Abstract
This paper describes the ‘push–pull’ or ‘stimulo-deterrent diversionary’ strategy in relation to current and potential examples from our own experiences. The push–pull effect is established by exploiting semiochemicals to repel insect pests from the crop (‘push’) and to attract them into trap crops (‘pull’). The systems exemplified here have been developed for subsistence farming in Africa and delivery of the semiochemicals is entirely by companion cropping, i.e. intercropping for the push and trap cropping for the pull. The main target was a series of lepidopterous pests attacking maize and other cereals. Although the area given to the cereal crop itself is reduced under the push–pull system, higher yields are produced per unit area. An important spin-off from the project is that the companion crops are valuable forage for farm animals. Leguminous intercrops also provide advantages with regard to plant nutrition and some of the trap crops help with water retention and in reducing land erosion. A major benefit is that certain intercrop plants provide dramatic control of the African witchweed (striga). Animal husbandry forms an essential part of intensive subsistence agriculture in Africa and developments using analogous push–pull control strategies for insect pests of cattle are exemplified.
Keywords: push–pull, stem borers, cereals, subsistence farming, cattle pests, disease vectors
1. Introduction
Many systems for integrating different pest control techniques have been developed. These often rely on improving cultural practices to minimize fertilizer and pesticide inputs (Glen et al. 1995; Van Emden & Peakall 1996; Waterlow et al. 1998; Brooks & Roberts 1999; Gurr et al. 2004). However, for subsistence farming in Africa, there are insufficient resources for using fertilizers and pesticides, and even hybrid seed may be economically inappropriate (Gurr et al. 2004; Hester & Harrison 2005). In addition to the poor economy of subsistence farming, there is an uncertainty of whether or not the rains will come, meaning that the farmer will be justifiably reluctant to invest in technologies that would undoubtedly improve the crop, because there may be no crop at all. It has been stated that ‘farmers in more than two million demonstration plots have shown that they can double and triple yields, using relatively modest packages of improved seeds, fertilizers and good management practices. And yet, average national yields for maize, sorghum, and millet have not improved appreciably, as farmers have found it difficult to adopt these improved packages of practices due to high input prices especially for fertilizer, and volatile market prices for grains’ (Borlaug 2004).
Although the situation for agriculture in industrialized countries is economically very different from subsistence farming in Africa or elsewhere in the world, these countries also need to respond to a policy, now almost universal, of reducing fertilizer, and particularly pesticide, inputs in favour of exploiting natural processes. Industrialized countries may, therefore, be faced with learning from successes in integrated pest management (IPM) established within developing countries and there is a great potential for this in the studies described here. Molecular techniques are available to move more rapidly in this direction, not only by creating genetically modified crops and farm animals (Royal Society Reports 2001, 2002) but also by better breeding programmes, both of which could apply to advancements from the work described here for all types of agriculture.
There are two main alternatives to pest control by the broad-spectrum eradicant pesticides currently in use. One method is the exploitation of semiochemicals, including pheromones, which are natural signals that affect changes in the behaviour or development of many organisms (Hardie & Minks 1999; Matthes et al. 2003). The second comprises biological control agents, which range from pathogens of pests to other antagonistic organisms, including predators and parasitoids (Powell & Pickett 2003). Plants bred conventionally for resistance, or genetically modified organisms (GMOs) expressing resistance traits, produce toxicants or agents which, by other modes of action, cause a lack of development or destruction of the pest and so fall into the conventional approach, i.e. incorporating toxic or direct physiological mechanisms. Semiochemicals, on the other hand, work by non-toxic modes of action and are often the same as, or closely related to, food components or naturally derived or nature-identical food additives. These could, therefore, be exploited as non-toxic agents from GMOs, or as a result of more conventional breeding (Pickett & Poppy 2001; Pickett et al. 2003). If the biological control agents act by antibiotic effects, then logically they should fall into the first category, i.e. those to which we are seeking alternatives. However, those acting by pathogenicity would contribute to the new generation of alternatives. Already, synthetic but nature-identical insect pheromones are in wide use around the world for controlling pests of high-value horticultural crops (Trumble & Alvarado-Rodriguez 1993; Trumble 1997; Boller & Hurter 1998; Howse et al. 1998; Agelopoulos et al. 1999). In addition, some other semiochemicals are available in insect pest control. However, there are undoubted problems in that the UK, and the European Union generally, has not yet managed, as have the USA, to produce appropriate registration arrangements for these materials, which is currently interfering with commercial development and thereby agricultural replacement of pesticides (Weatherston & Minks 1995; Jones 2002).
In Africa, and in subsistence cereal production, there are economic barriers to conventional pest control approaches. However, semiochemicals can be delivered by companion cropping, provided that there is an appropriate labour resource with which to manage the companion crops. Already, subsistence farmers in Africa use the method of intercropping known in East Africa by the Kiswahili ‘kilimo cha mchanganyiko’, with the farmers planting beans (Phaseolus spp.) in between their rows of maize. The agroeco systems in which these subsistence farmers operate are usually rich in species diversity and contain many other natural hosts for pests. Therefore, the concept of controlling pests, in this case lepidopterous stem borers that attack maize, sorghum and other cereal crops, by establishing a semiochemical-based push–pull system using companion crops (Pyke et al. 1987; Miller & Cowles 1990; Pickett et al. 1997; Smart et al. 1997) seemed highly appropriate. This coincided with the suggestion by Thomas Odhiambo, then Director of the International Centre of Insect Physiology and Ecology (ICIPE) in Kenya, to exploit habitat management to control these insects, and a growing interest in exploiting push–pull systems at what is now Rothamsted Research. These were brought together in 1993 with the establishment of Rothamsted International and through funding by the Gatsby Charitable Foundation and now the Kilimo Trust.
2. The stem borer pests of Africa
The stem borer pests of cereal crops in sub-Saharan Africa comprise the larvae of a number of members of the Lepidoptera, both indigenous species, as exemplified by the maize stalk borer Busseola fusca (Noctuidae), and non-indigenous, or introduced, stem borers such as the spotted stem borer, Chilo partellus (Crambidae). B. fusca is distributed throughout sub-Saharan Africa, whereas C. partellus is mainly found in Eastern and southeastern African countries (Kfir et al. 2002). Their feeding habits on maize and sorghum result in yield losses of up to 88%, depending on the cultivar planted, developmental stage of the plant at infestation, infestation rate and prevailing environmental conditions, among other factors (Kfir et al. 2002). These insects use a range of grasses (Khan et al. 1997b; Khan & Polaszek 1998; Rebe et al. 2004) including indigenous crops such as sorghum and the introduced maize. Whether on the crops or on wild grass hosts, the larvae of the stem borers are parasitized by a range of predominantly hymenopterous parasitoids such as Cotesia sesamiae (Braconidae). As with most lepidopterous hosts, these parasitic wasps search for plants containing the larvae by detecting volatile semiochemicals produced as a consequence of larval feeding damage.
