Abstract
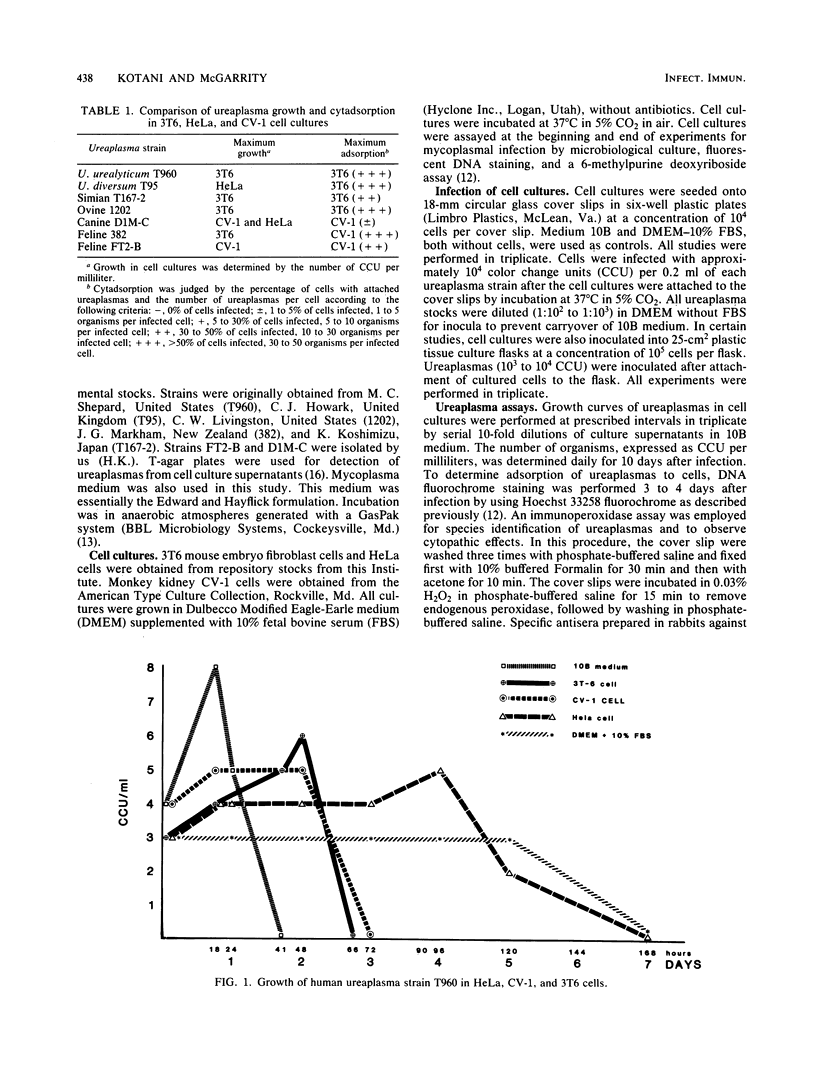
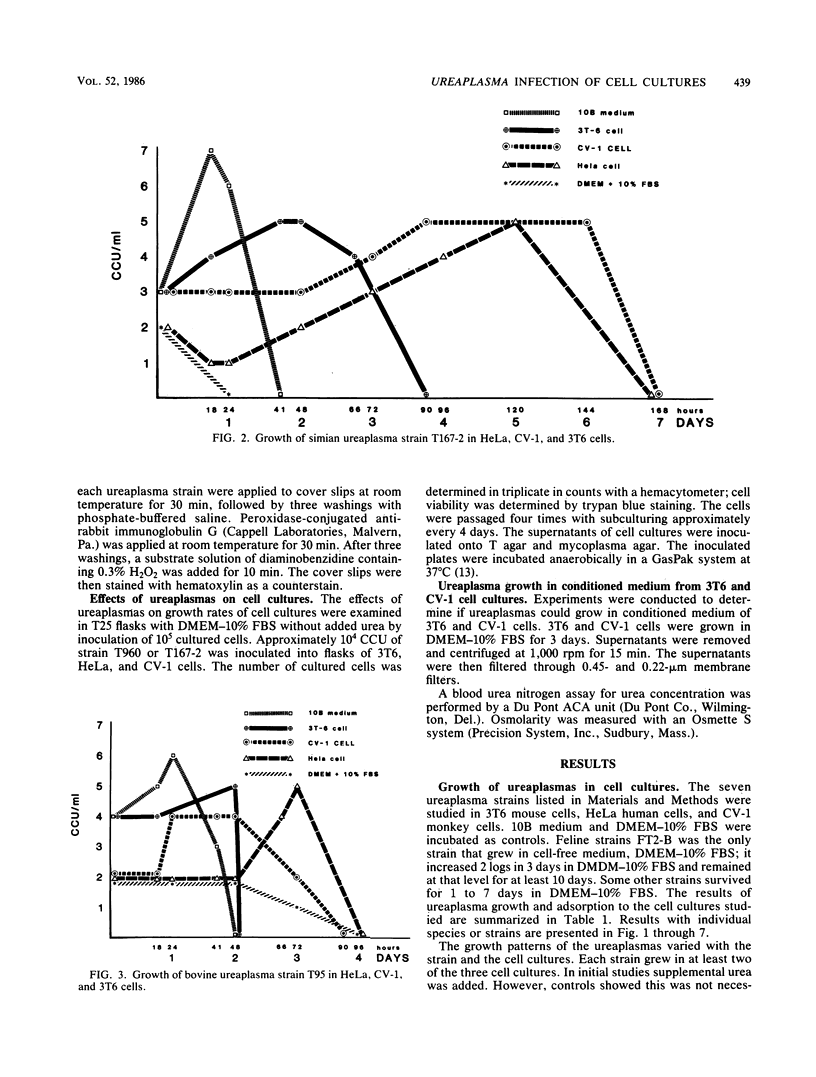
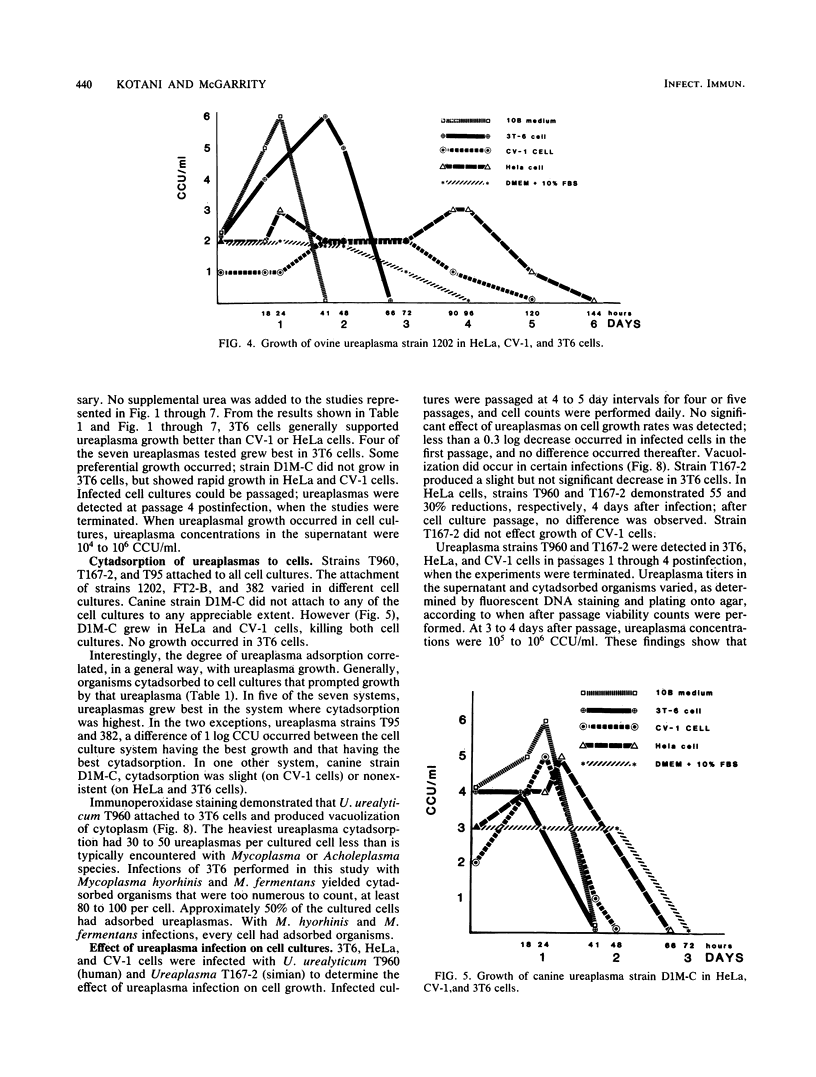
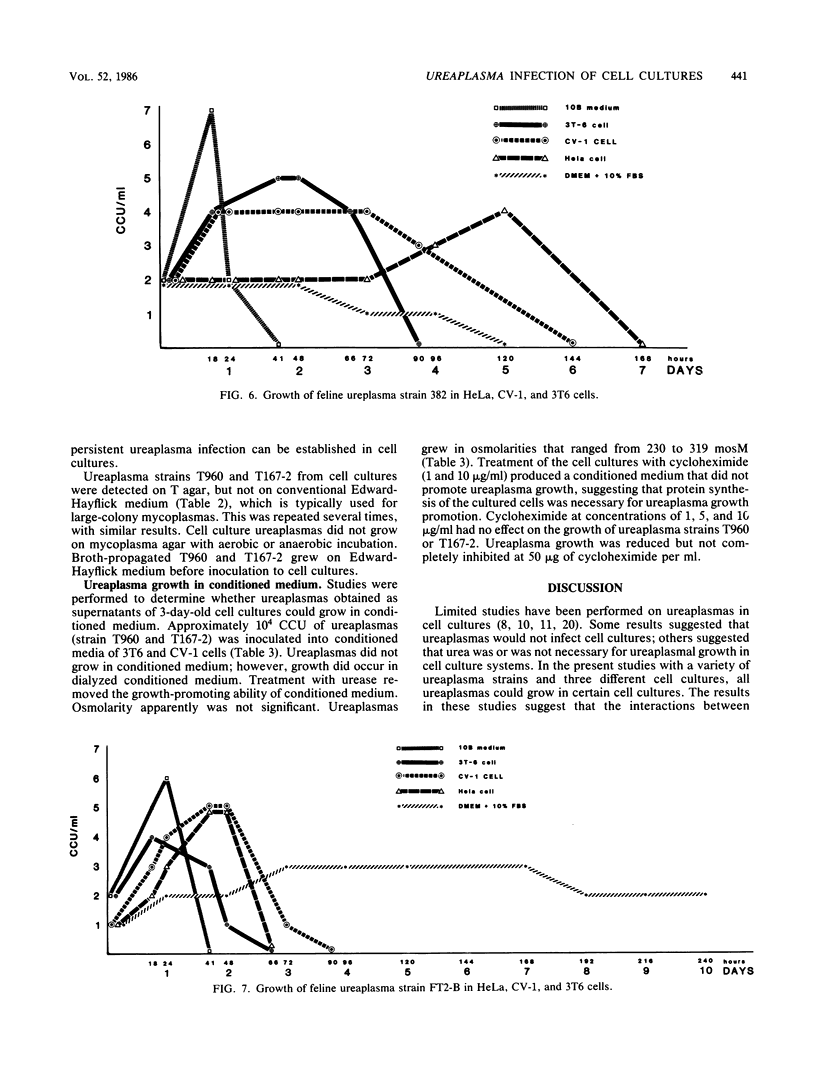
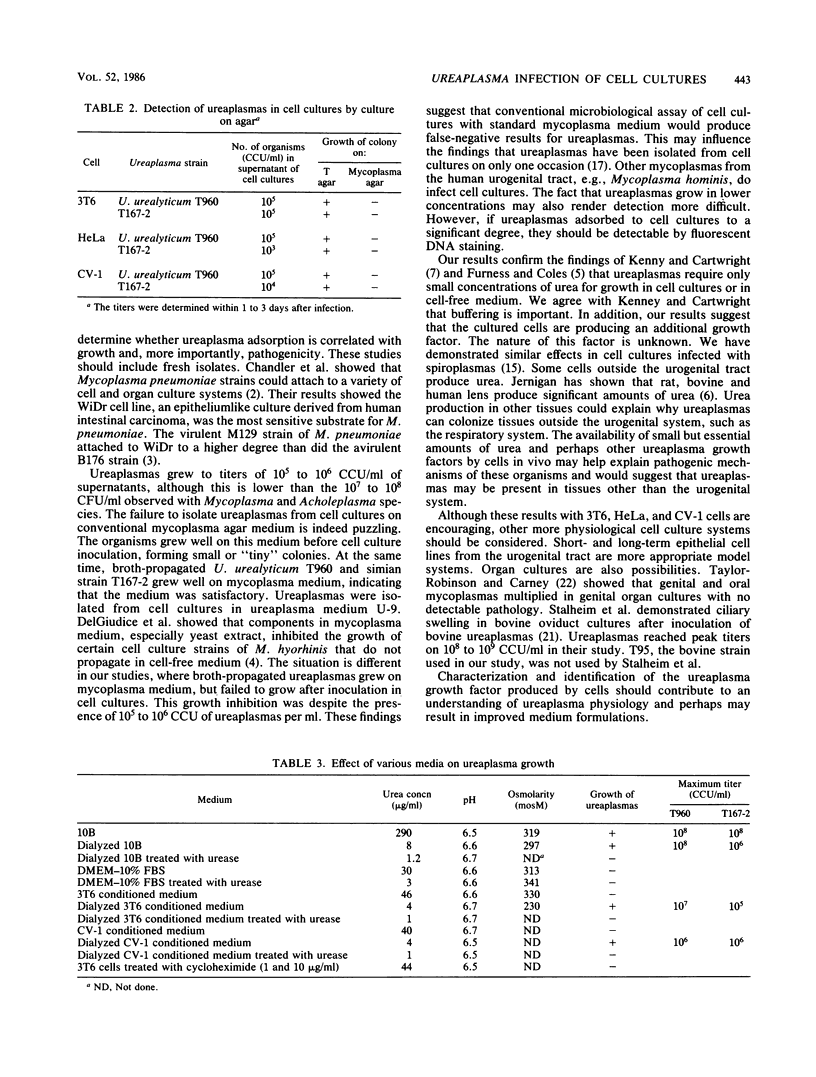
Studies were performed to characterize the effects of ureaplasmas in HeLa, 3T6, and CV-1 cell cultures. The ureaplasmas studied were human Ureaplasma urealyticum T960 (serotype VIII), bovine U. diversum T95, simian strain T167-2, ovine strain 1202, canine strain D1M-C, and feline strains 382 and FT2-B. FT2-B was the only ureaplasma to grow in the cell free culture medium, Dulbecco modified Eagle-Earle medium containing 10% fetal bovine serum. The growth pattern of the ureaplasmas varied in the different cell cultures, but each strain grew in at least two of the cell cultures, suggesting a requirement for a product of the cell culture and for low concentrations of urea. When growth occurred, organisms grew to concentrations that approached, but did not equal, those observed in 10B broth. Most, but not all, ureaplasmas grew quickly, reaching