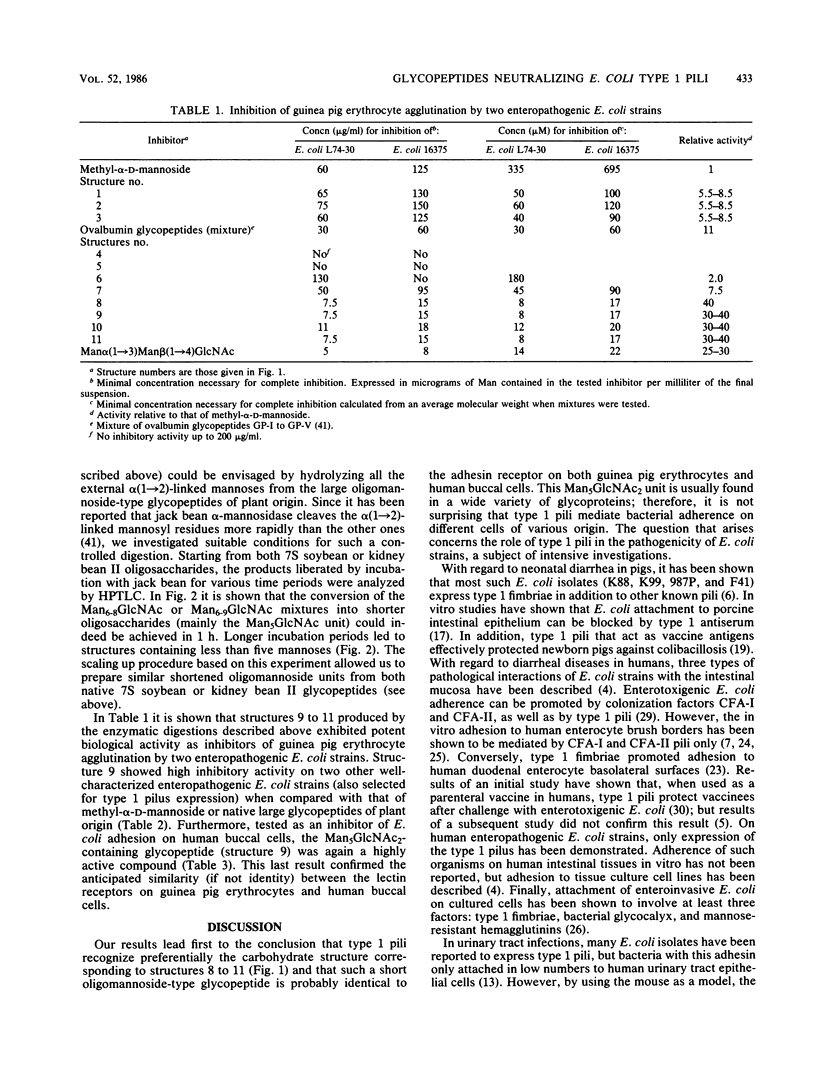
Various structurally defined glycopeptides of natural origin were tested as inhibitors of guinea pig erythrocyte agglutination by enteropathogenic Escherichia coli strains expressing type 1 pili. Besides hybrid-type glycoasparagines from ovalbumin which were not active, large oligomannoside-type carbohydrate chains from legume storage glycoproteins moderately inhibited hemagglutinations, whereas the short oligomannoside-type glycoasparagine from ovalbumin Man alpha(1----6) [Man alpha(1----3)]Man alpha(1----6)[Man alpha(1----3)] Man beta(1----4)GlcNAc beta(1----4)GlcNAc beta(1----N)Asn exhibited a potent activity. These results strongly suggested that the nonsubstitution of the alpha(1----3)-linked mannosyl residue from the N-linked glycopeptide core structure is the key determinant in the minimal structural requirement specific to this fimbrial lectin. Such Man5GlcNAc2-containing glycopeptides were obtained from larger N-linked carbohydrate chains, occurring abundantly in natural sources. The ability of jack bean alpha-mannosidase to cleave the alpha(1----2)-linked mannoses more rapidly than the others allowed the controlled digestion of large oligomannoside-type glycopeptides from legume storage glycoproteins. Such shortened glycopeptides of plant origin were prepared which strongly inhibited guinea pig erythrocyte agglutinations as well as bacterial adhesion on human buccal cells, thus confirming their similarity (if not identity) with the receptor of type 1 pili on mammalian cells. The importance of this preparation of a receptorlike compound that inhibits bacterial adhesion with regard to the research on the role of type 1 pili in E. coli pathogenicity is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Babu J. P., Giampapa C. S., Hasty D. L., Simpson W. A., Beachey E. H. Protection against Escherichia coli-induced urinary tract infections with hybridoma antibodies directed against type 1 fimbriae or complementary D-mannose receptors. Infect Immun. 1985 Jun;48(3):625–628. doi: 10.1128/iai.48.3.625-628.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson M., Medalia O., Schori L., Mirelman D., Sharon N., Ofek I. Prevention of colonization of the urinary tract of mice with Escherichia coli by blocking of bacterial adherence with methyl alpha-D-mannopyranoside. J Infect Dis. 1979 Mar;139(3):329–332. doi: 10.1093/infdis/139.3.329. [DOI] [PubMed] [Google Scholar]
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- Cheney C. P., Boedeker E. C. Adherence of an enterotoxigenic Escherichia coli strain, serotype O78:H11, to purified human intestinal brush borders. Infect Immun. 1983 Mar;39(3):1280–1284. doi: 10.1128/iai.39.3.1280-1284.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorland L., van Halbeek H., Vleigenthart J. F., Lis H., Sharon N. Primary structure of the carbohydrate chain of soybean agglutinin. A reinvestigation by high resolution 1H NMR spectroscopy. J Biol Chem. 1981 Aug 10;256(15):7708–7711. [PubMed] [Google Scholar]
- Eshdat Y., Sharon N. Recognitory bacterial surface lectins which mediate its mannose-specific adherence to eukaryotic cells. Biol Cell. 1984;51(2):259–266. doi: 10.1111/j.1768-322x.1984.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Carbohydrate specificity of the surface lectins of Escherichia coli, Klebsiella pneumoniae, and Salmonella typhimurium. Carbohydr Res. 1983 Aug 16;120:235–249. doi: 10.1016/0008-6215(83)88019-7. [DOI] [PubMed] [Google Scholar]