In spite of relatively wide host ranges for the pests, it would be expected that some grasses (Poaceae and related families) would be unsuitable hosts. In addition, some species growing in agroeco systems including non-poaceous crop plants would also be unsuitable hosts. Thus, our initial hypothesis was that it would be possible to identify hosts which are more strongly attractive to adult stem borers than the crop plants themselves. This would be based on such ‘super hosts’ releasing volatile semiochemicals that would act as pre-colonization cues and thus establish greater levels of oviposition by the gravid females. Work at Rothamsted and elsewhere had already shown that, although a number of the semiochemicals used in this process are common to many plants, specific responses in the olfactory system of the insects could allow particular patterns of host selection (Bruce et al. 2005). Furthermore, work also pioneered at Rothamsted had clearly demonstrated that additional compounds characterizing unsuitable hosts, or ratios of ubiquitous compounds typical of unsuitable hosts, could be used in host avoidance (Birkett et al. 2000; Pickett & Poppy 2001). Such avoidance is extremely important to herbivorous insects to reduce expenditure of resources in ovipositing on plants that will not eventually act as exploitable hosts.
The first phase, therefore, in developing a push–pull system against stem borers was to establish plots of as many grasses and other plants as possible which would be found in the targeted agroeco systems, and to determine their relative attractancy to pests. This was achieved by establishing triplicated plots, principally on members of the Poaceae but also Cyperaceae and Typhinae, as well as some leguminous crops and cattle forage plants, at the ICIPE Field Station at Mbita Point in the Suba District of Nyanza Province, on the banks of Lake Victoria (Khan et al. 1997b). From this preliminary work, it was established that molasses grass, Melinis minutiflora (Poaceae), was so unattractive that it received no significant oviposition. Although oviposition was used as a criterion for attractancy in this work, this is not rigorous at the behavioural level, as oviposition itself results in contact by the insect. However, the issue of choice between hosts and non-hosts had to be established economically and for early incorporation into farming systems. At the same time, two cattle forage grasses, Sudan grass, Sorghum vulgare sudanense (Poaceae), and Napier grass, Pennisetum purpureum (Poaceae), were found to be highly attractive, certainly more so than maize (Khan et al. 1997b).
3. The move towards push–pull
Field trials were initially established at Mbita Point and at the Kenyan Agricultural Research Institute's (KARI) field site at Kitale, Trans-Nzoia District, in which 50×50 m plots of maize were compared, in terms of stem borer attack, with a similarly sized plot incorporating a surround of two rows of Napier grass. A bare patch of ground was required between the maize and the Sudan or Napier grass, so that the trap crops would not take water or soil nutrients from the main crop. Where the maize was grown as a monocrop, there was a statistically significantly higher level of stem borer attack, as measured by cutting the stems and investigating for larval mining (16.8 and 27.5% in the treatment and control plots, respectively, in Suba District, Kenya, and 10 and 20.9% in the treatment and control plots, respectively, in Trans-Nzoia district, Kenya; Khan et al. 2001). Similar comparative trials were established using molasses grass, growing this as a one-to-one intercrop without changing the maize row spacing. Here, the reduction in stem borer damage was even more dramatic (for example, damage reduced from 39.2 to 4.6%; Khan et al. 1997b).
At this stage of preliminary success, the prospect of separate push or pull systems was introduced to the farming communities in Suba and Trans-Nzoia. This was achieved by farmers visiting the field station sites and by technical support staff visiting the farms of those expressing interest in developing this technology. It was quickly seen that the farmers were managing the push and pull systems independently and that they were highly satisfied with the improvement in control of stem borers. Technical staff from the programme continued to visit these farmers to measure damage and yields (table 1). The farmers themselves were quick to realize that they could exploit increased production of cattle forage, particularly in the case of Napier grass. By the end of 2000 more than 500 farmers had already joined the programme, and by the end of 2005 more than 4000 farmers were practising push–pull in Western and Central Kenya. The first investigation of stem borer host discrimination was made in 1994–1995 (Khan et al. 1997b), and by 1996–1997 farmers had started to put together the push and the pull (approx. 300 farmers in Uganda and 100 in Tanzania; Khan et al. 2000, 2001).
Table 1.
Stem borer damage and populations on maize in ‘push–pull’ plots in Trans-Nzoia and Suba districts of Kenya during the 1998 and 1999 long rains seasons.a (Adapted from Khan et al. (2001). T, treatment, i.e. designated companion planting; C, control. *Significant difference at p<0.05, **p<0.01, between treatment and control; —, not applicable.)
| year | location | maize+Napierb | maize+Napier+desmodiumc | maize+Napier+molasses grassc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| stem borer damage (%) | stem borers/40 maize plants | stem borer damage (%) | stem borers/40 maize plants | stem borer damage (%) | stem borers/40 maize plants | ||||||||
| T | C | T | C | T | C | T | C | T | C | T | C | ||
| 1998 | Trans-Nzoia | 8.3 | 18.8* | 18.6 | 37.3* | 4.8 | 21.2** | 7.7 | 45.4** | 6.5 | 18.9* | 8.3 | 51.6** |
| Suba | 14.9 | 25.7* | 16.9 | 35.9* | 6.7 | 29.6** | 8.0 | 39.4** | 7.9 | 22.1** | 11.5 | 43.1** | |
| 1999 | Trans-Nzoia | 11.7 | 23.1** | 22.6 | 49.6* | 9.7 | 26.5** | 13.6 | 41.8** | — | — | — | — |
| Suba | 18.7 | 29.3* | 22.7 | 425.8* | 13.5 | 36.6** | 19.7 | 57.4** | — | — | — | — | |
For each technology, data collected from 10 farmers.
Use of Napier grass as trap plant for stem borers.
Use of Napier grass as attractant to stem borers and desmodium or molasses grass to repel stem borers from maize field.
4. The scientific base
Although it had always been the intention to exploit repellent or non-host semiochemistry (push) and attractant semiochemistry (pull), we originally used simple oviposition measurements to determine the comparative attractancy between unsuitable and suitable hosts. In order to understand the process, it was necessary to investigate the underlying semiochemistry of the push and pull companion plants. This is an essential part of maintaining sustainability in the event that new planting material might be different, or may have genetically drifted away from that originally investigated. Thus, volatile compounds released by Sudan grass, Napier grass and other highly attractive hosts were captured by absorption onto a porous polymer. These volatiles were eluted from the polymer with a solvent and the samples subjected to gas chromatographic (GC) analysis coupled directly to a preparation from the moth antenna (an electroantennogram (EAG)), to enable identification of semiochemicals likely to have attractant activity at the levels released by the plant.