peak titers 2 days after infection. Canine strain D1M-C did not grow in 3T6, but showed rapid growth in HeLa and CV-1 cells, killing both cultures, In some systems, e.g., U. urealyticum T960 and simian strain T167-2, the infection persisted, and ureaplasmas could be recovered from cell cultures four passages after infection, when studies were terminated. The cell culture ureaplasmas grew on T agar, but not on mycoplasma agar medium.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chandler D. K., Collier A. M., Barile M. F. Attachment of Mycoplasma pneumoniae to hamster tracheal organ cultures, tracheal outgrowth monolayers, human erythrocytes, and WiDr human tissue culture cells. Infect Immun. 1982 Mar;35(3):937–942. doi: 10.1128/iai.35.3.937-942.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. K., Grabowski M. W., Barile M. F. Mycoplasma pneumoniae attachment: competitive inhibition by mycoplasmal binding component and by sialic acid-containing glycoconjugates. Infect Immun. 1982 Nov;38(2):598–603. doi: 10.1128/iai.38.2.598-603.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness G., Coles R. S. The urea requirement and urease production of some human species of T-mycoplasmas. Proc Soc Exp Biol Med. 1975 Dec;150(3):807–809. doi: 10.3181/00379727-150-39129. [DOI] [PubMed] [Google Scholar]
- Jernigan H. M., Jr Urea formation in rat, bovine, and human lens. Exp Eye Res. 1983 Dec;37(6):551–558. doi: 10.1016/0014-4835(83)90131-8. [DOI] [PubMed] [Google Scholar]
- Kenny G. E., Cartwright F. D. Effect of urea concentration on growth of Ureaplasma urealyticum (T-strain mycoplasma). J Bacteriol. 1977 Oct;132(1):144–150. doi: 10.1128/jb.132.1.144-150.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Studies on the nature of receptors involved in attachment of tissue culture cells to mycoplasmas. Br J Exp Pathol. 1969 Feb;50(1):66–75. [PMC free article] [PubMed] [Google Scholar]
- Masover G. K., Hayflick L. Dialysis culture of T-strain mycoplasmas. J Bacteriol. 1974 Apr;118(1):46–52. doi: 10.1128/jb.118.1.46-52.