- Firon N., Ofek I., Sharon N. Interaction of mannose-containing oligosaccharides with the fimbrial lectin of Escherichia coli. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1426–1432. doi: 10.1016/0006-291x(82)90947-0. [DOI] [PubMed] [Google Scholar]
- Hagberg L., Jodal U., Korhonen T. K., Lidin-Janson G., Lindberg U., Svanborg Edén C. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect Immun. 1981 Feb;31(2):564–570. doi: 10.1128/iai.31.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson R. E., Fusco P. C., Brinton C. C., Moon H. W. In vitro adhesion of Escherichia coli to porcine small intestinal epithelial cells: pili as adhesive factors. Infect Immun. 1978 Aug;21(2):392–397. doi: 10.1128/iai.21.2.392-397.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwahi T., Abe Y., Nakao M., Imada A., Tsuchiya K. Role of type 1 fimbriae in the pathogenesis of ascending urinary tract infection induced by escherichia coli in mice. Infect Immun. 1983 Mar;39(3):1307–1315. doi: 10.1128/iai.39.3.1307-1315.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayappa H. G., Goodnow R. A., Geary S. J. Role of Escherichia coli type 1 pilus in colonization of porcine ileum and its protective nature as a vaccine antigen in controlling colibacillosis. Infect Immun. 1985 May;48(2):350–354. doi: 10.1128/iai.48.2.350-354.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen M., Ehnholm C., Väisänen-Rhen V., Korhonen T., Pipkorn R., Kalkkinen N., Gahmberg C. G. Identification of the major human sialoglycoprotein from red cells, glycophorin AM, as the receptor for Escherichia coli IH 11165 and characterization of the receptor site. Eur J Biochem. 1985 Feb 15;147(1):47–52. doi: 10.1111/j.1432-1033.1985.tb08716.x. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Isaacson R. E. Proteinaceous bacterial adhesins and their receptors. Crit Rev Microbiol. 1983;10(3):229–260. doi: 10.3109/10408418209113564. [DOI] [PubMed] [Google Scholar]
- Knutton S., Lloyd D. R., Candy D. C., McNeish A. S. Adhesion of enterotoxigenic Escherichia coli to human small intestinal enterocytes. Infect Immun. 1985 Jun;48(3):824–831. doi: 10.1128/iai.48.3.824-831.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Lloyd D. R., Candy D. C., McNeish A. S. In vitro adhesion of enterotoxigenic Escherichia coli to human intestinal epithelial cells from mucosal biopsies. Infect Immun. 1984 May;44(2):514–518. doi: 10.1128/iai.44.2.514-518.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Lloyd D. R., Candy D. C., McNeish A. S. Ultrastructural study of adhesion of enterotoxigenic Escherichia coli to erythrocytes and human intestinal epithelial cells. Infect Immun. 1984 May;44(2):519–527. doi: 10.1128/iai.44.2.519-527.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutton S., Williams P. H., Lloyd D. R., Candy D. C., McNeish A. S. Ultrastructural study of adherence to and penetration of cultured cells by two invasive Escherichia coli strains isolated from infants with enteritis. Infect Immun. 1984 Jun;44(3):599–608. doi: 10.1128/iai.44.3.599-608.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama I. Isolation of a glycopeptide from a 7S protein in soybean globulins. Arch Biochem Biophys. 1969 Mar;130(1):370–373. doi: 10.1016/0003-9861(69)90046-0. [DOI] [PubMed] [Google Scholar]