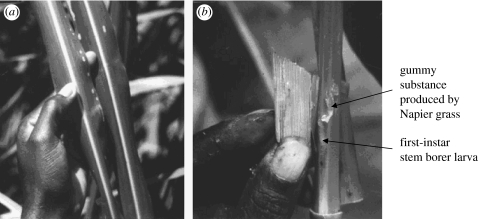
Subsequent analysis by GC-coupled mass spectrometry and other complementary studies showed a number of key compounds to be involved, including those typical of poaceous plants (Khan et al. 2000). An extension of the hypothesis expressed above was that the repellent (push) molasses grass would contain some additional compounds confirming its unacceptability. The five electrophysiologically active compounds identified from molasses grass were found to include (E)-ocimene and (E)-4,8-dimethyl-1,3,7-nonatriene, immediately suggesting the mechanism by which this plant repelled adult stem borers. These two compounds are well known to be produced by a range of plants, including maize (Turlings et al. 1990), when damaged by larval feeding. Therefore, these compounds were acting as cues denoting stem borer colonization, which incoming gravid females need to avoid to prevent cannibalism of their eggs and young larvae. For each compound, the electrophysiological studies were supported by behavioural work (Khan et al. 2000), which confirmed the attractiveness of the first group of compounds and the repellency of the additional cues from molasses grass. Indeed, early in the work, the value of this knowledge in establishing quality control was clear, in that molasses grass from the Suba District did not perform as well as the planting material obtained subsequently from the Thika region of Central Kenya. Larval survival studies also showed that, specifically on Napier grass, there was a low rate of survival compared with maize and other hosts, caused by a sticky exudation from the grass which often killed later instar larvae (figure 1).
Figure 1.
(a) Feeding marks of stem borer larvae on Napier leaves and (b) production of sticky exudate by Napier grass tissue in response to penetration by first- and second-instar stem borer larvae. Adapted from Khan & Pickett (2004).
In the field, although fewer stem borer larvae were found on maize where molasses grass was used as the intercrop, i.e. only the push component, and also in the full push–pull approach, there was increased parasitism as a consequence of increased foraging by the parasitoids. This could be explained by the release of (E)-ocimene and (E)-4,8-dimethyl-1,3,7-nonatriene by the molasses grass, which, as well as repelling adult stem borers, would also be expected to increase parasitoid foraging. As has already been stated, these compounds are released from grasses highly infested with stem borer larvae. Indeed, behavioural experiments showed that the (E)-4,8-dimethyl-1,3,7-nonatriene, at a similar level to that released by the molasses grass, caused the same level of attraction as the molasses grass itself and the volatiles isolated by entrainment onto the porous polymer (Khan et al. 1997a).
Further studies, arising from attempts to understand why some wild grasses are more attractive than cultivated crop plants, have shown diurnal variation in the volatiles released by these plants. For stem borer oviposition, we identified over 30 physiologically active compounds in the volatile profiles emitted by four poaceous plant species: blue thatching grass (Hyparrhenia tamba), Napier grass, sorghum and maize (Chamberlain et al. 2006). The total quantities of volatiles collected hourly, over a 9 h period, from P. purpureum and H. tamba showed a hundredfold increase in the first hour of the scotophase. Thereafter, the amount decreased rapidly to the levels present during the photophase. Although the onset of the scotophase also triggered an increase in the quantities of volatiles collected from two cultivars of sorghum, and two out of three cultivars of maize, these increases were much less dramatic than that in the two wild grasses, being only 10 times as much as in the last hour of the photophase. Analysis of the volatiles, by gas chromatography, showed that up to 95% of the increase in volatiles at the onset of the scotophase was due to just four compounds, the green leaf volatiles hexanal, (E)-2-hexenal, (Z)-3-hexen-1-ol and (Z)-3-hexen-1-yl acetate, with the latter dominating the volatile profile. Gravid female stem borer moths are known to seek oviposition sites during the first 6 h of the scotophase, with most of the activity in the first 2 h. Although further behavioural studies are required, the combination of these two factors, i.e. the electrophysiological response to green leaf volatiles and the coincidence of production of large amounts of these compounds with time of oviposition flights by moths, suggests that the insects are using the increase in green leaf volatiles occurring at nightfall for host location. These factors would also explain why the wild grasses are more attractive than the cultivated crop plants for egg laying, since these plants produce much larger amounts of the green leaf volatiles at nightfall than do the maize and sorghum.
5. Expansion of the push–pull system
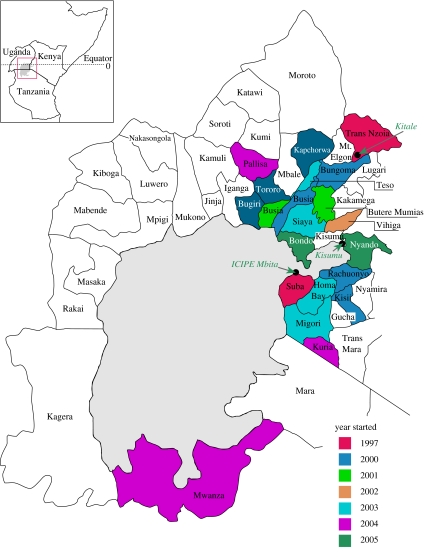
After the successes in 1995, ICIPE, in collaboration with KARI and the Kenyan Ministry of Agriculture, began to expand into new regions. Extremes were represented by the Suba District (arid and infertile) and Trans-Nzoia (wet and highly fertile), but regions displaying intermediate conditions were also encountered, for example at Kakamega and at Bungoma on the northern shore of Lake Victoria (figure 2).
Figure 2.
Districts where push–pull is practised by the farmers and year they started. Adapted from Gatsby Occasional Paper (2005), courtesy Green Ink.
At each site, there were clear advantages to the farmers from the livestock forage provided by the push–pull approach and, in the Suba District, the number of graded cows increased from 4 to 350. On farmers' days, or ‘barazas’, there were discussions on the system and some modifications were made to allow for current agricultural practices. However, the main feature of the push–pull approach was to have two to three rows of the trap crop, preferably Napier grass but sometimes Sudan grass, growing around the maize plot at a distance of 1 m, the maize itself being normally spaced but with one row of molasses grass planted between five and seven rows of the crop. Kiswahili-speaking farmers considered that a better term for the system would be ‘vuta sukuma’ (‘pull–push’). This terminology was used in a radio programme, Tembea na Majeera, where a storyline explained the system, together with some stories of how farmers had travelled by bus between communities to transfer the technology. The farmers also explained that they would prefer to have the option of growing an edible bean as the intercrop, as was the tradition, rather than a cattle forage such as the molasses grass. A survey was therefore made of a wide range of leguminous plants to see if there were opportunities for these to act as effective intercrops. Certainly, cowpea, Vigna unguiculata (Leguminosae=Fabaceae), gave a statistically significant reduction in stem borers when grown one-to-one with maize, but this was a weak effect compared with that of molasses grass. However, although not an edible bean, the forage legume silverleaf, Desmodium uncinatum (Fabaceae), was seen to give promising results and, by the main rainy season of 1997, silverleaf grown one-to-one with maize was widely employed in the Suba District. During these trials, it was also noted that, where this intercrop was used, the parasitic African witchweed or striga, Striga hermonthica (Scrophulariaceae), was controlled to an extent readily visible to technical staff and farmers alike. In regions with a high striga level, use of silverleaf or other species of desmodium was therefore taken up avidly by the farmers. This effect was investigated in a quasi-Latin square six-replicated field trial, together with fertilizer and ground cover treatments, which definitively demonstrated that the control was through an allelopathic or allelobiotic effect (Khan et al. 2002).