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masover G. K., Namba M., Hayflick L. Cytotoxic effect of a T-strain mycoplasma (Ureaplasma urealyticum) on cultured normal human cells (wi-38). Exp Cell Res. 1976 May;99(2):363–374. doi: 10.1016/0014-4827(76)90594-2. [DOI] [PubMed] [Google Scholar]
- Mazzali R., Taylor-Robinson D. The behaviour of T-mycoplasmas in tissue culture. J Med Microbiol. 1971 Feb;4(1):125–138. doi: 10.1099/00222615-4-1-125. [DOI] [PubMed] [Google Scholar]
- McGarrity G. J., Coriell L. L. Detection of anaerobic mycoplasmas in cell cultures. In Vitro. 1973 Jul-Aug;9(1):17–18. doi: 10.1007/BF02615983. [DOI] [PubMed] [Google Scholar]
- Megraud F., Gamon L. B., McGarrity G. J. Characterization of Spiroplasma mirum (suckling mouse cataract agent) in a rabbit lens cell culture. Infect Immun. 1983 Dec;42(3):1168–1175. doi: 10.1128/iai.42.3.1168-1175.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata M., Kotani H., Yamamoto K. Serological comparison of bovine ureaplasmas. Nihon Juigaku Zasshi. 1979 Dec;41(6):629–637. doi: 10.1292/jvms1939.41.629. [DOI] [PubMed] [Google Scholar]
- SHEPARD M. C., LUNCEFORD C. D. EFFECT OF PH ON HUMAN MYCOPLASMA STRAINS. J Bacteriol. 1965 Feb;89:265–270. doi: 10.1128/jb.89.2.265-270.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi K. K. On the incidence of mycoplasma contamination in cell cultures. Zentralbl Bakteriol Orig A. 1972 Apr;219(4):550–554. [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D. Serological typing of Ureaplasma urealyticum isolates from urethritis patients by an agar growth inhibition method. J Clin Microbiol. 1978 Nov;8(5):566–574. doi: 10.1128/jcm.8.5.566-574.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalheim O. H., Proctor S. J., Gallagher J. E. Growth and effects of ureaplasmas (T mycoplasmas) in bovine oviductal organ cultures. Infect Immun. 1976 Mar;13(3):915–925. doi: 10.1128/iai.13.3.915-925.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Carney F. E., Jr Growth and effect of mycoplasmas in Fallopian tube organ cultures. Br J Vener Dis. 1974 Jun;50(3):212–216. doi: 10.1136/sti.50.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]