- Levine M. M. Adhesion of enterotoxigenic Escherichia coli in humans and animals. Ciba Found Symp. 1981;80:142–160. doi: 10.1002/9780470720639.ch10. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Black R. E., Brinton C. C., Jr, Clements M. L., Fusco P., Hughes T. P., O'Donnell S., Robins-Browne R., Wood S., Young C. R. Reactogenicity, immunogenicity and efficacy studies of Escherichia coli type 1 somatic pili parenteral vaccine in man. Scand J Infect Dis Suppl. 1982;33:83–95. [PubMed] [Google Scholar]
- Neeser J. R., Schweizer T. F. A quantitative determination by capillary gas-liquid chromatography of neutral and amino sugars (as O-methyloxime acetates), and a study on hydrolytic conditions for glycoproteins and polysaccharides in order to increase sugar recoveries. Anal Biochem. 1984 Oct;142(1):58–67. doi: 10.1016/0003-2697(84)90516-5. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H. Mannose binding and epithelial cell adherence of Escherichia coli. Infect Immun. 1978 Oct;22(1):247–254. doi: 10.1128/iai.22.1.247-254.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J., Finne J., Achtman M., Väisänen V., Korhonen T. K. Escherichia coli strains binding neuraminyl alpha 2-3 galactosides. Biochem Biophys Res Commun. 1983 Mar 16;111(2):456–461. doi: 10.1016/0006-291x(83)90328-5. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J., Cohen L. S. Antipili antibody affords protection against experimental ascending pyelonephritis. J Clin Invest. 1979 Jul;64(1):333–336. doi: 10.1172/JCI109458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Dreyer J. S., Schauer S. Effect of pili on susceptibility of Escherichia coli to phagocytosis. Infect Immun. 1979 Apr;24(1):218–223. doi: 10.1128/iai.24.1.218-223.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit H., Gaastra W., Kamerling J. P., Vliegenthart J. F., de Graaf F. K. Isolation and structural characterization of the equine erythrocyte receptor for enterotoxigenic Escherichia coli K99 fimbrial adhesin. Infect Immun. 1984 Nov;46(2):578–584. doi: 10.1128/iai.46.2.578-584.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svanborg Edén C., Bjursten L. M., Hull R., Hull S., Magnusson K. E., Moldovano Z., Leffler H. Influence of adhesins on the interaction of Escherichia coli with human phagocytes. Infect Immun. 1984 Jun;44(3):672–680. doi: 10.1128/iai.44.3.672-680.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai T., Yamashita K., Ogata-Arakawa M., Koide N., Muramatsu T., Iwashita S., Inoue Y., Kobata A. Structural studies of two ovalbumin glycopeptides in relation to the endo-beta-N-acetylglucosaminidase specificity. J Biol Chem. 1975 Nov 10;250(21):8569–8575. [PubMed] [Google Scholar]
- Tsai P. K., Frevert J., Ballou C. E. Carbohydrate structure of Saccharomyces cerevisiae mnn9 mannoprotein. J Biol Chem. 1984 Mar 25;259(6):3805–3811. [PubMed] [Google Scholar]
- Väisänen V., Korhonen T. K., Jokinen M., Gahmberg C. G., Ehnholm C. Blood group M specific haemagglutinin in pyelonephritogenic Escherichia coli. Lancet. 1982 May 22;1(8282):1192–1192. doi: 10.1016/s0140-6736(82)92264-4. [DOI] [PubMed] [Google Scholar]