6. The push–pull system and striga control
Problems were encountered in providing sufficient silverleaf seed for the expanding programme. Previously, most of the companion crops had been provided through vegetative propagation, except for some seed from molasses grass, and the farmers could easily trade such planting material between themselves. However, for the desmodium, seed production needed to become part of the programme. Silverleaf and other Desmodium species are now grown in the push–pull system on a one-to-one basis (figure 3) and after harvesting the maize, the desmodium plants, growing perennially, are allowed to set seed which the farmer can harvest or sell. The desmodium is then cut down to the ground, fed to cattle or ensilaged with Napier grass and, for the next season, the maize is sown into a drill cut by a hoe (‘jembe’ in Kiswahili). Although this practice provided some seed, further resources were needed, so women's groups were resourced to establish monocultures of silverleaf and other Desmodium species for seed production (figure 4).
Figure 3.
Maize : D. uncinatum at 1 : 1 in full push–pull in Suba district.
Figure 4.
Women's group responsible for producing D. uncinatum seed.
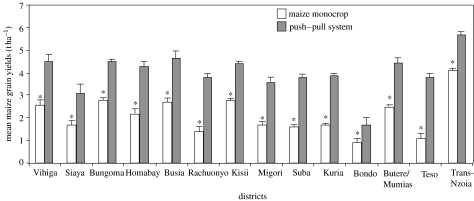
At this point, the services of a Small to Medium Enterprise, Western Seeds, were brought to bear in a collaborative effort in which they bought certified seed and then sold it on to new farmers, or to farmers expanding their smallholdings. One enterprising region, in Muranga, Muragua and Kirinyaga districts in Central Kenya, unable to obtain sufficient seed and working somewhat independently of the main programme, devised an ingenious way in which to propagate silverleaf desmodium vegetatively by taking cuttings. The regions currently under push–pull in Kenya, Uganda and Tanzania, as a consequence of expansion driven by the effective control of stem borers and now striga, are given in figure 2. This was achieved by a combination of processes involving the research and extension communities. In Kenya, the main approach for dissemination of the push–pull technology was through 110 farmer-teachers, in 12 districts in Western Kenya, working as village extension staff in teaching new farmers how to plant push–pull fields. Each farmer-teacher taught an average of 10 new farmers every year and was provided with a notebook, a bicycle and a bicycle maintenance allowance of Ksh 750 ($10) per month. The ICIPE technician met with the farmer-teachers every month to evaluate their progress. Within 2 years, these farmer-teachers were able to teach push–pull technology to 2000 new farmers. Many farmers have agreed to maintain plots without companion cropping and, for 14 districts in Kenya, there are comparative data on yields (figure 5).
Figure 5.
Yield differences in push–pull and control plots in 14 districts in Kenya during the 2005 long rains. Within a district, bars marked by asterisk are significantly lower (p<0.05, t-test).
Cost analysis has been done (table 2) which shows that the return on labour and investment is better than for other systems. These data do not yet include the value to production of cattle and dairy goats, now taking in components of the push–pull system in other regions.
Table 2.
Economics of push–pull strategy as compared to farmer's practice in six districts in Kenya in 2004. (a, b and c represent data averages for 7, 4 and 3 years, respectively. All the parameters studied were significantly lower in the farmers' practice than in the push–pull technology in all districts (*p<0.05).)
| total labour cost ($) ha−1 | total variable cost ($) ha−1 | total gross revenue ($) ha−1 | gross benefit ($) ha−1 | |||||
|---|---|---|---|---|---|---|---|---|
| district | push–pull | farmers' practice | push–pull | farmer's practice | push–pull | farmer's practice | push–pull | farmer's practice |
| Trans-Nzoiaa | 223±1.2 | 128±1.5 | 493±1.6 | 374±2.0 | 1,290±27.7 | 628±32.4 | 797±28.0 | 254±31.0* |
| Subaa | 167±1.6 | 134±0.4 | 278±1.1 | 250±0.7 | 679±10.2 | 329±5.9 | 401±9.9 | 79±5.7* |
| Bungomab | 247±3.8 | 222±2.3 | 331±3.9 | 300±2.8 | 867±22.6 | 415±8.6 | 536±21.3 | 115±9.9* |
| Busiab | 222±1.7 | 118±0.3 | 321±1.9 | 243±0.6 | 862±11.9 | 418±2.9 | 541±12.7 | 175±2.9* |
| Kisiib | 184±1.8 | 140±1.1 | 246±2.1 | 210±1.0 | 733±6.4 | 334±15.7 | 487±5.3 | 134±15.9* |
| Vihigac | 227±1.9 | 128±1.0 | 359±2.3 | 331±1.5 | 785±12 | 423±7.1 | 426±13.4 | 92±7.0* |
Further socio-economic analysis is still required, since there are aspects which are noticeable but which have yet to be quantified. For example, improved family cohesiveness, which could help issues such as local security all the way through to the problems of HIV, has been observed. In terms of transferring this technology, the main effort now will be to expand into regions not yet covered and also to investigate exit strategies. It is imperative that, in devising exit strategies, links remain in place for technological intervention. The need for this has been clearly demonstrated by the recent discovery of a new phytoplasma stunting disease in the Napier grass. This is affecting Napier grass production, whether in the push–pull system or when grown as a monoculture for dairy herds, from Ethiopia down to Malawi. Through the links between Africa and Rothamsted, the causative agent was identified and now, in collaboration with Africa, also involving the University of Cardiff, the vector is being identified. Having identified the vector, which is a homopteran leafhopper, a realistic programme of breeding for resistance in Napier grass can be embarked upon. The push–pull system can be taken up beyond this particular project, provided that farmers can acquire the necessary knowledge and techniques for establishing the companion crops and, for example, the Sasakawa Africa Foundation in Ethiopia under Marco Quinones (2006, personal communication) has successfully established Desmodium species as intercrops.
The introduction of Bt maize, i.e. employing transgenic insect resistance, and maize resistant to herbicides such as the imidazolinones for control of striga, are important developments. These, by definition, are delivered by hybrid maize seed and may not be suitable for all socio-economic conditions, particularly those subsistence farmers targeted here for the programmes controlling stem borers and striga. However, where appropriate, integration with these techniques should be considered, and preliminary work in South Africa on Bt maize and push–pull (Midega et al. 2006), and in Kenya and Uganda on herbicide-tolerant maize and push–pull, have shown promise. In any event, the juxtaposition of insect pest and weed control in systems integrating push–pull with other approaches gives the farmer the best possible situation from which to make informed choices.
7. Long-term opportunities for biotechnological exploitation
It may be considered that producing a maize variety capable of releasing (E)-ocimene and (E)-4,8-dimethyl-1,3,7-nonatriene from the intact plants, as is the case with molasses grass, would be a way forward. Maize is already capable of producing both compounds, but only does so, at sufficient levels for crop protection, at high levels of infestation. None the less, if release could be enhanced and linked to earlier and more minor stages of infestation, perhaps even just oviposition, then the maize would protect itself more effectively. Such efficient protection may allow selection among the stem borers to rely more heavily on other cues, and the parasitic wasps could be selected for cues more closely associated with their hosts. However, the integrated push–pull system involves other means of pest population reduction, i.e. the trap crop, which would mitigate against immediate selection pressures. The gene for (E)-ocimene synthesis in Arabidopsis and some other plants (Dudareva et al. 2003; Fäldt et al. 2003; Arimura et al. 2004) is known, but not yet the gene for the synthesis of (E)-4,8-dimethyl-1,3,7-nonatriene. However, this is being studied at Rothamsted and in Wageningen, the Netherlands, and perhaps at other centres working on plant stress and plant molecular biology. Maize already contains certain compounds, the hydroxamic acids or benzoxazinones (Frey et al. 1997), which are antibiotic against pests and diseases, and at Rothamsted there is an active plant breeding programme exploiting this pathway against pests and diseases in wheat (Ruth Gordon-Weeks 2006, personal communication), and at Charles Sturt University, Wagga Wagga, Australia (Jim Pratley 2005, personal communication) against weeds. This could easily be exploited in a plant breeding programme for the benefit of African farmers, where it would be possible to deliver the genetics into open pollinated land races, thereby fitting in more closely with current practice.
Perhaps the most pressing target is the control of striga by Desmodium species. This has been demonstrated to involve germination stimulation, comparable to that induced by host plants of striga, and a post-germination inhibitory effect measured by inhibition of seed radicle development in the striga. Many legumes cause germination of striga seeds but, apparently, only the Desmodium genus produces highly effective inhibitory compounds. Characterization of these compounds demonstrates that they are unusual C-glycosylated flavonoids. Since legumes are rich in flavonoids, the specific enzymes affecting the C-glycosylation would need to be introduced into edible bean legumes in the first instance. This process could be helped by the advanced genetics already being applied to Medicago truncatula (Lamblin et al. 2003) and Lotus japonicus (Kato et al. 2003; Kouchi et al. 2004), and to other technologies now available for studying plants for which the full genomic sequence has not yet been determined, as with the Desmodium species. If this type of approach to weed control continues to be successful and can be exploited using biotechnology, as proposed, then there are lessons for such approaches in industrialized agricultural cropping situations.
8. Prospects for push–pull in controlling livestock pests and disease vectors
Combinations of repellent and attractant semiochemistry may also find use in push–pull tactics for controlling livestock pests and disease vectors. Several possibilities are currently being explored. The adults of the brown ear tick, Rhipicephalus appendiculatus, the vector of the cattle disease East Coast Fever (Theileria), have been shown to use push–pull semiochemistry to locate bovid ears (Wanzala et al. 2004). Thus, odour collected from the anal region repels the tick and that from ears will attract it. Interestingly, in a related species, Rhipicephalus eversti, which prefers to feed around the anal region, the two semiochemicals perform the opposite functions. Preliminary experiments with odour collections from the anal region of cattle have shown that it is possible to mask the attraction of the ear to the brown ear tick. A large proportion of the ticks that were released on different regions of cattle failed to locate their feeding site and eventually dropped off (Sika & Hassanali 1996, unpublished observation). Characterization of the attractant semiochemicals may allow the development of a push–pull tactic that combines the use of a source of a synthetic or botanical tick repellent at the ear and an attractant-baited trap treated with fungal pathogen or acaricide located on the back of the animal.
Tsetse flies (vectors of animal and human sleeping sickness) show a gradation of feeding preference on different vertebrate animals and appear to use push–pull semiochemistry actively to avoid some hosts and to locate those which are preferred (Gikonyo et al. 2000). Identification of a series of kairomones for savannah tsetse from preferred hosts (Hall et al. 1984; Owaga et al. 1988) facilitated the development of baited traps and targets (the latter impregnated with insecticides) effective in large-scale suppression of these species (Vale et al. 1988; Brightwell et al. 1991). Several synthetic and natural repellents, including a constituent of bovid odours, 2-methoxyphenol, have been evaluated but were found not to be sufficiently effective in protecting cattle in the field (Torr et al. 1996). However, recent identification of a potent repellent blend from waterbuck, Kobus defassa (Gikonyo et al. 2002, 2003), which is refractory to tsetse, may provide much better protection for cattle and an effective push component in the push–pull approach for faster and more effective suppression of tsetse populations, particularly where cattle are the dominant source of a blood meal for the flies. A preliminary experiment undertaken on the Kenyan coast, comparing the effects of protecting cattle with a synthetic repellent (push), baited traps (pull) and a combination of these two (push–pull), suggests a better performance of the push–pull approach in suppressing tsetse (Spala et al., in preparation). Studies on fly preferences within a herd of one breed show consistent differences in fly loads of cattle (Jensen et al. 2004) and this has been shown to be a consequence of those cattle which have fewer flies producing additional compounds. These compounds have been identified and can be used in a slow release formulation to repel flies from cattle (Birkett et al. 2004). The push–pull could be established by combining this approach with highly attractive individuals treated frequently with, for example, a bioinsecticide such as pyrethrum.
Push–pull may also find a useful application in controlling malaria vectors, particularly zoophilic species like Anopheles arabiensis. The use of animals to divert (pull) mosquitoes from feeding on and transmitting disease to human beings (zooprophylaxis) has been considered as a possible tool in reducing mosquito numbers and levels of malaria (WHO 1982). Indeed, changing agricultural practices, resulting in more effective diversion of mosquitoes to farm animals, may have been an important factor contributing to the disappearance of malaria from Europe and parts of North America (Bruce-Chwatt 1985). However, in Africa and Asia, livestock keeping has been associated with increased malaria prevalence, particularly where cattle sheds are close to human dwellings (Bouma & Rowland 1995; Seyoum et al. 2002). A major handicap is that no methodical scientific study has been undertaken on the effects of the relative proportion and spatial relation of the two hosts, and the extent of mixing of their competing odour plumes on the degree of diversion. A recent theoretical study suggests that effective zooprophylaxis is dependent on such factors (Nedorezov et al. 2005). In addition, incorporation of a push component in households, in the form of repellent fumigants from readily available local plants, could have a significant effect on malaria incidence.
9. Concluding remarks on integrated pest management
Clearly, by accepting farmer practices such as kilimo cha mchanganyiko, but by underpinning these practices with sound hypothesis-driven science, new technologies with which to alleviate poverty in subsistence agrarian communities can be developed. Since these communities need somewhat different technologies from those already developed for agriculture in industrialized countries, there are lessons being learned which could, none the less, be applied to the latter. Certainly, organic farming in industrialized countries could benefit, but also, by forcing routes other than simple heterologous plant gene expression, this approach could help to develop a new generation of GM-protected plants, with an exciting intervening period of development by more conventional plant breeding techniques. After pioneering work by others (Pyke et al. 1987; Miller & Cowles 1990), we now see push–pull technology established as a reliable IPM system. Push–pull is also applicable without needing conventional pesticides, as do many IPM practices or even genetic modification, although better breeding and GMOs can easily be incorporated with mutual benefits. Still more resources are needed to further the expansion of these technologies. To this end, the newly formed Kilimo Trust is funding studies into second generation research questions associated with large-scale extension and diffusion of push–pull technologies among smallholder farmers, and to develop partnerships with extension services in East Africa. Constraints mainly revolve around the farmers themselves and the need to produce clean stands of the companion crops. This rigour is best transmitted farmer-to-farmer, but there must be technical involvement to maintain standards and to ‘troubleshoot’, and to link any serious problems from the latter to a science input in order to solve the problem, as with the Napier grass stunting disease. Key to long-term promulgation is the need to identify locally growing and agronomically suitable companion crops for the full push–pull approach, and to deal with new problems and any selection for resistance by the insect pests or weeds to the semiochemical strategies underpinning the push–pull system described here. Given these issues, this programme will continue to contribute to establishing food security in sub-Saharan Africa and the inevitable benefits this will have on the social and political situations there. We hope that it will inspire similar efforts in the control of other pests, pathogens and weeds of crops, as well as of livestock pests and disease vectors.
Acknowledgments
Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council (BBSRC), UK. Additional funding was provided under the BBSRC Biological Interactions in the Root Environment (BIRE) initiative. The International Centre of Insect Physiology and Ecology appreciates the long-standing core support from the Governments of Sweden, Switzerland, Denmark, Norway, Finland, France and Kenya. The push–pull project was primarily funded by The Gatsby Foundation, with additional support from The Rockefeller Foundation. From 2006 onwards, the push–pull project in Eastern Africa is funded by the Kilimo Trust (Uganda).
Footnotes
One contribution of 16 to a Theme Issue ‘Sustainable agriculture I’.
References
- Agelopoulos N, et al. Exploiting semiochemicals in insect control. Pest. Sci. 1999;55:225–235. doi:10.1002/(SICI)1096-9063(199903)55:3<225::AID-PS887>3.0.CO;2-7 [Google Scholar]
- Arimura G, Ozawa R, Kugimiya S, Takabayashi J, Bohlmann J. Herbivore-induced defense response in a model legume: two-spotted spider mites, Tetranychus urticae, induce emission of (E)-β-ocimene and transcript accumulation of (E)-β-ocimene synthase in Lotus japonicus. Plant Physiol. 2004;135:1976–1983. doi: 10.1104/pp.104.042929. doi:10.1104/pp.104.042929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkett M.A, et al. New roles for cis-jasmone as an insect semiochemical and in plant defense. Proc. Natl Acad. Sci. USA. 2000;97:9329–9334. doi: 10.1073/pnas.160241697. doi:10.1073/pnas.160241697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkett M.A, et al. The role of volatile semiochemicals in mediating host location and selection by nuisance and disease-transmitting cattle flies. Med. Vet. Entomol. 2004;18:313–322. doi: 10.1111/j.0269-283X.2004.00528.x. doi:10.1111/j.0269-283X.2004.00528.x [DOI] [PubMed] [Google Scholar]
- Boller, E. F. & Hurter, J. 1998 The marking pheromone of the cherry fruit fly: a novel non-toxic and ecologically safe technique to protect cherries against cherry fruit fly infestation. In Proc. Second Int. Symp. on Insect Pheromones, Wageningen, The Netherlands, 30 March–3 April 1998
- Borlaug, N. E. 2004 President's Address. Sasakawa Africa association, annual report 2003–2004, 1–2. (Writers and eds: A. McNab & C. Dowswell).
- Bouma M, Rowland M. Failure of passive zooprophylaxis: cattle ownership in Pakistan is associated with a higher prevalence of malaria. Trans. R. Soc. Trop. Med. Hyg. 1995;89:351–353. doi: 10.1016/0035-9203(95)90004-7. doi:10.1016/0035-9203(95)90004-7 [DOI] [PubMed] [Google Scholar]
- Brightwell R, Dransfield R.D, Kyorku C.A. Development of a low-cost tsetse trap and odour baits for Glossina pallidipes and G. longipennis in Kenya. Med. Vet. Entomol. 1991;5:153–164. doi: 10.1111/j.1365-2915.1991.tb00536.x. [DOI] [PubMed] [Google Scholar]
- Brooks G.T, Roberts T.R, editors. Pesticide chemistry and bioscience, the food-environment challenge. Royal Society of Chemistry; Cambridge, UK: 1999. [Google Scholar]
- Bruce T, Wadhams L.J, Woodcock C.M. Insect host location: a volatile situation. Trends Plant Sci. 2005;10:269–274. doi: 10.1016/j.tplants.2005.04.003. doi:10.1016/j.tplants.2005.04.003 [DOI] [PubMed] [Google Scholar]
- Bruce-Chwatt L.J, editor. Essential malarology. 2nd edn. Heinemann; London, UK: 1985. [Google Scholar]
- Chamberlain K, Khan Z.R, Pickett J.A, Toshova T, Wadhams L.J. Diel periodicity in the production of green leaf volatiles by wild and cultivated host plants of stemborer moths, Chilo partellus and Busseola fusca. J. Chem. Ecol. 2006;32:565–577. doi: 10.1007/s10886-005-9016-5. doi:10.1007/s10886-005-9016-5 [DOI] [PubMed] [Google Scholar]
- Dudareva N, Martin D, Kish C.M, Kolosova N, Gorenstein N, Fäldt J, Miller B, Bohlmann J. (E)-β-Ocimene and myrcene synthase genes of floral scent biosynthesis in snapdragon: function and expression of three terpene synthase genes of a new terpene synthase subfamily. Plant Cell. 2003;15:1227–1241. doi: 10.1105/tpc.011015. doi:10.1105/tpc.011015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fäldt J, Arimura G.I, Gershenzon J, Takabayashi J, Bohlmann J. Functional identification of AtTPS03 as (E)-β-ocimene synthase: a monoterpene synthase catalyzing jasmonate- and wound-induced volatile formation in Arabidopsis thaliana. Planta. 2003;216:745–751. doi: 10.1007/s00425-002-0924-0. [DOI] [PubMed] [Google Scholar]
- Frey M, et al. Analysis of a chemical plant defense mechanism in grasses. Science. 1997;277:696–699. doi: 10.1126/science.277.5326.696. doi:10.1126/science.277.5326.696 [DOI] [PubMed] [Google Scholar]
- Gatsby Charitable Foundation 2005 The quiet revolution: push-pull technology and African farmer. Gatsby Occasional Paper. The Gatsby Charitable Foundation, Allington House, London SW1E 5AE, UK.
- Gikonyo N.K, Hassanali A, Njagi P.G.N, Saini R.K. Behaviour of Glossina morsitans morsitans Westwood (Diptera: Glossindae) on waterbuck Kobus defasa Ruppel and feeding membranes smeared with waterbuck sebum indicates the presence of allomones. Acta Trop. 2000;77:295–303. doi: 10.1016/s0001-706x(00)00153-4. doi:10.1016/S0001-706X(00)00153-4 [DOI] [PubMed] [Google Scholar]
- Gikonyo N.K, Hassanali A, Njagi P.G.N, Gitu P.M, Midiwo J.O. Odour composition of preferred (buffalo and ox) and non-preferred (waterbuck) hosts of some savanna tsetse flies. J. Chem. Ecol. 2002;28:961–973. doi: 10.1023/a:1015205716921. doi:10.1023/A:1015205716921 [DOI] [PubMed] [Google Scholar]
- Gikonyo N.K, Hassanali A, Njagi P.G.N, Saini R.K. Responses of Glossina morsitans morsitans to blends of electroantennographically active compounds in the odors of its preferred (buffalo and ox) and non-preferred (waterbuck) hosts. J. Chem. Ecol. 2003;29:2331–2345. doi: 10.1023/a:1026230615877. doi:10.1023/A:1026230615877 [DOI] [PubMed] [Google Scholar]
- Glen D.M, Greaves M.P, Anderson H.M, editors. Ecology and integrated farming systems. Wiley; Chichester, UK: 1995. [Google Scholar]
- Gurr G.M, Wratten S.D, Altieri A, editors. Ecological engineering for pest management, advances in habitat manipulation for arthropods. CAB International; Wallingford, UK: 2004. [Google Scholar]
- Hall D.R, Beevor P.S, Cork A, Nesbitt B.F, Vale G.A. 1-Octen-3-ol: a potent olfactory stimulant and attractant for tsetse isolated from cattle odours. Insect Sci. Appl. 1984;5:153–163. [Google Scholar]
- Hardie J, Minks A.K, editors. Pheromones of non-lepidopteran insects associated with agricultural plants. CAB International; Wallingford, UK: 1999. [Google Scholar]
- Hester R.E, Harrison R.M, editors. Sustainability in agriculture. Royal Society of Chemistry; Cambridge, UK: 2005. [Google Scholar]
- Howse P.E, Stevens I.D.R, Jones O.T, editors. Insect pheromones and their use in pest management. Chapman and Hall; London, UK: 1998. [Google Scholar]
- Jensen K.-M.V, Jespersen J.B, Birkett M.A, Pickett J.A, Thomas G, Wadhams L.J, Woodcock C.M. Variation in the load of the horn fly, Haematobia irritans, in cattle herds is determined by the presence or absence of individual heifers. Med. Vet. Entomol. 2004;18:275–280. doi: 10.1111/j.0269-283X.2004.00506.x. doi:10.1111/j.0269-283X.2004.00506.x [DOI] [PubMed] [Google Scholar]
- Jones, O. T. 2002 An analysis of potential barriers to the commercial development of semiochemicals for crop protection. Defra deskop study.
- Kato T, Sato S, Nakamura Y, Kaneko T, Asamizu E, Tabata S. Structural analysis of a Lotus japonicus genome. V. Sequence features and mapping of sixty-four TAC clones which cover the 6.4 Mb regions of the genome. DNA Res. 2003;10:277–285. doi: 10.1093/dnares/10.6.277. doi:10.1093/dnares/10.6.277 [DOI] [PubMed] [Google Scholar]
- Kfir R, Overholt W.A, Khan Z.R, Polaszek A. Biology and management of economically important lepidopteran cereal stemborers in Africa. Annu. Rev. Entomol. 2002;47:701–731. doi: 10.1146/annurev.ento.47.091201.145254. doi:10.1146/annurev.ento.47.091201.145254 [DOI] [PubMed] [Google Scholar]
- Khan Z.R, Pickett J.A. The ‘push–pull’ strategy for stemborer management: a case study in exploiting biodiversity and chemical ecology. In: Gurr G.M, Wratten S.D, Altieri M.A, editors. Ecological engineering for pest management: advances in habitat manipulation for arthropods. CAB International; Wallingford, UK: 2004. pp. 155–164. [Google Scholar]
- Khan Z.R, Polaszek A. Host plants. In: Polaszek A, editor. African cereal stemborers: economic importance, taxonomy, natural enemies and control. CAB International; Wallingford, UK: 1998. pp. 3–10. [Google Scholar]
- Khan Z.R, Chiliswa P, Ampong-Nyarko K, Smart L.E, Polaszek A, Wandera J, Mulaa M.A. Utilisation of wild gramineous plants for the management of cereal stemborers in Africa. Insect Sci. Appl. 1997a;17:143–150. [Google Scholar]
- Khan Z.R, et al. Intercropping increases parasitism of pests. Nature. 1997b;388:631–632. doi:10.1038/41681 [Google Scholar]
- Khan Z.R, Pickett J.A, Vandenberg J, Wadhams L.J, Woodcock C.M. Exploiting chemical ecology and species diversity: stemborer and Striga control for maize and sorghum in Africa. Pest Man. Sci. 2000;56:957–962. doi:10.1002/1526-4998(200011)56:11<957::AID-PS236>3.0.CO;2-T [Google Scholar]
- Khan Z.R, Pickett J.A, Wadhams L.J, Muyekho F. Habitat management strategies for the control of cereal stemborers and Striga in maize in Kenya. Insect Sci. Appl. 2001;21:375–380. [Google Scholar]
- Khan Z.R, Hassanali A, Overholt W, Khamis T.M, Hooper A.M, Pickett J.A, Wadhams L.J, Woodcock C.M. Control of witchweed Striga hermonthica by intercropping with Desmodium spp. and the mechanism defined as allelopathic. J. Chem. Ecol. 2002;28:1871–1885. doi: 10.1023/a:1020525521180. doi:10.1023/A:1020525521180 [DOI] [PubMed] [Google Scholar]
- Kouchi H, et al. Large-scale analysis of gene expression profiles during early stages of root nodule formation in a model legume, Lotus japonicus. DNA Res. 2004;11:263–274. doi: 10.1093/dnares/11.4.263. doi:10.1093/dnares/11.4.263 [DOI] [PubMed] [Google Scholar]
- Lamblin A.F.J, et al. MtDB, a database for personalized data mining of the model legume Medicago truncatula transcriptome. Nucleic Acids Res. 2003;31:196–201. doi: 10.1093/nar/gkg119. doi:10.1093/nar/gkg119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes, M., Napier, J. A., Pickett, J. A. & Woodcock, C. M. 2003 New chemical signals in plant protection against herbivores and weeds. In Proc. BCPC Int. Congress—Crop Science & Technology 2003, 10–12 November, SECC Glasgow, 9C-4, pp. 1227–1236.
- Midega C.A.O, Khan Z.R, Vandenberg J, Ogol C.K.P.O, Pickett J.A, Wadhams L.J. Maize stemborer predator activity under ‘push–pull’ system and Bt-maize: a potential component in managing Bt-resistance. Int. J. Pest Man. 2006;52:1–10. doi:10.1080/09670870600558650 [Google Scholar]
- Miller J.R, Cowles R.S. Stimulo-deterrent diversionary cropping: a concept and its possible application to onion maggot control. J. Chem. Ecol. 1990;16:3197–3212. doi: 10.1007/BF00979619. doi:10.1007/BF00979619 [DOI] [PubMed] [Google Scholar]
- Nedorezov, L. V., Hassanali, A. & Sadykov, A. M. 2005 Individual-based model of mosquito choices up odour plumes to alternative hosts. In Proc. Fifth Eur. Conf. on Ecological Modelling (ed. A. S. Komarov), pp. 136–137.
- Owaga M.L.A, Hassanali A, McDowell P.G. The role of 4-cresol and 3-n-propylphenol in the attraction of tsetse to buffalo urine. Insect Sci. Appl. 1988;9:95–100. [Google Scholar]
- Pickett J.A, Poppy G.M. Switching on plant genes by external chemical signals. Trends Plant Sci. 2001;6:137–139. doi: 10.1016/s1360-1385(01)01899-4. doi:10.1016/S1360-1385(01)01899-4 [DOI] [PubMed] [Google Scholar]
- Pickett J.A, Wadhams L.J, Woodcock C.M. Developing sustainable pest control from chemical ecology. Agric. Ecol. Environ. 1997;64:149–156. doi:10.1016/S0167-8809(97)00033-9 [Google Scholar]
- Pickett J.A, Rasmussen H.B, Woodcock C.M, Matthes M, Napier J.A. Plant stress signalling: understanding and exploiting plant–plant interactions. Biochem. Soc. Trans. 2003;31:123–127. doi: 10.1042/bst0310123. [DOI] [PubMed] [Google Scholar]
- Powell W, Pickett J.A. Manipulation of parasitoids for aphid pest management: progress and prospects. Pest Man. Sci. 2003;59:149–155. doi: 10.1002/ps.550. doi:10.1002/ps.550 [DOI] [PubMed] [Google Scholar]
- Pyke B, Rice M, Sabine B, Zalucki M. The push–pull strategy—behavioural control of Heliothis. Aust. Cotton Grower. 1987;4:7–9. [Google Scholar]
- Rebe M, Vandenberg J, McGeoch M.A. Colonization of cultivated and indigenous graminaceous host plants by Busseola fusca (Fuller) (Lepidoptera: Noctuidae) and Chilo partellus (Swinhoe) (Lepidoptera: Crambidae) under field conditions. Afr. Entomol. 2004;12:187–199. [Google Scholar]
- Royal Society policy statements and reports 2001 The use of genetically modified animals. Ref 05/01.
- Royal Society policy statements and reports 2002 Genetically modified plants for food use and human health—an update. Ref 04/02.
- Seyoum A, Balcha M, Balkew A.A, Gebre-Michael T. Impact of cattle keeping on human biting rate of Anopheline mosquitoes and malaria transmission around Ziway, Ethiopia. East Afr. Med. J. 2002;79:485–490. doi: 10.4314/eamj.v79i9.9121. [DOI] [PubMed] [Google Scholar]
- Smart L.E, Pickett J.A, Powell W. Push–pull strategies for pest control. Grain Legumes. 1997;15:14–15. [Google Scholar]
- Torr S.J, Mangwiro T.N.C, Hall D.R. Responses of Glossina pallidipes (Diptera: Glossinidae) to synthetic repellents in the field. Bull. Entomol. Res. 1996;86:609–616. [Google Scholar]
- Trumble J.T. In: Insect pheromone research: new directions. Cardé R, Minks A, editors. Chapman and Hall; London, UK: 1997. pp. 397–410. ch. 35. [Google Scholar]
- Trumble J.T, Alvarado-Rodriguez B. Development and economic evaluation of an IPM program for fresh market tomato production in Mexico. Argric. Ecol. Envion. 1993;43:267–284. doi:10.1016/0167-8809(93)90091-3 [Google Scholar]
- Turlings T.C.J, Tumlinson J.H, Lewis W.J. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. doi:10.1126/science.250.4985.1251 [DOI] [PubMed] [Google Scholar]
- Vale G.A, Lovemore D.F, Flint S, Cockbill G.F. Odour-baited targets to control tsetse flies, Glossina spp. (Diptera: Glossindae), in Zimbabwe. Bull. Entomol. Res. 1988;78:31–49. [Google Scholar]
- Van Emden H.F, Peakall D.B. Chapman and Hall; London, UK: 1996. Beyond silent spring, integrated pest management and chemical safety. [Google Scholar]
- Wanzala W, Sika N.F.K, Gule S, Hassanali A. Attractive and repellent host odours guide ticks to their respective feeding sites. Chemoecology. 2004;14:229–232. doi:10.1007/s00049-004-0280-6 [Google Scholar]
- Waterlow J.C, Armstrong D.G, Fowden L, Riley R, editors. Feeding a world population of more than eight billion people—a challenge to science. Oxford University Press; Oxford, UK: 1998. [Google Scholar]
- Weatherston I, Minks A.K. Regulation of semiochemicals—global aspects. Integr. Pest Manage. Rev. 1995;1:1–13. doi:10.1007/BF00140330 [Google Scholar]
- WHO. World Health Organisation; Geneva, Switzerland: 1982. Manual on environmental management for mosquito control with special emphasis on malaria vectors. [PubMed] [Google Scholar]